Zeste基因增强子同源物基因2与肿瘤发展的研究进展
黄 嫄,王晓春
·综 述·
Zeste基因增强子同源物基因2与肿瘤发展的研究进展
黄 嫄,王晓春
多聚梳抑制复合体2作为一种表观遗传调节因子可选择性催化组蛋白H3第27位赖氨酸三甲基化,从而诱导靶基因转录抑制。Zeste基因增强子同源物2(enhancer of zeste homolog 2,EZH2)是多聚梳抑制复合体2中具有酶活性的亚基,在肿瘤触发、进展、转移及耐药性方面有重要作用。EZH2与其他表观遗传修饰酶相互协调介导基因沉默,EZH2超表达是多种实体肿瘤晚期和转移性的标志,EZH2的表达与活性受多种肿瘤相关转录因子的调节,各位点氨基酸残基的磷酸化状态可影响EZH2的催化活性,EZH2基因突变在血液系统恶性肿瘤中频繁发生,除通过经典作用即催化抑癌基因启动子区组蛋白H3第27位赖氨酸甲基化来抑制转录外,EZH2还具有诱导基因活化功能。因此,EZH2成为肿瘤治疗的一个理想靶点,其特异性抑制剂EPZ6438正处于临床Ⅰ/Ⅱ期试验阶段。
Zeste基因增强子同源物2;多聚梳抑制复合体2;肿瘤;转录抑制;基因活化
在真核生物中,组蛋白的转录后修饰在调节染色质结构和基因表达过程中有重要作用。这种表观遗传改变与遗传学改变是有区别的,一旦发生遗传学改变,DNA序列通常无法恢复正常,而表观遗传改变则可被特异性抑制剂逆转。由于表观遗传异常在人类肿瘤中很常见,并且在肿瘤进展中有重要作用,因此对表观遗传改变的研究有利于对药物开发和肿瘤治疗[1]。
Zeste基因增强子同源物2(enhancer of zeste homolog 2,EZH2)是多聚梳群基因家族成员之一,EZH2蛋白在胚胎发育早期普遍表达,具有维持基因转录抑制的作用。EZH2对细胞增殖也是必需的,其高表达导致细胞进入S期。除了维持胚胎正常发育作用之外,EZH2在衰老细胞中低表达,p53基因通过下调EZH2的表达而使细胞发生复制性衰老。EZH2还能增强原代细胞的生长能力,在多种原发性肿瘤中超表达。研究显示,EZH2在肿瘤触发、进展、转移及耐药性方面有重要作用[2]。因此,EZH2成为潜在的抗肿瘤药物作用的靶点。作者对当前EZH2的致瘤作用,EZH2蛋白表达与活性的调节机制,EZH2与癌基因信号转导通路新进展以及EZH2靶向治疗及其发展潜力作一综述。
1 EZH2基因、蛋白结构与生物学功能
人类EZH2基因定位于染色体7q35,全长近40 kb,含20个外显子,外显子长度从41~323 bp不等,内含子长度从150~17 700 bp不等[3],编码一个由746个氨基酸残基组成的组蛋白赖氨酸甲基转移酶家族蛋白。EZH2蛋白主要的功能性结构域有CXC结构域(富含半胱氨酸结构域)、SET结构域(suvar3-9,enhancer of zeste,trithorax)、非编码RNA结合结构域。其中外显子17~20编码高度保守的SET结构域,EZH2介导的转录抑制依赖完整的SET结构域[4]。当甲基供体和底物进入SET结构域的侧面时,甲基供体与底物结合并将甲基基团转移至底物上,转移后与SET结构域分离以便结合下一个甲基供体,故EZH2催化3个连续的甲基化反应,分别形成单甲基化、二甲基化和三甲基化组蛋白H3第2位赖氨酸(histone H3 at lysine2,H3K2)[5]。
多聚梳抑制复合体2(polycomb repressive complex 2,PRC2)作为重要的染色质修饰因子,在所有生物体(无论植物、果蝇还是人类)中都是保守的[6]。人类PRC2包含EZH2、胚胎外胚层发育蛋白(embryonic ectoderm development,EED)和Zeste基因抑制子基因12(suppressorof Zeste12,SUZ12)3个核心亚基。WD40-重复蛋白EED与EZH2的N末端残基相互作用,含有锌指结构的SUZ12则介导EZH2与核小体的结合,其中任何一个核心亚基发生突变将影响EZH2的稳定性与催化活性[7-8]。