Preparation, thermal and mechanical properties of POSS/PI hybrid films for cryogenic applications
WEI Shaohua, WU Xiaojun, DU Kai, YI Yong, YIN Qiang*
(1.Joint Laboratory for Extreme Conditions Matter Properties, Southwest University of Science and Technology, Mianyang621010, Sichuan, China; 2.Research Center of Laser Fusion, China Academy of Engineering Physics,Mianyang 621900, Sichuan, China)
Preparation, thermal and mechanical properties of POSS/PI hybrid films for cryogenic applications
WEI Shaohua1,2, WU Xiaojun2, DU Kai1,2, YI Yong1, YIN Qiang2*
(1.JointLaboratoryforExtremeConditionsMatterProperties,SouthwestUniversityofScienceandTechnology,Mianyang621010,Sichuan,China; 2.ResearchCenterofLaserFusion,ChinaAcademyofEngineeringPhysics,Mianyang621900,Sichuan,China)
A series of polyhedral oligomeric silsesquioxane (POSS)/polyimide (PI) hybrid films were prepared by the sol-gel technique.In these nanocomposite materials, different quality ra-tios of theγ-glycidyloxypropylsilsesquioxane (G-POSS) cage mixture was introduced to the poly(pyromellitic dianhydride-co-4, 4′-oxydianiline), amic acid solution (PAA) and the precursor of polyimide.Then, the intermediate products were cast to the glass templates and circled to be nanocomposite films at a temperature program.Particular mechanical and thermal properties of the G-POSS/PI nanocomposite films were investigated, i.e., the tensile strength of the hybrid films at cryogenic temperature (77 K) were obviously higher than that at room temperature.The highest tensile strength of the G-POSS/PI films was 239.38 MPa by incorporating 3% G-POSS at 77 K, and 101.3 MPa with 5% G-POSS at room temperature, respectively.With the raise of G-POSS content, the tensile modulus of the films increased, while the elongation at break of these hybrid films decreased in tensile tests and it was also found that the thermal degradation temperatures of these materials had an obvious decrease which was attributed to the weak thermal resistance of the organic groups of G-POSS molecule.As a consequence, the hybrid film with lower than 5% POSS content (e.g., 3%) would be more possibly used as a kind of novel material at cryogenic temperature compared with pure PI film in the cryogenic refrigeration technology and inertial confinement fusion (ICF) physics experiments.
G-POSS/polyimide; mechanical properties; cryogenic applications
Aromatic polyimides (PIs) have been widely studied and applied in aerospace and microelectro-nic industries at extreme conditions since Kapton films were released by Du Pont Corporation (USA) in 1960s.[1]From then on, much work has been done by many researchers related to the to-pping characteristics of PIs, for instance, excellent thermal stability, outstanding mechanical properties, eximious chemical resistance, and low dielectric constant.[2]However, with the rapid development in some special applications such as the cryogenic refrigeration technology and inertial confinement fusion (ICF) physics experiment etc., further improvement of properties of PIs is of paramount importance and worthy of deeper investigation.[3-8]In ICF physics experiments, for example, the polymer capsule is contained in a radiation case named hohlraum.Then, some specific material for the fabrication of windows of hohlraum and also different membranes are needed.A promising approach is doping the polyimide with rigid nanoparticles, which is a type of hybrid material with the strengthening particles are in the order of nanometer.The fillers such as nanoparticles,[9-10]nanotubes,[11-14]graphene[15-17]and silica network[18-21]were introduced to the pristine polyimide and the hybrid systems exhibited better characterization in many reports, nevertheless, with the increase of the amount of the fillers, defects also increase.
Polyhedral oligomeric silsesquioxane (POSS), which is consisted of a rigid and cubic silica core (SiO1.5)nsurrounded by organic functional groups, is a kind of advanced organic/inorganic silica hybrid material.In recent years, POSS/PIs hybrid nanocomposites have been received a great deal of attention,[22-29]but few studies of the applications of POSS/PI hybrid films at cryogenic temperature were reported.
In this work, theγ-glycidyloxypropylsilsesquioxane (G-POSS) cage mixture is chosen for its ability of self-addition through the epoxy groups[20]to make a reticular structure in the poly(pyromellitic dianhydride-co-4,4′-oxydianiline), amic acid (PAA) solution.Moreover, it can react with the hydroxyl carboxyl of PAA chains to enhance the crossing density of the hybrid system at elevated temperatures to improve the compatibility of the two components.[18]As it is well known, the mechanical property of a composite material strongly depends on the compatibility and interfacial action between the filler and the matrix.Therefore, we intend to improve the dispersion and interfacial action of the POSS/PI hybrid system through the epoxy groups, and explore its mechanical properties at cryogenic temperature.
