介孔γ-Al2O3负载的高分散Ni-Ce-Zr氧化物的制备及其二氧化碳甲烷化研究
聂望欣 邹秀晶 汪学广 丁伟中 鲁雄刚
(上海大学材料科学与工程学院,省部共建高品质特殊钢冶金与制备国家重点实验室,上海市钢铁冶金新技术开发应用重点实验室,上海200072)
介孔γ-Al2O3负载的高分散Ni-Ce-Zr氧化物的制备及其二氧化碳甲烷化研究
聂望欣 邹秀晶*汪学广*丁伟中 鲁雄刚
(上海大学材料科学与工程学院,省部共建高品质特殊钢冶金与制备国家重点实验室,上海市钢铁冶金新技术开发应用重点实验室,上海200072)
采用柠檬酸(CA)-浸渍法制备了介孔γ-Al2O3(γ-MA)负载的高分散Ni-Ce-Zr氧化物,并将其用于二氧化碳甲烷化反应。研究了柠檬酸加入量对催化剂物理化学性质及催化性能的影响。结果表明,柠檬酸的加入可明显提高Ni-Ce-Zr氧化物在γ-Al2O3表面的分散性,同时可以增加镍氧化物与载体间的相互作用。制备材料经氢气还原后得到Ni-Ce-Zr/γ-MA催化剂,镍纳米颗粒均匀分散于γ-Al2O3表面。Ni-Ce-Zr/γ-MA催化剂在二氧化碳甲烷化反应中表现出了较高的反应活性和几乎100%的甲烷选择性。反应活性随CA/(Ni+Ce+Zr)摩尔比的增加而增加,主要是由于镍颗粒尺寸的减小和Ni-Ce-ZrOx物种电子和结构性质的提高。CA/(Ni+Ce+ Zr)摩尔比为1的Ni-Ce-Zr/γ-MA催化剂在反应300 h内活性仅降低7%,并且没有明显积碳。表明催化剂在二氧化碳甲烷化反应中具有优异的反应稳定性和抗积碳性能。
镍催化剂;柠檬酸辅助;混合金属氧化物;二氧化碳;甲烷化
1 Introduction
Human activities have brought about a severe rise in CO2emissions in the atmosphere.Because of its impact on the environment through the greenhouse effect,CO2fixation has attracted considerable interest in achieving a low carbon economy and society1.With regards to upcoming energy challenges,CO2conversion to biofuels and high-value chemicals seems to be a more attractive and promising approach.Considering that the consumption of fuels is two orders of magnitude higher than chemicals′one,CO2has to be mainly converted into energy carriers such as methane,methanol,and so on2-4.
CO2methanation(Sabatier reaction,CO2+4H2⇌CH4+2H2O, ΔH298K=-164.7 kJ·mol-1)shows substantial advantages over CO2conversion to other fuels because methane can be injected directly into existing natural gas pipelines,or be used as a raw material for production of chemicals.As a reversible exothermic reaction,CO2methanation is required to perform at lower temperatures in order to obtain methane yield as high as possible.However,low temperature is favorable for CO disproportionation(2CO⇌CO2+C, ΔH298K=-172.4 kJ·mol-1),resulting in coke deposition or forming subcarbonyl species.Up to now,it remains a great challenge to develop a highly active and stable catalyst for CO2methanation.
Ni-based catalysts are preferred as promising candidates for the CO2methantion due to their high intrinsic activity and low cost2-12. However,supported Ni catalysts frequently suffer from severe deactivation due to particle sintering,interaction of metal particles with carbon monoxide,formation of mobile nickel subcarbonyls, and coke deposition.Numerous investigations have been directed toward improving metal-support interaction,adding promoters, and adopting improved preparation methods2-4.Ni-Ce-Zr mixed oxides are believed to be one of the potential catalysts for CO2methanation due to their high oxygen storage capacity and properties to activate CO22,3.Nevertheless,these Ni catalysts are frequently insufficient to meet the demands of the reaction because of low surface areas,which preclude dispersion of the active metals.Some efforts have been devoted to introducing Ni-Ce-Zr mixed oxides in the support with high surface area for the dispersion of active metals.γ-Alumina was usually used as support of Ni-Ce-Zr oxides due to the large surface area and stability13-15. Synthesis of highly dispersed Ni catalyst requires a strong interaction between support and nickel oxide species.Numerous studies have been conducted to increase the dispersion of supported metal oxide by modifying the impregnation solution on support,such as using organic metal precursors and adding organic chelating additives.Citric acid(CA)has frequently been chosen as a chelating agent of metal ions in the catalyst preparation because of its excellent ability to chelate metal ions16-19.The use of chelating agents has several implications in the material properties:morphology of active phase,dispersion of metal oxides in the support,and the promotion of highly active phases20-27.
In this work,mesoporous γ-alumina supported Ni-Ce-Zr mixed oxides(Ni-Ce-Zr/γ-MA)were prepared by a CA-assisted impregnation method.Metal oxide species were uniformly dispersed on mesoporous γ-alumina surface.Their catalytic behavior for CO2methanation was investigated in detail.The obtained catalysts showed excellent catalytic activity and almost 100%CH4selectivity in the temperature range of 150-400°C.
2 Experimental
2.1Catalyst synthesis
All reagents were analytical-grade,purchased from Sinopharm Chemical Reagent Co.,Ltd.and used as received without purification.