研究显示,EZH2、DNA甲基转移酶(DNA methyltransferases,DNMTs)[9]与组蛋白去乙酰化酶(histone deacetylases,HDACs)[10-11]三者在结构和功能上都有联系。如图1[1],PRC2催化组蛋白H3第27位赖氨酸三甲基化(trimethylatedhistone H3 lysine 27,H3K27me3)并沉默靶基因,但若K27处于乙酰化状态,则需要HDACs先将组蛋白去乙酰化。HDACs通过对H3K27以及其他赖氨酸残基包括H3K29、H3K14和H4K8去乙酰化,使赖氨酸侧链的ε-氨基基团暴露,易于被PRC2甲基化。在细胞分化过程中,需甲基化标志的基因先招募PRC2至启动子区,随后DNMTs催化该基因CpG岛发生超甲基化,使靶基因染色质永久沉默。因此,PRC2可以与HDACs协同改变组蛋白标志,使之从乙酰化修饰转为甲基化修饰,PRC2也能招募DNMTs使染色质更致密。在结肠癌、前列腺癌、肝癌、肺癌、卵巢癌和乳腺癌中均发现EZH2、DNMTs和HDACs三者功能相关联[12]。
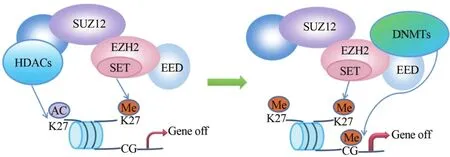
图1 表观遗传沉默酶协作模式
2 EZH2在肿瘤中的表达调控
EZH2在多种肿瘤中超表达。实体瘤中EZH2超表达与肿瘤侵袭性、转移及不良预后有关[12-16]。调节EZH2表达的转录因子在细胞增殖、肿瘤形成和干细胞低分化状态维持等过程中有重要作用。如前列腺癌中,Myc基因与EZH2启动子结合并直接激活转录,且EZH2的表达水平与Myc的表达水平呈正相关[17]。而在恶性胶质瘤肿瘤干细胞中,c-Myc表达反过来受到EZH2的正调节,内在机制尚不清楚[17]。除Myc之外,另一个细胞周期调节因子E2F也正调节EZH2转录[18-19];反之,EZH2对pRb-E2F通路也有重要调节作用。在乳腺癌和前列腺癌中[16,20],ANCCA(AAA+nuclear coregulator cancer associated),一种包含溴结构域的三磷酸腺苷酶、E2F的结合蛋白,可增强E2F诱导的EZH2转录。
3 EZH2的磷酸化
磷酸化状态可改变EZH2的催化活性与稳定性。Cha等[21]报道,EZH2蛋白具有一个高度保守的蛋白激酶B磷酸化位点——第21位丝氨酸(serine 21,S21)。蛋白激酶B催化EZH2 S21磷酸化,该位点磷酸化后EZH2对组蛋白H3的亲和力降低,导致H3K27me3减少,基因转录去抑制。S21磷酸化对EZH2的H3K27me3非依赖性功能至关重要[21]。在雄激素非依赖性前列腺癌细胞中,pS21 EZH2是雄激素受体的转录共激活因子,对雄激素非依赖性生长有重要作用[22]。酪氨酸激酶磷酸化EZH2第641位酪氨酸(tyrosine 641,Y641),该位点磷酸化后EZH2与β-转导重复相容蛋白的相互作用增强,诱导EZH2蛋白降解[23]。细胞周期蛋白依赖性激酶(cyclin-dependent kinase,CDK)1/2磷酸化EZH2的多个苏氨酸位点,包括第345位苏氨酸(threonine 345,T345)、T416和T487[24-26]。CDK介导的EZH2磷酸化位点与效应具有多样性,因细胞类型与细胞状态而异。T345磷酸化可促进EZH2和HOTAIR基因相互作用,而T416磷酸化则诱导核抑制蛋白磷酸酶1与EZH2结合,核抑制蛋白磷酸酶1可抑制蛋白磷酸酶1对EZH2的去磷酸化作用。T345和T416磷酸化均对EZH2招募至特异性靶基因座至关重要[24,26]。CDK1磷酸化 T487,诱导 EZH2从PRC2解离,导致EZH2失活、肿瘤细胞的浸润性降低[25]。与之相反,T345磷酸化则促进细胞迁移与增殖[27]。EZH2的 T345和 T487磷酸化后,诱导EZH2发生泛素化,蛋白质降解[28]。
4 EZH2基因突变