1 Experimental
1.1 Materials
All materials were commercially available.The poly(pyromellitic dianhydride-co-4,4′-oxydianiline), amic acid solution (15.0%-16.0% in NMP, 50-70 poise) was purchased from the SIGMA-ALDRICH CO.(USA), and used without further purification.1-Methyl-2-pyrrolidinone (NMP, analytical reagent grade) was obtained from Xiya Chemical Industry Co.Ltd.(Shandong, China) and dried over molecular sieves before using.The G-POSS Mixture was purchased from Hybrid Plastics (USA).Scheme 1a shows mole-cular structure of the PAA chain unit formed by condensation polymerization of pyromellitic dianhydryde (PMDA) and oxydianiline (ODA).The PI chain unit structure was shown in Scheme 1b and the structure of G-POSS monomer was shown in Scheme 1c.
1.2 Preparation of pure polyimide film
The sol-gel technique was employed for the synthesis of pure PI and hybrid films.The main steps were as follows: 15 g poly amic acid solution

Scheme 1 Schematic presentation of polyamic acid unit (a), PMDA-ODA PI unit (b) and G-POSS molecure (c)
was added to a 250 mL four necks flask equipped with nitrogen (N2) inlet and mechanical stirrer.Some proper NMP was added to the flask and the solid concentration was kept at about 5.5%.The mixed solution was stirred at -10 ℃ under N2(99.999%) for about 6 h to get a transparent and homogeneous solution.The pure polyimide film was prepared by casting the solution onto a glass template.After the majority of solvent volatilized at 80 ℃, the poly amic acid film (PAA) was tore off from the glass template and heated at 100, 150, 200, 250 and 300 ℃ for 1 h respectively to obtain light yellow transparent film.
1.3 Preparation of G-POSS/PI hybrid films
In a 100 mL dried beaker, the required quality ratio of G-POSS mixture and some NMP were placed.Then the container was settled in an ultrasonic unit and processed at room temperature for 0.5 h to gain achromatous solution.The solution was added to the PAA precursor and the system was stirred for another 6 h under flowing N2.The rest steps of preparing the PI-POSS hybrid films were the same as the pure PI film, and yellow or brown films was obtained.
1.4 Characterization
Diffuse reflectance infrared Fourier transform (DRIFT-IR) spectra of films were obtained by a Nicolet 6700 FT-IR spectrometer (Nicolet Instrument Corporation, USA).The film samples were dried in a vacuum at 110 ℃ before they were tested.At least 32 scans at a resolution of 4 cm-1were performed for each sample.
Thermal gravimetric analysis (TGA) was ca-rried out with the instrument (TGA, Pyris 1 PE) at a heating rate of 10 ℃/min from 100 to 800 ℃ under a continuous argon (99.999%) flow.
Mechanical properties of 45-50 μm thick G-POSS/PI films at room and cryogenic temperature were measured according to the specification of ISO 527-3:1 995 at a strain rate of 5 mm/min, respectively.Specimens having a constant width of 13 mm in the gauge region were cut into rectangle shape samples.The initial distance between grips was 100 mm.Tests were performed using a universal testing machine (KDⅢ-5, Kaiqiangli testing Instruments Co., Ltd).Six samples of each G-POSS/PI film were tested and standard deviations were recorded.
Images of the fracture surfaces of the films were obtained using an environmental scanning electron microscopy (SEM, MERLIN|VP Compact ZEISS, German) with the acceleration voltage of 10 kV.The samples were coated with a layer of gold for 150 s in vacuum conditions.