Mesoporous γ-alumina was prepared via one-pot template-free partial hydrolysis route,as proposed in our previous study28. Typically,0.1 mol of Al(NO3)3·9H2O was dissolved in 50 mL of deionized water at 70°C,and was dropped slowly with 1 mol·L-1(NH4)2CO3aqueous solution under vigorous magnetic stirring until a formation of transparent gel,and then the gel beaker was covered with plastic film and aged at 30°C for 48 h.After this,the crude gel was dispersed in an open glass dish at 100°C for 24 h. The as-prepared solid was treated at 200°C in air for 10 h to remove ammonium nitrate,and further calcined at 500°C for 10 h in air at a heating rate of 1°C·min-1.
Ni-Ce-Zr/γ-MA materials were prepared by the impregnation method in the presence of citric acid.In a typical synthesis,Ni (NO3)2·6H2O,Ce(NO3)3·6H2O,Zr(NO3)4·5H2O and required amounts of citric acid were dissolved in 10 mL of deionized water at room temperature.5 g γ-MA was added in the above solution and stirred at room temperature for 2 h,and then continuously stirred at 80°C until the water was evaporated out.The obtained solid was dried at 100°C for 24 h and calcined at 500°C for 10 h.The nominal compositions of the Ni-Ce-Zr/γ-MAcatalysts were fixed at 15%(w,mass fraction)Ni and 10%(w)Ce-ZrO2with a Ce/Zr molar ratio of 0.7:0.3,respectively,which were confirmed to be optimum for CO2methanation in the preliminary experiments.The amounts of CAwith different CA/(Ni+Ce+Zr)molar ratios in the range of 0-2 were utilized.The final materials were denoted as Ni-Ce-Zr/γ-MA-n,where n represents CA/(Ni+Ce+ Zr)molar ratios in the reaction solutions.
2.2Catalyst characterization
Powder X-ray diffraction(XRD)was performed with a Bruker AXS D8Advance(Germany)diffractometer using Cu Kαradiation at 40 kV and 40 mA.N2adsorption and desorption isotherms weremeasured using a Micromeritics ASAP 2020 Sorptometer (America)at liquid nitrogen temperature(-196°C).Before the measurement,each sample was degassed at 300°C for 6 h.The specific surface areas(SBET)were evaluated using the Brunauer-Emmett-Teller(BET)method in the relative pressure(p/p0)range from 0.05 to 0.25.Pore size distribution curves were calculated using the desorption branch of the isotherms and the Barrett-Joyner-Halenda(BJH)method.The pore sizes(Dp)were obtained from the peak positions of the distribution curves.The pore volumes(Vp)were takenat the p/p0of 0.990single point.Temperatureprogrammed reduction with H2(H2-TPR)was performed on a fixed-bed reactor.Prior to the measurement,0.1 g of the sample placed in a quartz reactor was first pretreated in an Ar stream(30 mL·min-1)at 200°C for 0.5 h to remove moisture and other absorbed impurities.After this,H2-TPR was conducted with a gas mixture of 5%(volume fraction)H2in Ar at 30 mL·min-1.The temperature was raised to 1000°C with a heating rate of 10°C· min-1.The amount of H2uptake was measured with a thermal conductivity detector(TCD).Thermogravimetric(TG)analysis of the used catalysts was carried out on a Netzsch STA 4449 F3 thermogravimetric analyzer(Germany).The samples were first treated at 50°C for 0.5 h,and then were heated to 800°C in an air flow of 30 mL·min-1at a heating rate of 10°C·min-1.Transmission electron microscopy(TEM)micrographs were acquired with a JEOL JEM-2010F field emission microscope(Japan)operating at 200 kV.
2.3Catalyst test and analysis
CO2methanation was performed at atmospheric pressure in a vertical continuous-flow fixed-bed reactor(inner diameter of 8 mm and a length of 800 mm)at atmospheric pressure.The reaction gases were controlled using mass flow controllers.The actual temperature of the catalyst was monitored using a thermocouple placed in the middle of the catalyst bed.200 mg of catalyst diluted with 800 mg of quartz particles(60-80 mesh)was placed between two layers of quartz wool in the center of the reactor.Prior to the reaction,the catalyst was first reduced in situ at 600°C at a heating rate of 10°C·min-1under a 20 mL·min-1flow of H2for 2 h and then cooled to the set reaction temperature (150-400°C).The effluent gas was cooled in a condenser at room temperature and passed a drierite bed to remove all water.Finally, the dried gas products were analyzed using an on-line chromatogram(GC)-TCD chromatograph using TDX-01 and Molecular Sieve 5A packed column.The flow rate of the outlet gas was measured by a soap flow meter.The overall mass balance was more than 98%on the basis of carbon in the starting reactants.
On the basis of the carbon balance in the effluent gas under assumption of no carbon deposition,the conversion of CO2(XCO2) and the selectivities of CH4(SCH4)and CO(SCO)were calculated with the formulae shown as follows:
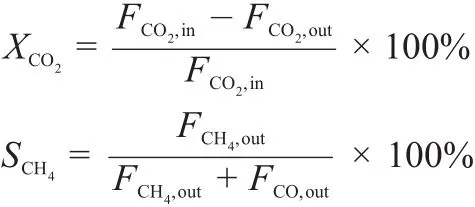
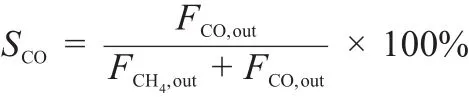
where,Fi,inand Fi,outwere the flow rate of i component(i=CO2, CH4,CO)in the feed and effluent gas,respectively.
The turnover frequencies(TOFs)of the Ni-Ce-Zr/γ-MA-n catalysts for the CO2methanation are defined as the number(n) of converted CO2molecules per surface metal Ni atom and hour. In order to precisely determine the intrinsic reaction rate on the metal active sites,the CO2methanation was carried out at a high gas hourly space velocity(GHSV)to obtain a low CO2conversion (≤20%).For this,a 50 mg catalyst powder diluted with 200 mg of quartz powder was used for the reaction.