2010 年,肿瘤基因组测序确定了EZH2杂合型体细胞错义突变在滤泡性淋巴瘤中发生率为7%,而在生发中心B细胞型弥漫性大B细胞淋巴瘤中发生率高达22%[29]。突变分别位于EZH2的SET结构域第641位酪氨酸(Y641N、F、S或H)、第677位丙氨酸突变为甘氨酸(alanine 677 glycin,A677G)和第687位丙氨酸突变为缬氨酸(alanine 687 valine,A687V)[29-31]。与EZH2超表达不同,EZH2基因突变导致H3K27me3丰度在全基因组范围内显著上升[32]。此种功能获得性突变的原理是:野生型EZH2催化H3K27单甲基化的活性较强,但其在后续的二甲基化、三甲基化反应中催化活性较弱;与之相反,Y641突变后EZH2修饰未甲基化组蛋白的能力减弱,而修饰单甲基化、二甲基化组蛋白的能力增强[29,33]。因此,在杂合型突变中,EZH2催化3次甲基化的能力均增强。A687V与Y641突变效果类似,而发生A677G突变的EZH2催化组蛋白3次甲基化的能力都增强[30-31]。在B细胞发育过程中,EZH2在生发中心细胞内表达并建立一个抑制性二价基因座特异性染色质环境,使细胞周期检查点与分化因子基因沉默,B细胞生长分裂;B细胞成熟后,EZH2失活,细胞周期检查点与分化因子基因重新活化,B细胞离开生发中心,分化为浆细胞并停止分裂。在生发中心的B细胞若发生Y641突变,H3K27三甲基化增强,导致EZH2靶基因的沉默放大,B细胞分化阻滞,细胞增殖增强,促进肿瘤形成[33]。
与在淋巴瘤中的情况相反,在T细胞型急性淋巴细胞白血病与髓系恶性肿瘤中,EZH2基因发生了一系列错义突变、无义突变和框移突变[34-36]。这些基因改变常常是纯合性的,分布在整个基因范围内,通常会导致EZH2失去组蛋白甲基转移酶活性。因此,含有EZH2基因失活突变的细胞系内H3K27me3水平整体下降。在T细胞型急性淋巴细胞白血病中,PRC2通常与该病的主要驱动因子NOTCH1基因竞争特异性靶基因,EZH2发生失活性突变后间接促进了NOTCH1驱动的癌基因活化[36]。在髓样肿瘤中,EZH2基因突变在骨髓增生异常综合征与骨髓增生性肿瘤中更常见,与不良转归有关,而在急性粒细胞性白血病中罕见[34,37-39]。在裸鼠模型中发现,EZH2发生失活性突变后导致H3K27me3锐减,癌基因如Hmga2、Pbx3、Lmo1和Myc靶基因转录抑制,导致骨髓增生异常综合征或骨髓增生性肿瘤样表型[40]。
EZH2突变对H3K27me3的影响截然不同,说明EZH2的靶基因因组织类型而异,同时显示出组蛋白标志的平衡对细胞内稳态的重要性。此外,尽管抑制EZH2在淋巴瘤中具有治疗作用,在其他类型细胞中却可能是有害的,所以在开展此种表观遗传治疗之前应进行综合性临床前机制的研究。
5 EZH2与肿瘤相关的靶基因
EZH2在肿瘤细胞增殖、迁移、浸润和上皮间质转化中有重要作用,这些过程都与肿瘤发生、进展和转移有关。更重要的是,EZH2与干细胞尤其是肿瘤干细胞特性和肿瘤起始细胞功能密切相关。到目前为止,已经发现了多种EZH2靶基因,其中大部分是抑癌基因。子宫内膜癌中,抑癌基因APC受EZH2的调节:YY1基因招募EZH2至APC启动子,EZH2三甲基化启动子区H3K27导致APC表观沉默[41]。另一个靶基因是p57,在卵巢癌中,抑制EZH2可上调p57的表达水平,降低卵巢癌细胞的增殖和转移能力[15]。CDK抑制因子1C编码肿瘤抑制蛋白p57KIP2。在多种乳腺癌细胞系,CDK抑制因子表达减少与EZH2超表达及H3K27me3增加有关。跨膜受体E-钙黏蛋白维持上皮细胞的黏附性和完整性,其下调可增强肿瘤的侵袭性。EZH2可抑制E-钙黏蛋白的表达,从而导致乳腺癌、胰腺癌、前列腺癌及卵巢癌的浸润与转移[42-43]。
6 EZH2的H3K27me3非依赖性功能
除基因抑制功能外,研究显示EZH2也有基因活化的功能[22,44-46]。Xu等[22]报道,在去势抵抗性前列腺癌中发现一组EZH2相关基因,它们不与PRC2亚基SUZ12结合,也不发生H3K27三甲基化。这些基因大多在EZH2敲除后下调。提示EZH2是这些基因的激活因子,而且这一作用不依赖PRC2,还发现这种功能转变依赖EZH2的S21磷酸化和完整的甲基转移酶结构域。EZH2可能通过甲基化雄激素受体或其他相关蛋白而介导转录激活,这是EZH2的一个新的功能——非组蛋白甲基化。