2 Results and discussion
2.1 FT-IR
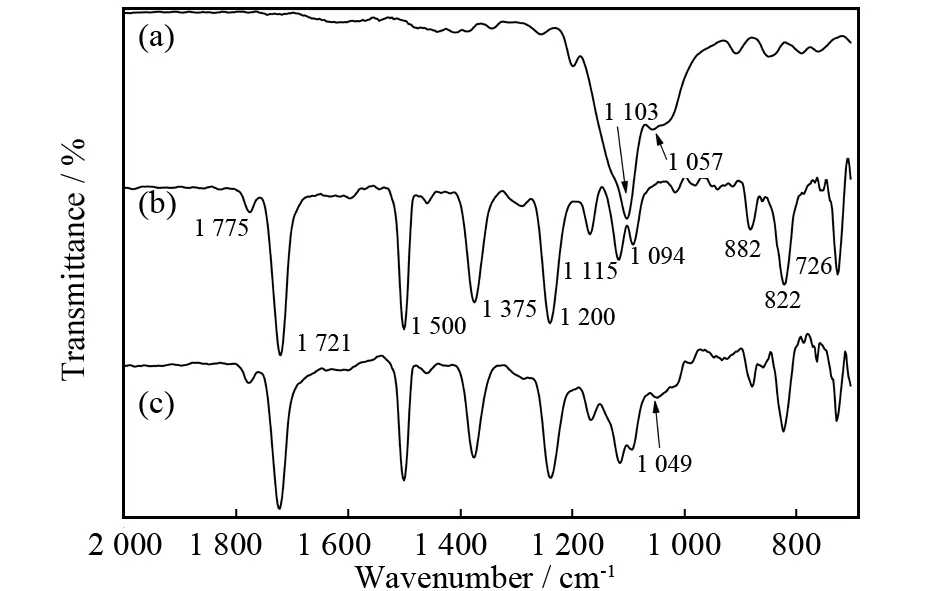
FT-IR spectra of the G-POSS cage mixture, pure polyimide film and 20% G-POSS/PI hybrid film between 4 000-700 cm-1are showed in Fig.1, 2 000-700 cm-1are showed in Fig.2.The sharp and strong peaks of 1 775 cm-1(C=O sy-mmetric stretching), 1 721 cm-1(C=O asymmetric stretching), 1 375 cm-1(imide groups C-N stretching), 726 cm-1(C=O bending) were observed in the pure polyimide and the 20% G-POSS/PI sample films.The wide and strong absorption at wavenumbers 1 200-1 000 cm-1in the spectrum of G-POSS corresponds to Si-O-Si stretching vibration.[25, 28]The peak of 1 103 cm-1which belongs to Si-O-Si cage stretching vibration was difficult to distinguish from the 20% G-POSS/PI film, but another representative band belonged to Si-O-Si network stretching vibration which changed from 1 057 cm-1to 1 049 cm-1was obvious.This change resulted from the higher crosslink density was produced in circled program, which leaded to a tighter network of the G-POSS.Similar results were obtained in ref.18, with the molecule of G-POSS was solidified in hybrid film, the position of absorption position decreased to lower wavenumber.It is noted that the absorption bands at 3 000-3 500 cm-1(Si-OH, H2O) in the curve of G-POSS disappeared in the 20% G-POSS/PI hybrid film which was resulted from self-condensation reaction and removal of absorbed water in the G-POSS material.[18]

2.2 Thermal properties
Fig.3 presents TGA curves of G-POSS, pure PI and various G-POSS/PI nanocomposite films under argon atmosphere.A decrease in the decomposition temperature (Td) was found for G-POSS/PI nanocomposites relative to the pure PI due to the lower degradation temperature of organic groups of G-POSS.Char yields of these G-POSS/PI hybrid materials remained nearly the same as the pure PI.More detailed information can be found in the Table.1.Fig.4 showed TGA curves of 10% G-POSS/PI hybrid film and mixture of G-POSS and pure PI.It was observed that theTdof 10% G-POSS/PI hybrid film had been improved, which was attributed to the reaction between epoxy groups and hydroxyl carboxyl of PAA chains.In our study, the main concern was the performance at low temperature which was significantly lower than theTds of the hybrid films.Therefore, the decrease of the thermal property of the film was not an obstacle where we used in the physics experiment.