On the assumption that all Ni ions were completely reduced into metallic Ni atoms and formed spherical particles with a diameter (dXRD)determined by the Scherrer equation to the Ni(200)diffraction peaks,and the distance between metal atoms in Ni crystallites was assumed to be 0.249 nm.TOF(h-1)was calculated by the equation as follows:
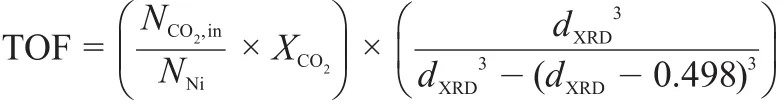
where,NCO2is the flow rate of CO2in mol·h-1in the feed;NNiis the mole number of Ni in 50 mg catalyst.
3 Results and discussion
3.1Physicochemical properties of Ni-Ce-Zr/γ-MA-nmaterials
N2sorption measurement was first employed to investigate textural properties of the prepared materials.Fig.1 presents the N2adsorption-desorption isotherms and BJH pore size distributions of the prepared γ-MA support and Ni-Ce-Zr/γ-MA-n(n=0,0.5, 1.0,2.0)materials.The γ-MAsupport exhibited characteristic type IV isotherms with apparent hysteresis loops and narrow pore size distribution in the range of 3.0-4.5 nm,which was indicative of the mesoporous structures,as reported in the previous study28. After the incorporation of Ni-Ce-Zr oxides,N2sorption isotherms and pore size distributions showed no observable change.This result indicated that the mesoporous structures of the γ-MA ma-terials were maintained in the Ni-Ce-Zr/γ-MA-n samples calcined at 500°C,which were also illustrated by the TEM images(not shown).

Fig.1 (a)N2adsorption-desorption isotherms and (b)BJH pore size distributions of the prepared γ-MAsupport and Ni-Ce-Zr/γ-MA-n materials
Table 1 summarizes the specific surface areas,pore volumes and pore diameters of the γ-MAand Ni-Ce-Zr/γ-MA-n(n=0,0.5, 1.0,2.0)materials.The γ-MA support had a specific surface area of 348 m2·g-1,a pore volume of 0.30 cm3·g-1,and a pore size of 3.4 nm.The incorporation of Ni-Ce-Zr oxides resulted in a remarkable loss of specific surface areas and pore volumes mainly as a result of the increase in the density of the materials,but pore sizes of the Ni-Ce-Zr/γ-MA-n samples were still retained at (3.5±0.2)nm.However,the Ni-Ce-Zr/γ-MA-n(n=0.5,1.0,2.0) presented much larger specific surface areas(~300 m2·g-1)and pore volumes(~0.27 cm3·g-1)than the Ni-Ce-Zr/γ-MA-0 sample (172 m2·g-1,0.16 cm3·g-1).This result could be attributed to the surface blockage of parts of pores in the γ-MA support resulted from the agglomeration of Ni-Ce-Zr oxides on the surface of the Ni-Ce-Zr/γ-MA-0 sample29-31.
Fig.2 illustrates the XRD patterns of the Ni-Ce-Zr/γ-MA-n(n= 0,0.5,1.0,2.0)materials.All the Ni-Ce-Zr/γ-MA-n samples showed three diffraction peaks around 2θ=37.6°,45.8°,66.7°, corresponding to the reflections for γ-Al2O3,similar to γ-MA28.In the case of Ni-Ce-Zr/γ-MA-0 sample,other two-group strong diffraction peaks were observed,which were indicative of the formation of large crystallites:one group at 2θ=28.6°,33.1°, 47.6°,56.4°could be assigned to the Ce-rich Ce-ZrO2solid solution formed by the dissolution of ZrO2into cubic CeO2structure32;the other at 2θ=37.3°,43.3°,62.9°,75.4°was corresponding to cubic NiO phase.Upon addition of CAin the reaction solution,the diffraction intensities of either the Ce-ZrO2solid solution or the NiO phase sharply attenuated,and finally completely disappeared when the CA/(Ni+Ce+Zr)molar ratio was increased to 1.0.These results demonstrated that the presence of CA in the precursor solutions significantly promoted the dispersion of Ni-Ce-Zr oxide species on the surface of the γ-MAsupport. This kind of promotion effect of CA in the reaction solutions on the dispersion of metal oxide species on the support surfaces seemed plausible to be explained by the following aspects.In the mixed aqueous solution of Ce,Zr,and Ni nitrates and CA,metal ions reacted with CAmolecules to constitute metal-CAcomplexes, which could effectively inhibit the hydrolysis of metal ions and thermal decomposition.The metal-CA complexes with higher melting points were homogeneously immobilized on the surface of γ-MA in the dried procedure.Upon calcinations at elevated temperatures,metal-CA complexes were in situ decomposed to monolayer-dispersed metal oxide species on the support surface, or interact with each other at the molecular scale to produce highlydispersed microcrystals in the γ-MAframeworks.In the case of the preparation process in the absence of CA,the molecules of metal nitrates without complexation could move freely in the host pores in the immobilized procedure because they had relatively lower melting points(56.7°C for Ni(NO3)2;96°C for Ce(NO3)3).The metal nitrates moved towards the pore mouths and agglomerated at the pore mouths or on the exterior surface due to the capillary forces in the drying and heat-treating process,and finally produced larger metal oxide crystallites,blocking the pores of the support20.