乳腺癌中,EZH2通过2种不同的方式诱导基因转录,取决于细胞内雌激素受体(estrogen receptor,ER)的水平。在ER-阴性基底细胞样乳腺癌细胞MDA-MB-231中,EZH2与核因子-κB的2个亚基RelA及RelB形成一个三元复合体,组成性激活核因子-κB靶基因如白介素-6和肿瘤坏死因子的转录[44]。在ER-阳性管腔型乳腺癌MCF-7细胞中,EZH2在cyclin B1与c-Myc启动子区作为一个桥梁物理性连接ERα与Wnt信号通路的2个元件——E-钙黏蛋白与T细胞因子,反式激活雌激素靶基因与Wnt信号通路,促进细胞周期进展[45-46]。EZH2的反式激活结构域Ⅱ位于N末端,是转录因子与转录中介体复合物连结的平台,通过与RNA聚合酶Ⅱ相互作用并诱导转录。EZH2对基因的反式激活作用不依赖PRC2的其他亚基,也不依赖SET结构域与甲基转移酶活性。在2种乳腺癌中,虽然EZH2都具有转录激活功能,但机制不同。
7 靶向EZH2的治疗
由于EZH2是肿瘤细胞增殖、迁移、浸润和干细胞特性保持的重要调节因子,因此EZH2是肿瘤治疗的一个理想靶点。DZNep(3-deazaneplanocin A)是一种s腺苷同型半胱氨酸(S-adenosylhomocysteine,SAH)水解酶抑制剂,SAH参与蛋氨酸循环[47]。如图2[48],DZNep抑制SAH水解酶,引起SAH水平升高,通过旁路途径阻断蛋氨酸循环,间接抑制PRC2的活性,下调H3K27me3水平,重新活化PRC2的靶基因。DZNep诱导肿瘤细胞发生凋亡,但对正常细胞没有影响[47]。为研究EZH2在肿瘤中的作用,该药被广泛用于各种肿瘤的临床前和体外研究,并已证实能有效抑制细胞增殖和肿瘤生长[49-51]。DZNep对乳腺癌易感蛋白1缺失型乳腺癌细胞的杀伤效果是乳腺癌易感蛋白1成熟型乳腺癌细胞的20倍,内在机制尚不清楚[52]。

图2 DZNep作为SAH水解酶抑制剂可间接降低EZH2和H3K27me3水平
开发的几种针对EZH2的高度选择性小分子抑制剂,如GSK126、EPZ005678、EI1、和EPZ6438[53-56]。这些抑制剂在淋巴瘤患者中对含Y641突变型EZH2的作用比对野生型EHZ2的作用大。当前,除EPZ6438正在B细胞淋巴瘤和晚期实体瘤患者中进行临床Ⅰ/Ⅱ期试验外,其余均处于临床前实验阶段。EZH2抑制后,肿瘤细胞对其他抗癌药物敏感性提高,如HDACs抑制剂、伊马替尼、吉西他滨、紫杉醇、顺铂,提示联合用药可能疗效更好。
除特异性EZH2抑制剂外,一些饮食性天然成分也可以下调EZH2,包括ω-3多不饱和脂肪酸、姜黄素和表没食子儿茶素没食子酸酯。Dmiri等[57]报道,ω-3多不饱和脂肪酸可诱导EZH2蛋白泛素化,发生蛋白酶体介导的EZH2蛋白降解,下调EZH2蛋白在乳腺癌细胞中的表达与活性。ω-3多不饱和脂肪酸可以使被EZH2沉默的抑癌基因重新表达,如E-钙黏蛋白与胰岛素样生长因子结合蛋白,最终降低乳腺癌的浸润性。姜黄素是姜黄根粉末中的一种天然成分,可调节EZH2水平并诱导G1期阻滞,从而抑制MDA-MD-435乳腺癌细胞增殖。分裂原活化蛋白激酶通路参与姜黄素介导的EZH2水平下调,有助于姜黄素的抗乳腺癌细胞增殖效应[58]。研究显示,一种主要的绿茶多酚表没食子儿茶素没食子酸酯,在皮肤癌细胞中诱导EZH2发生蛋白酶体依赖性降解,且在与DZNep联合用药时抗癌效果更好[59]。
8 总结与展望
如图3[2],EZH2的经典作用是甲基化H3K27来介导基因沉默;但研究显示EZH2甲基化底物不限于组蛋白[46],即EZH2具有甲基化非组蛋白底物从而激活基因转录的作用;此外,EZH2还可通过非甲基化作用直接反式激活基因转录,即EZH2具有甲基转移酶非依赖性功能[47-48]。这些经典作用以外的功能在肿瘤发生时有重要作用。特异性靶向EZH2抑制剂通过结合至EZH2催化活性位点直接抑制其酶活性,从而使组蛋白的甲基化水平整体性降低,其中EPZ6438正在B细胞淋巴瘤和晚期实体瘤患者中进行临床Ⅰ/Ⅱ期试验,但该类小分子抑制剂具有潜在的不良反应。EZH2在维持细胞正常状态中也有重要作用,因此未来应重点解决如何在某种特定肿瘤细胞或某个特定微环境中有效控制EZH2表达的调节环路。