Table 1 Effects of G-POSS content on the thermal properties of G-POSS/PI hybrid films

G-POSSContent/%0151020Td/℃421.59401.86355.27326.75300.29Td(5%loss)/℃591.11579.08552.36517.59415.29


2.3 Mechanical properties
Typical tensile strength curves for the films are shown in Fig.5.It is observed that the tensile strength of G-POSS/PI hybrid films and the parent PI at 77 K are generally higher than that at room temperature.This is because on the one hand, the PI molecules are frozen at 77 K, leading to a tighter array and stronger interface adhesion, which results in a higher tensile strength than that at room temperature.On the other hand, the reaction between epoxy groups and hydroxyl carboxyl minor matters of PAA chains forms a network with the core of G-POSS, which increases the crosslink density, also enhancing the intensity of the tensile strength of the nanocomposites.Fig.5 also displays that the tensile strength of the hybrid films at both cryogenic and room temperature increases with the increase of G-POSS content, and is higher than that of the pure PI film when G-POSS content is lower than 5% at cryogenic temperature and 10% at room temperature respectively, and then decreases with the increase of G-POSS content.The similar result is observed in PI-MMT and PI-mica hybrid films with low fillers content.[29]The tensile strength of the hybrid films exhibits the maximum value of 239.38 MPa with 18.38% increase at 3% G-POSS, and then decreased with the increase of G-POSS content.The improvement of the tensile strength of the G-POSS/PI hybrid films at low G-POSS content is attributed to the fact that the load can be effectively transferred to the G-POSS through the PI matrix because of the strong interfacial action between G-POSS and PI matrix.The depravation of tensile strength at higher G-POSS content is possibly caused by the bad dispersion and the aggregation of G-POSS in the PI matrix as shown in Fig.8.




Fig.6 shows the tensile modulus of G-POSS/PI hybrid films as a function of G-POSS content at room and cryogenic temperature.It could be seen that the modulus of hybrid films displays an increasing tendency with the increase of G-POSS content at both room and cryogenic temperature.This is mainly attributed to the fact that the Young’s modulus of G-POSS is higher than that of PI matrix.Moreover, the modulus at cryogenic temperature is higher than that at room temperature because of the tighter arrangement of the PI matrix.
Fig.7 reveals that the elongation at break of G-POSS/PI hybrid films.It can be seen that the elongation at break at cryogenic temperature is much lower than that at room temperature.That might resulted from the increase of the crosslink density of the system and a brittle fracture mechanism at cryogenic temperature.Furthermore, it is found that when the G-POSS content is lower than 5%, the elongation at break of the hybrid films is more than 5% at 77 K, showing some ductility for cryogenic engineering applications.This indicates that the polyimide molecules show definite mechanical and flexible properties at low temperature.
2.4 Morphologies of the fracture surfaces of G-POSS/PI hybrid films
On the fracture surface of hybrid film samples (Fig.8a), we can clearly observe some plastic deformed veins which correspond to shrinkage deformation of the film as a homogenous material.These microstructure characteristics generally lead to lower strength and modulus of the film though it has a better plastic deformation capability.With adding G-POSS, the morphological change is obvious on the fracture section; there is a significant enhancement in the mechanical properties of hybrid films.The relative high tensile strength and modulus of hybrid films could be attributed partly to the fine microstructures found in hybrid composite films (Fig.8b).However, with the G-POSS content increases, the G-POSS aggregation degree would increase, which will causes a pronounced drop in the tensile strength and ductility of the hybrid films with higher G-POSS contents, though the incorporation of G-POSS nanoparticles have enhanced the Young’s modulus of hybrid films (Fig.8c, d).With the film of high G-POSS content (20%).The film has a brittle fracture mechanism.This is because the adhesion between the G-POSS aggregations and matrix became weaker which are consistent with Figs.5-7.
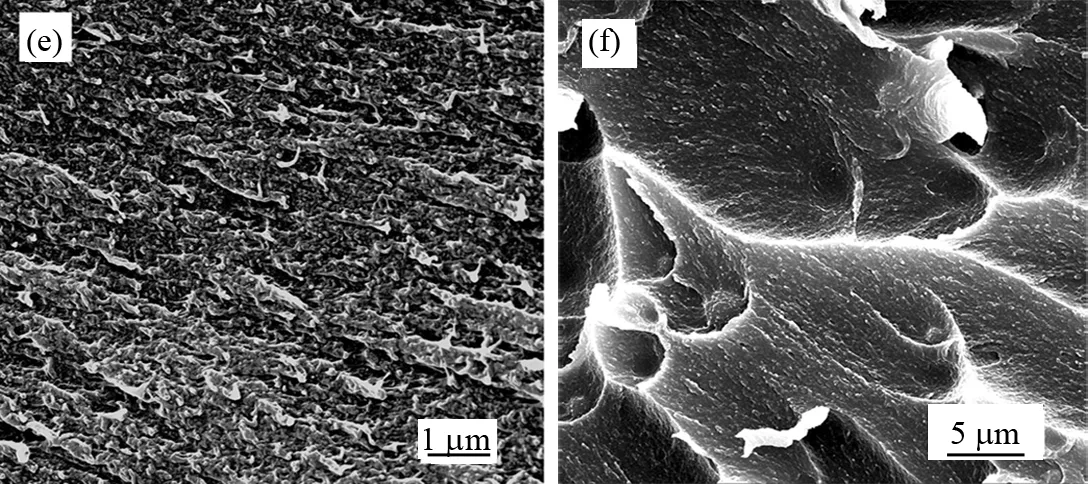
As shown in Fig.9, with a tighter array and stronger interface adhesion at 77 K, both of the pure PI (e) and 20% G-POSS/PI (f) films exhibit more coarser fracture surfaces than those at room temperature (Fig.8a and d).