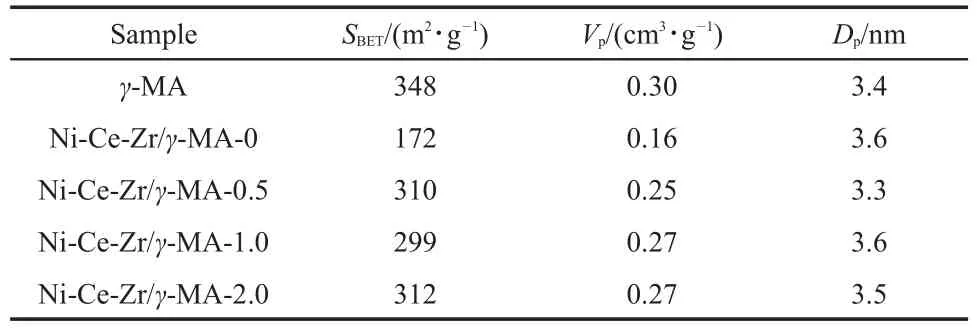
Table 1 Textural properties for the prepared materials
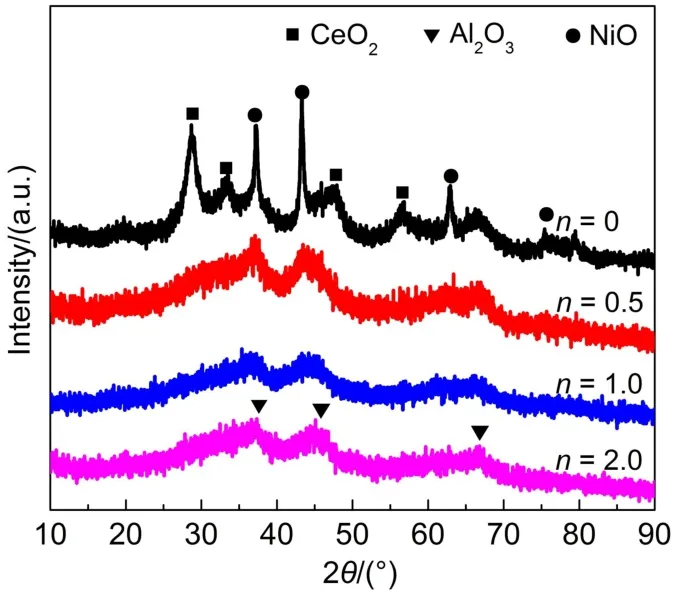
Fig.2 XRD patterns of the prepared Ni-Ce-Zr/γ-MA-n materials calcined at 500°C

Fig.3 H2-TPR profiles of the prepared Ni-Ce-Zr/γ-MA-n materials
It is common knowledge that Ni-based catalysts should first be pre-reduced to generate metallic Ni active sites.Thus,it is important to investigate the reducibility of the prepared materials and the Ni crystallite sizes formed after the reduction,which strongly affect the catalytic activity,the stability and the resistance to carbon deposition of the catalysts for CO2methanation.Fig.3 displays the TPR profiles of Ni-Ce-Zr/γ-MA-n(n=0,0.5,1.0,2.0) materials calcined at 500°C.It could be seen that the presence of CA in the reaction solutions had significant influence on types of Ni species,the reducibility of Ni2+ions and the interaction of Ni species with the support.In terms of the Ni-Ce-Zr/γ-MA-0 sample, the H2-TPR profile showed two distinct H2consumption peaks in the temperature range of ca 350-650°C and 650-900°C,respectively.The former was ascribed to the reduction of free NiOcrystallites;and the latter was associated with the reduction of surface nickel aluminate-like species on the γ-MAsupport,which had a strong interaction with the support5-7.However for the Ni-Ce-Zr/γ-MA-n(n=0.5,1.0,2.0)materials,a broadened H2consumption occurred in the range of 450-750°C,and meanwhile, the reduction peaks for the NiO crystallites and surface nickel aluminate-like species in the Ni-Ce-Zr/γ-MA-0 sample completely disappeared.These results demonstrated that CA in the precursor solutions not only promoted the dispersion of metal oxide species on the surface of the γ-MA support,but also significantly improved the interaction between Ni species and the support,as observed in the XRD patterns of Fig.2 and the TPR result of Fig.3, due to the mixing of metal ions at atomic level on the support surface15.The apparent asymmetry of H2consumption profiles in the shape revealed considerable heterogeneity of NiO species on the support surface,which might be associated with the distribution of the Ce-ZrO2species on the surfaces.

Fig.4 XRD patterns of the Ni-Ce-Zr/γ-MA-n materials reduced at 600°C for 2 h
Fig.4 illustrates the XRD patterns of the Ni-Ce-Zr/γ-MA-n(n= 0,0.5,1.0,2.0)materials reduced with H2at 600°C for 2 h,at which they showed the optimum catalytic performance in the preliminary study and applied for the CO2methanation in the present paper.It could be seen that all the reduced Ni-Ce-Zr/γ-MA-n catalysts exhibited three new diffraction peaks at 44.5°,51.8°, and 76.4°,corresponding to Ni(111),(200),and(220)reflections, respectively.This result demonstrated that Ni2+ions in the samples were reduced to metallic Ni crystallites.The peak intensity of Ni crystallites in the Ni-Ce-Zr/γ-MA-0 catalyst was much stronger than those of Ni crystallites in the Ni-Ce-Zr/γ-MA-n(n=0.5,1.0, 2.0)catalysts by the CA-assisted impregnation method,which was in consistence with the dispersion of Ni oxide species shown in Fig.2.This result suggested that the Ni crystallite sizes formed strongly depended on the dispersion of the Ni oxide species on the surface of the Ni-Ce-Zr/γ-MA-n materials.The diffraction peaks associated with the Ce-ZrO2solid solution had no discernible change,indicating that the dispersion of the Ce-ZrO2species on the surface was maintained during the reduction process.Table 2 lists the apparent Ni crystallite sizes estimated from broadening of the Ni(200)reflection using the Scherrer formula.Upon the addition of CA in the precursor solutions,the Ni crystallite sizes rapidly declined from 11.5 nm for the Ni-Ce-Zr/γ-MA-0 to 5.4 nm at n=0.5,and further to 5.1 nm at n=2.0.The promotion effect of the Ni dispersion was further confirmed by the TEM results displayed in Fig.5 and Table 2,which exhibited that the darker Ni nanoparticles were homogeneously distributed throughout the mesoporous alumina frameworks,and the addition of CA significantly improved the dispersion of metallic Ni particles.