图3 EZH2在人类肿瘤中的多种作用
【参考文献】
[1]Tan JZ,Yan Y,Wang XX,et al.EZH2:biology,disease,and structure-based drug discovery[J].Acta Pharmacol Sin,2014,35(2):161-174.
[2]Yamaguchi H,Hung MC.Regulation and role of EZH2 in cancer[J].Cancer Res Treat,2014,46(3):209-222.
[3]Cardoso C,Mignon C,Hetet G,et al.The human EZH2 gene: genomic organisation and revised mapping in 7q35 with in the critical region for malignant myeloid disorders[J].Eur J Hum Genet,2000,8(3):174-180.
[4]Copeland RA,Solomon ME,Richon VM.Protein methyltransferases as a target class for drug discovery[J].Nat Rev Drug Discov,2009,8(9):724-732.
[5]Xiao B,Wilson JR,Gamblin SJ.SET domains and histone methylation[J].Curr Opin Struct Biol,2003,13(6):699-705.
[6]O’Meara MM,Simon JA.Inner workings and regulatory inputs that control Polycomb repressive complex 2[J].Chromosoma,2012,121(3):221-234.
[7]Montgomery ND,Yee D,Montgomery SA,et al.Molecular and functional mapping of EED motifs required for PRC2-dependent histoneme thylation[J].J Mol Biol,2007,374 (5):1145-1157.
[8]Pasini D,Bracken AP,Jensen MR,et al.Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity[J].EMBO J,2004,23(20):4061-4071.
[9]Viré E,Brenner C,Deplus R,et al.The Polycomb group protein EZH2 directly controls DNA methylation[J].Nature,2006,439(7078):871-874.
[10]Wang H,Wang L,Erdjument-Bromage H,et al.Role of histone H2A ubiquitination in Polycomb silencing[J].Nature,2004,431(7010):873-878.
[11]van der Vlag J,Otte AP.Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation[J].Nat Genet,1999,23(4):474-478.
[12]Lee HW,Choe M.Expression of EZH2 in renal cell carcinoma as a novelprognostic marker[J].Pathol Int,2012,62 (11):735-741.