3 Conclusions
Different quality ratios of G-POSS cage mixture are introduced to the PMDA-ODA polyimide and a series of nanocomposite films are obtained.We investigate the relationship between the additive amounts of G-POSS and thermal and mechanical properties of hybrid systems.It is observed that the existence of epoxy groups reduce theTds and the elongation at break of the films, but increase the crosslink density of the systems to improve the compatibility of the two components.The tensile strength increased when G-POSS content is lower than 5% compared to the pure PI film at both room and cryogenic temperature.This work demonstrates that it is possible to use sol-gel technique to process special membrane materials used in the ICF experiments.And application of these hybrid films at cryogenic temperature is ongoing in our group.
[1] WILSON D, STENZENBERGER H D, HERGENROTHER P M.Polyimides [M].London: Chapman & Hall, 1990: 10-20.
[2] DING M X.Isomeric polyimides [J].Prog Polym Sci, 2007, 32: 623-668.
[3] YAMAOKA H, MIYATA K, YANO O.Cryogenic properties of engineering plastic films [J].Cryogenics, 1995, 35: 787-789.
[4] MARSACQ D, DUFOUR B, BLONDEL B, et al.High-performance aromatic polyimides for inertial confinement fusion experiments [J].Polym Int, 2000, 49: 1021-1023.
[5] FORBES R.Recent contributions to the use of polyimides in the fabrication of ICF and IFE targets [J].Fusion Sci Technol, 2004, 45: 197-201.
[6] POUSSARD L, ANSELMI E, BLONDEL B, et al.Synthesis and characterization of fully deuterated upilex type polyimides [J].Fusion Sci Technol, 2006, 49: 707-713.
[7] BERANT T P, BITTNER D N, CARTER S, et al.Plastic deformation and helium permeation in thin polyimide windows [J].Fusion Sci Technol, 2009, 55: 343-348.
[8] KOOHMAREH G A.Synthesis and characterization of new disperse-red functionalized polyimide for use as nonlinear optical material [J].Des Monomers Polym, 2012, 15: 275-288.
[9] LIU L Z, LI Y Y, WENG L, et al.Effect of Al2O3-coated SiO2on properties of Al2O3-coated SiO2/PI composite films [J].Iran Polym J, 2014, 23: 987-994.
[10] BABANZADEH S, ATAEI S M, MAHJOUB A.Effect of nanosilica on the dielectric properties and thermal stability of polyimide/SiO2nonohybrid [J].Des Monomers Polym, 2013, 16: 417-424.
[11] LI Y Q, PAN Q Y, LI M, et al.Preparation and mechanical properties of novel polyimide-T-silica hybrid films [J].Compo Sci Technol, 2007, 67: 54-60.
[12] SO H H, CHO J W, SAHOO N G.Effect of carbon nanotubes on mechanical and electrical properties of polyimide/carbon nanotubes nanocomposites [J].Eur Polym J, 2007, 43: 3750-3756.
[13] YUEN S M, MA C C M, LIN Y Y, et al.Preparation, morphology and properties of acid and amine modified multiwalled carbon nanotube-polyimide composite [J].Compo Sci Technol, 2007, 67: 2564-2573.
[14] ABDEHGAH R M, ASHOURI D, MOUSAVIAN S.In situ preparation of high performance polyimide nanocomposites based on functionalized multiwalled carbon nanotubes [J].Des Monomers Polym, 2013, 16: 108-115.
[15] KIM G Y, CHOI M C, LEE D, et al.2D-Aligned graphene shees in transparent polyimide/graphene nanocomposite films based on noncovalent interactions between poly(amic acid) and graphene carboxylic acid [J].Macromol Mater Eng, 2012, 297: 303-311.