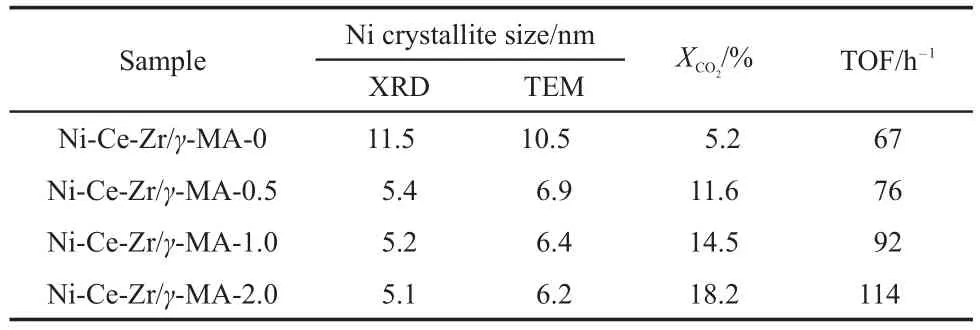
Table 2 Ni crystallite sizes of the Ni-Ce-Zr/γ-MA-n catalysts reduced at 600°C and TOF values for the CO2methanation
3.2Catalysis reaction
The CO2methanation was conducted at atmospheric pressure in the range of 150-400°C.The Ce-Zr/γ-MAmaterials had been proven to be inactive for the CO2methanation,indicating that the metallic Ni was responsible for the catalytic reactivity.In the reaction process,no other products were observed besides H2,CO, CO2,and CH4in the exit gas.Fig.6 illustrates that all the Ni-Ce-Zr/ γ-MA-n(n=0,0.5,1.0,2.0)catalysts had excellent catalytic activities and the CH4selectivities of≥99.7%(not shown).The CO2conversions rapidly increased with elevating the reaction temperature and exhibited the maximum values at~300°C. Obviously over the whole range of reaction temperature,the CO2conversions over the Ni-Ce-Zr/γ-MA-n(n=0.5,1.0,2.0)catalystswere much higher than those over the Ni-Ce-Zr/γ-MA-0 prepared without CA in the reaction solution,and at 300°C,could reach~92%,close to chemical equilibrium value(~93%).These results demonstrated that CA in the reaction solution significantly enhanced the catalytic activity of the Ni-Ce-Zr/γ-MA-n catalysts for the CO2methanation,likely due to the promotion of the dispersion of Ni species.
Fig.5 TEM images of the Ni-Ce-Zr/γ-MA-n materials
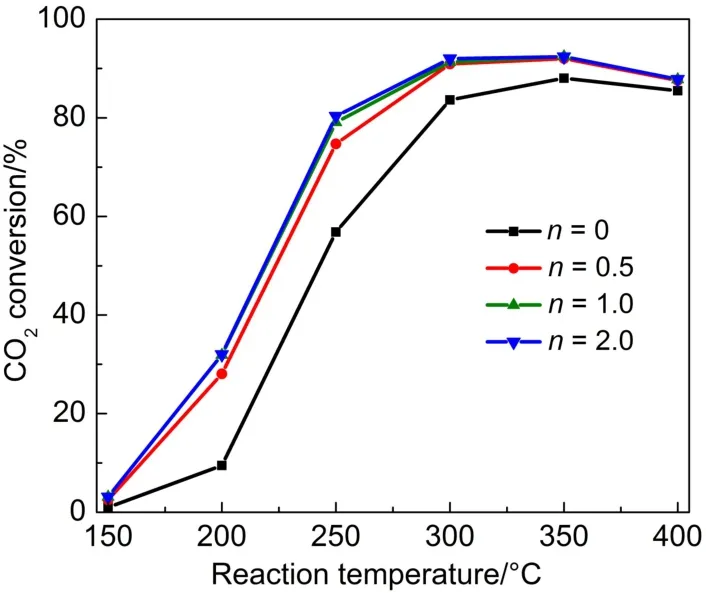
Fig.6 Activities as a function of reaction temperature over the Ni-Ce-Zr/γ-MA-n catalysts for CO2methanation
In order to further analyze the interrelation between the catalytic properties and the Ni active sites over the Ni-Ce-Zr/γ-MA-n for the CO2methanation,TOFs,which reflect the intrinsic activity of the active sites in the catalyst,were measured at a low CO2conversion(≤20%),where the possibility of either the external or internal diffusion limitations were almost completely eliminated by the experiments of varying the GHSVs and the CO2methanation could be considered to be governed by chemical kinetics. Table 2 summarizes the CO2conversions and the corresponding TOFs over the Ni-Ce-Zr/γ-MA-n(n=0,0.5,1.0,2.0)catalysts for the CO2methanation under the reaction conditions:GHSV,60000 mL·g-1·h-1,H2/CO2,4/1(molar ratio),reaction temperature, 250°C,and reaction time,1 h.It could be seen that the TOF of Ni active sites over the Ni-MgO/γ-MA-0 catalyst was lower than those over the Ni-Ce-Zr/γ-MA-n(n=0.5,1.0,2.0).However,in the case of the Ni-Ce-Zr/γ-MA-n(n=0.5,1.0,2.0)with the similar Ni crystallite sizes,the TOFs were found to increase with the CA/(Ni+Ce+Zr)molar ratio in the reaction solutions.It was known in the N2sorption results of Fig.1 that all the Ni-Ce-Zr/γ-MA-n catalysts had the mesoporous frameworks with similar pore sizes and pore size distributions,which could be assumed to have the approximate kinetics of heat transfer and mass transport in the reaction process.Therefore,it could be concluded that the intrinsic rates for the CO2methanation over the Ni-Ce-Zr/γ-MA-n catalysts not only depended on the Ni crystallite sizes or the number of surface Ni atoms,but also had a strong correlation with the amounts of CAused in the reaction solution.It was speculated that the presence of CA might affect the electronic and structure natures of Ni-Ce-ZrOxspecies formed on the support surface32,resulting in the improvement of the catalytic properties of Ni active sites.

Fig.7 Stability of Ni-Ce-Zr/γ-MA-1.0 catalyst for CO2methanation
The Ni-Ce-Zr/γ-MA-1.0 catalyst was selected to examine the long-term stability for the CO2methanation.Fig.7 illustrates the catalytic properties as a function of reaction time at the given reaction conditions.It could be seen that the catalyst exhibited the initial LPG conversion of~82.3%,where the CO2methanation was controlled by chemical kinetics.During the test period of~300 h,the CO2conversions only showed a slight decline to 76.6%with a deactivation degree of~7%,and the CH4selectivity almost kept unchanged,which was indicative of an excellent catalytic stability.
It has been established that carbon deposition and Ni sintering were mainly responsible for the deactivation of Ni-based catalysts during CO2methanation3.Thus,the carbon deposition on the Ni-Ce-Zr/γ-MA-1.0 catalyst used for the CO2methanation at 300°C for 300 h was investigated by the TG profile displayed in Fig.8. Only trace amount of carbon was deposited on the catalyst,indicating that the Ni-Ce-Zr/γ-MA-1.0 catalyst had excellent anticoking ability for the CO2methantion.Fig.9 illustrates that the XRD patterns of the Ni-Ce-Zr/γ-MA-1.0 catalyst before and after the reaction.It could be seen that the XRD patterns of the used catalyst almost remained unchanged.This result indicated that after the reaction,the mesoporous γ-Al2O3framework and the dispersion of metal species were still retained.This point was alsorevealed by the TEM image in Fig.10 for the spent Ni-Ce-Zr/γ-MA-1.0 catalyst,in which the spent catalyst showed a homogenous wormhole-like mesoporous structure and highly dispersed Ni nanoparticles with a mean Ni particle size of 6.9 nm,similar to the counterpart before the reaction.

Fig.8 Thermogravimetric(TG)profile of the spent Ni-Ce-Zr/γ-MA-1.0 catalyst for CO2methanation at 300°C for 300 h
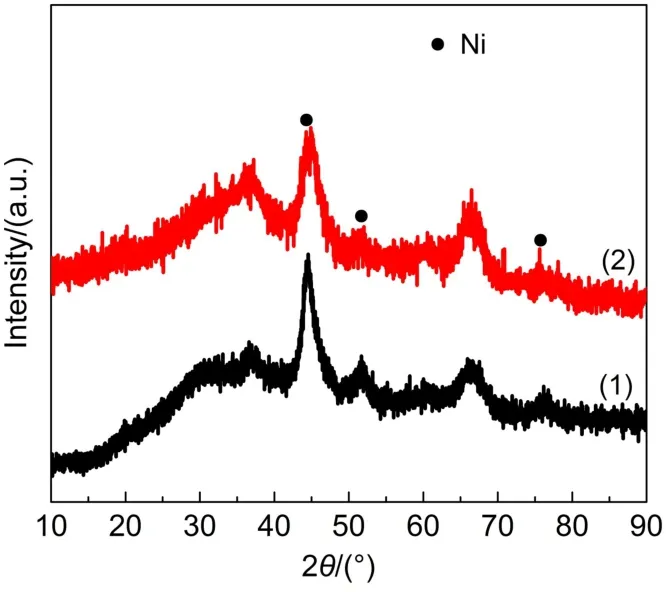
Fig.9 XRD patterns of the Ni-Ce-Zr/γ-MA-1.0 catalyst
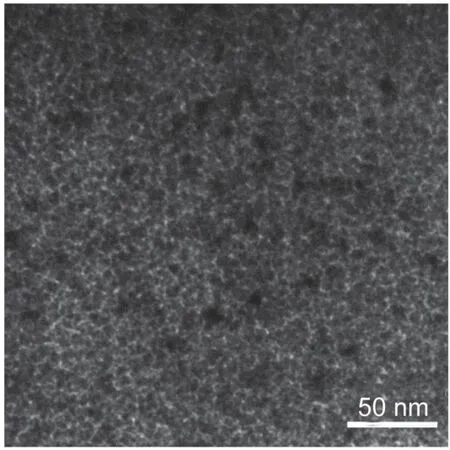
Fig.10 TEM image of the spent Ni-Ce-Zr/γ-MA-1.0 catalyst for CO2methanation at 300°C for 300 h
4 Conclusions
Mesoporous γ-alumina supported Ni-Ce-Zr mixed oxides(Ni-Ce-Zr/γ-MA)were prepared by the citric acid-assisted impregnation method.The addition of citric acid in the precursor solution strongly affected on dispersion of metal oxide species,reducibility of Ni2+ions and the Ni crystallite sizes formed,resulting in the improvement of catalytic properties of the Ni-Ce-Zr/γ-MAcatalysts for the CO2methanation.Compared with the Ni-Ce-Zr/γ-MA catalyst prepared by the citric acid-free impregnation method,the catalysts by the citric acid-assisted method showed much higher catalytic activity,and could achieve the CO2conversion close to chemical equilibrium value at 300°C due to both the decrease in the Ni crystallite size and the variation in the electronic and structure natures of Ni-Ce-ZrOxspecies.The Ni-Ce-Zr/γ-MA-1.0 catalyst was demonstrated to have excellent catalytic activity,longterm stability and high anti-coking ability for the CO2methanation.