[16] YOONESSI M, SHI Y, SCHEIMAN D A, et al.Graphene polyimide nanocomposites; thermal, mechanical, and high-temperature shape memory effects [J].ACS Nano, 2012, 6: 7644-7655.
[17] DAI W, YU J, WANG Y, et al.Enhanced thermal and mechanical properties of polyimide-graphene composites [J].Macromol Res, 2014, 22: 983-989.
[18] KIOUL A, MASCIA L.Compatibility of polyimide-silicate ceramers induced by alkoxysilane silane coupling agents [J].J Non-Cryst Solids, 1994, 175: 169-186.
[19] WANG S, AHMAD Z, MARK J E.Polyimide-silica hybrid materials modified by incorporation of an organically substituted alkoxysilane [J].Chem Mater, 1994, 6: 943-946.
[20] MASCIA L, KIOUL A.Influence of siloxane composition and morphology on properties of polyimide-silica hybrids [J].Polymer, 1995, 36: 3649-3659.
[21] SHANG X Y, ZHU Z K, YIN J, et al.Compatibility of soluble polyimide/silica hybrids induced by a coupling agent [J].Chem Mater, 2002, 14: 71-77.
[22] LEU C M, CHANG Y T, WEI K H.Synthesis and dielectric properties of polyimide-tethered polyhedral oligomeric silsesquioxane (POSS) nanocomposites via POSS-diamine [J].Macromolecules, 2003, 36: 9122-9127.
[23] LEU C M, CHANG Y T, WEI K H.Polyimide-side-chain tethered polyhedral oligomeric silsesquioxane nanocomposites for low-dielectric film applications [J].Chem Mater, 2003, 15: 3721-3727.
[24] LEU C M, REDDY G M, WEI K H, et al.Synthesis and dielectric properties of polyimide-chain-end tethered polyhedral oligomeric silsesquioxane nanocomposites [J].Chem Mater, 2003, 15: 2261-2265.
[25] VERKER R, GROSSMAN E, GOUZMAN I, et al.TriSilanolPhenyl POSS-polyimide nanocomposites Structure-properties relationship [J].Compo Sci Technol, 2009, 69: 2178-2184.
[26] VERKER R, GROSSMAN E, ELIAZ N.Erosion of POSS-polyimide films under hypervelocity impact and atomic oxygen: The role of mechanical properties at elevated temperatures [J].Acta Mater, 2009, 57: 1112-1129.
[27] VERKER R, GROSSMAN E, ELIAZ N.Effect of the POSS-polyimide nanostructure on its mechanical and electrical properties [J].Compo Sci Technol, 2012, 72: 1408-1415.
[28] LEE Y J, HUANG J M, KUO S W, et al.Polyimide and polyhedral oligomeric silsesquioxane nanocomposites for low-dielectric applications [J].Polymer, 2005, 46: 173-181.
[29] ZHANG Y H, FU S Y, LI R K, et al.Investigation of polyimide-mica hybrid films for cryogenic applications [J].Compo Sci Technol, 2005, 65: 1743-1748.
[责任编辑:张普玉]
笼形低聚倍半硅氧烷/聚酰亚胺杂化薄膜的合成、热性能及低温力学性能研究
魏少华1,2,吴小军2,杜 凯1,2,易 勇1,尹 强2*
(1.西南科技大学 极端条件物质特性联合实验室,四川 绵阳 621010; 2.中国工程物理研究院 激光聚变研究中心,四川 绵阳 621900)
通过掺杂不同质量分数的八缩水甘油醚基笼形低聚倍半硅氧烷(G-POSS),以溶胶凝胶法制备得到一种新型聚酰亚胺(PI)杂化薄膜.通过红外反射光谱(DRIFT-IR)、扫描电子显微镜(SEM)表征了其结构与薄膜断面形貌,以热重(TG)和机械性能分析研究了薄膜的耐热性与常温和低温(77 K)下的力学性能.结果表明,在掺杂量低于5%时,该杂化薄膜耐热性保持稳定,同时在常温和低温下都表现出优于纯PI膜的拉伸强度,其中在G-POSS掺杂量为3%时,杂化薄膜的拉伸强度为239.38 MPa(77 K),比纯PI膜提升了17%.这是由于在低温条件下,聚合物分子链被冻结,G-POSS粒子与PI基底间的排列更加紧密,同时界面作用力更大.
笼形低聚倍半硅氧烷/聚酰亚胺;机械性能;低温应用
O633
A
1008-1011(2016)06-0771-08