(1) Abe,T.;Tanizawa,M.;Watanabe,K.;Taguchi,A.Energy Environ.Sci.2009,2,315.doi:10.1039/b817740f
(2) Wang,W.;Wang,S.P.;Ma,X.B.;Gong,J.L.Chem.Soc.Rev. 2011,40,3703.doi:10.1039/c1cs15008a
(3) Gao,J.J.;Liu,Q.;Gu,F.N.;Liu,B.;Zhong,Z.Y.;Su,F.B. RSC Adv.2015,5,22759.doi:10.1039/c4ra16114a
(4) Aziz,M.A.A.;Jalil,A.A.;Triwahyono,S.;Ahmad,A.Green Chem.2015,17,2647.doi:10.1039/c5gc00119f
(5) Chang,F.W.;Kuo,M.S.;Tsay,M.T.;Hsieh,M.C.Appl.Catal. A 2003,247,309.doi:10.1016/S0926-860X(03)00181-9
(6) Perkas,N.;Amirian,G.;Zhong,Z.Y.;Teo,J.;Gofer,Y.; Gedanken,A.Catal.Lett.2009,130,455.doi:10.1007/s10562-009-9952-8
(7) Zhang,R.B.;Liang,L.;Zeng,X.R.;Shang,J.Y.;Wang,T.; Cai,J.X.Acta Phys.-Chim.Sin.2012,28,1951.[张荣斌,梁蕾,曾宪荣,商金艳,汪 涛,蔡建信.物理化学学报,2012,28, 1951.]doi:10.3866/PKU.WHXB201206041
(8) Song,H.L.;Yang,J.;Zhao,J.;Chou,L.J.Chin.J.Catal.2010, 31,21.doi:10.1016/S1872-2067(09)60036-X
(9) Lu,B.W.;Kawamoto,K.Fuel 2013,103,699.doi:10.1016/j. fuel.2012.09.009
(10) Ocampo,F.;Louis,B.;Kiwi-Minsker,L.;Roger,A.C.Appl. Catal.A 2011,392,36.doi:10.1016/j.apcata.2010.10.025
(11) Cai,W.;Zhong,Q.;Zhao,Y.X.Catal.Commun.2013,39,30. doi:10.1016/j.catcom.2013.04.025
(12) Fan,Z.G.;Sun,K.H.;Rui,N.;Zhao,B.R.;Liu,C.J.J.Energy Chem.2015,24,655.doi:10.1016/j.jechem.2015.09.004
(13) Guo,Z.L.;Huang,L.Q.;Chu,W.;Luo,S.Z.Acta Phys.-Chim. Sin.2014,30,723.[郭章龙,黄丽琼,储 伟,罗仕忠.物理化学学报,2014,30,723.]doi:10.3866/PKU.WHXB201402242
(14) Liu,H.Z.;Zou,X.J.;Wang,X.G.;Lu,X.G.;Ding,W.Z. J.Nat.Gas Chem.2012,21,703.doi:10.1016/S1003-9953(11) 60422-2
(15) Luisetto,I.;Tuti,S.;Battocchio,C.;Lo Mastro,S.;Sodo,A. Appl.Catal.A 2015,500,12.doi:10.1016/j.apcata.2015.05.004
(16) Boullosa-Eiras,S.;Zhao,T.J.;Vanhaecke,E.;Chen,D.; Holmen,A.Catal.Today 2011,178,12.doi:10.1016/j. cattod.2011.08.029
(17) Villarreal,A.;Ramirez,J.;Caero,L.C.;Villalon,P.C.; Gutierrez-Alejandre,A.Catal.Today 2015,250,60.doi: 10.1016/j.cattod.2014.03.035
(18) Chen,J.J.;Labruyere,V.;Mauge,F.;Quoineaud,A.A.;Hugon, A.;Oliviero,L.J.Phys.Chem.C 2014,118,30039. doi:10.1021/jp510470g
(19) Liu,X.Q.;Li,S.H.;Sun,M.T.;Yu,C.L.;Huang,B.C.Acta Phys.-Chim.Sin.2016,32,1236.[刘小青,李时卉,孙梦婷,喻成龙,黄碧纯.物理化学学报,2016,32,1236.]doi:10.3866/ PKU.WHXB201602251
(20) Hu,Y.;Wang,X.G.;Tan,M.W.;Zou,X.J.;Ding,W.Z.;Lu,X. G.ChemCatChem 2016,8,1055.doi:10.1002/cctc.201501384
(21) Klimova,T.E.;Valencia,D.;Mendoza-Nieto,J.A.;Hernandez-Hipolito,P.J.Catal.2013,304,29.doi:10.1016/j. jcat.2013.03.027
(22) Castillo-Villalon,P.;Ramirez,J.;Vargas-Luciano,J.A.J.Catal. 2014,320,127.doi:10.1016/j.jcat.2014.09.021
(23) Dik,P.P.;Klimov,O.V.;Koryakina,G.I.;Leonova,K.A.; Pereyma,V.Y.;Budukva,S.V.;Gerasimov,E.Y.;Noskov,A.S. Catal.Today 2014,220-222,124.doi:10.1016/j. cattod.2013.07.004
(24) Liu,G.L.;Geng,Y.X.;Pan,D.M.;Zhang,Y.;Niu,T.;Liu,Y. Fuel Process Technol.2014,128,289.doi:10.1016/j. fuproc.2014.07.010
(25) Greluk,M.;Rybak,P.;Slowik,G.;Rotko,M.;Machocki,A. Catal.Today 2015,242,50.doi:10.1016/j.cattod.2014.07.032
(26) Greluk,M.;Rotko,M.;Machocki,A.Catal.Lett.2016,146, 163.doi:10.1007/s10562-015-1628-y
(27) Wu,H.D.;Duan,A.J.;Zhao,Z.;Qi,D.H.;Li,J.M.;Liu,B.; Jiang,G.Y.;Liu,J.;Wei,Y.C.;Zhang,X.Fuel 2014,130,203. doi:10.1016/j.fuel.2014.04.038
(28) Shang,X.F.;Wang,X.G.;Nie,W.X.;Guo,X.F.;Zou,X.J.; Ding,W.Z.;Lu,X.G.J.Mater.Chem.2012,22,23806. doi:10.1039/c2jm35508f
(29) Mendoza-Nieto,J.A.;Robles-Mendez,F.;Klimova,T.E.Catal. Today 2015,250,47.doi:10.1016/j.cattod.2014.05.002
(30) Calderon-Magdaleno,M.A.;Mendoza-Nieto,J.A.;Klimova,T. E.Catal.Today 2014,220-222,78.doi:10.1016/j. cattod.2013.06.002
(31) Li,B.T.;Qian,X.Y.;Wang,X.J.Int.J.Hydrog.Energy 2015, 40,8081.doi:10.1016/j.ijhydene.2015.04.104
(32) Ye,Q.;Wang,R.P.;Xu,B.Q.Acta Phys.-Chim.Sin.2006,22, 33.[叶 青,王瑞璞,徐柏庆.物理化学学报,2006,22,33.] doi:10.3866/PKU.WHXB201206041
Preparation of Highly Dispersed Ni-Ce-Zr Oxides over Mesoporous γ-Alumina and Their Catalytic Properties for CO2Methanation
NIE Wang-Xin ZOU Xiu-Jing*WANG Xue-Guang*DING Wei-Zhong LU Xiong-Gang
(Shanghai Key Laboratory of Advanced Ferrometallurgy,State Key Laboratory of Advanced Special Steel, School of Materials Science and Engineering,Shanghai University,Shanghai 200072,P.R.China)
Highly dispersed Ni-Ce-Zr mixed oxides supported on mesoporous γ-alumina(Ni-Ce-Zr/γ-MA)were prepared by a citric acid(CA)-assisted impregnation method and evaluated as catalysts for the methanation of CO2with H2.The effects of the CAcontent of the reaction solution on the physicochemical properties and the catalytic performance of the Ni-Ce-Zr/γ-MAcatalysts were investigated in detail.The addition of CApromoted the dispersion of the Ni-Ce-Zr oxide species on the γ-alumina surface and improved the interactions between the Ni oxide species and the support,resulting in the formation of homogeneously dispersed Ni nanoparticles in the γ-MAframeworks upon reduction with H2.The resulting Ni-Ce-Zr/γ-MAcatalysts were highly active and showed almost 100%selectivity for CH4during the methanation of CO2at temperatures in the range of 150-400°C.Notably,the catalytic activity increased as the molar ratio of CA/(Ni+Ce+Zr)increased in the range of 0-2.This effect was most likely caused by the associated decrease in the Ni particle size and the improved electronic and structural properties of the Ni-Ce-ZrOxspecies.The results of a stability test for the Ni-Ce-Zr/γ-MAcatalyst prepared with a CA/(Ni+Ce+Zr)molar ratio of 1.0 showed that there was only a 7%decrease in the CO2conversion following a reaction time of 300 h at 300°C with negligible coke deposition,indicating excellent catalytic stability and good anti-coking ability of these systems for the methanation of CO2.
Nickel catalyst;Citric acid-assistance;Mixed metal oxide;Carbon dioxide;Methanation
O643
10.3866/PKU.WHXB201607291
Received:June 2,2016;Revised:July 28,2016;Published online:July 29,2016.
*Corresponding authors.ZOU Xiu-Jing,Email:xjzou@shu.edu.cn.WANG Xue-Guang,Email:wxg228@shu.edu.cn;Tel:+86-21-56338244.
The project was supported by the Innovation Program of Shanghai Municipal Education Commission,National Key Basic Research Program of China(973)(2014CB643403),National Science Fund for Distinguished Young Scholars,China(51225401),and Basic Major Research Program of Science and Technology Commission Foundation of Shanghai,China(14JC1491400).
上海市教育委员会科研项目,国家重点基础研究发展规划项目(973)(2014CB643403),国家杰出青年科学基金(51225401)及上海市科委基础重点项目(14JC1491400)资助