[13]Deb G,Thakur VS,Gupta S.Multifaceted role of EZH2 in breast and prostate tumorigenesis:epigenetics and beyond [J].Epigenetics,2013,8(5):464-476.
[14]Chen Y,Xie D,Yin Li W,et al.RNAi targeting EZH2 inhibits tumor growth and liver metastasis of pancreatic cancer in vivo[J].Cancer Lett,2010,297(1):109-116.
[15]Guo J,Cai J,Yu L,et al.EZH2 regulates expression of p57 and contributes to progression of ovarian cancerin vitro and in vivo[J].Cancer Sci,2011,102(3):530-539.
[16]Kalashnikova EV,Revenko AS,Gemo AT,et al.ANCCA/ ATAD2 overexpression identifies breast cancer patients with poor prognosis,acting to drive proliferation and survival of triple-negative cells through control of B-Myb and EZH2[J].Cancer Res,2010,70(22):9402-9412.
[17]Koh CM,Iwata T,Zheng Q,et al.Myc enforces overexpression of EZH2 in early prostatic neoplasia via transcriptional and post-transcriptional mechanisms[J].Oncotarget,2011,2(9):669-683.
[18]Lu C,Han HD,Mangala LS,et al.Regulation of tumor angiogenesis by EZH2[J].Cancer Cell,2010,18(2):185-197.
[19]Bracken AP,Pasini D,Capra M,et al.EZH2 is downstream of the pRB-E2F pathway,essential for proliferation and amplified in cancer[J].EMBO J,2003,22(20):5323-5335.
[20]Duan Z,Zou JX,Yang P,et al.Developmental and androgenic regulation of chromatin regulators EZH2 and ANCCA/ ATAD2 in the prostate Via MLL histone methylase complex[J].Prostate,2013,73(5):455-466.
[21]Cha TL,Zhou BP,Xia W,et al.Akt-mediated phosphorylation of EZH2 suppresses methylatio n of lysine 27 in histone H3[J].Science,2005,310(5746):306-310.
[22]Xu K,Wu ZJ,Groner AC,et al.EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent[J].Science,2012,338(6113):1465-1469.
[23]Sahasrabuddhe AA,Chen X,Chung F,et al.Oncogenic Y641 mutations in EZH2 prevent Jak2/β-TrCP-mediated degradation[J].Oncogene,2015,34(4):445-454.
[24]Kaneko S,Li G,Son J,et al.Phosphorylation of the PRC2 component Ezh2 is cell cycle-regulated and up-regulates its binding to ncRNA[J].Genes Dev,2010,24(23):2615-2620.
[25]Wei Y,Chen YH,Li LY,et al.CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells[J].Nat Cell Biol,2011,13(1):87-94.
[26]Minnebo N,Görnemann J,O’Connell N,et al.NIPP1 maintains EZH2 phosphorylation and promoter occupancy at proliferation-related target genes[J].Nucleic Acids Res,2013,41(2):842-854.
[27]Chen S,Bohrer LR,Rai AN,et al.Cyclin-dependent kinases regulate epigenetic gene silencing through phosphorylation of EZH2[J].Nat Cell Biol,2010,12(11):1108-1114.
[28]Wu SC,Zhang Y.Cyclin-dependent kinase 1(CDK1)-mediated phosphorylation of enhancer of zeste2(Ezh2)regulates its stability[J].J Biol Chem,2011,286(32):28511-28519.
[29]Morin RD,Johnson NA,Severson TM,et al.Somatic mutations altering EZH2(Tyr641)in follicular and diffuse large B-cell lymphomas of germinal-center origin[J].Nat Genet,2010,42(2):181-185.
[30]McCabe MT,Graves AP,Ganji G,et al.Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27(H3K27)[J].Proc Natl Acad Sci USA,2012,109 (8):2989-2994.
[31]Majer CR,Jin L,Scott MP,et al.A687V EZH2 is a gain-offunction mutation found in lymphoma patients[J].FEBS Lett,2012,586(19):3448-3451.
[32]Béguelin W,Popovic R,Teater M,et al.EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation[J].Cancer Cell,2013,23 (5):677-692.
[33]Sneeringer CJ,Scott MP,Kuntz KW,et al.Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3(H3K27) in human B-cell lymphomas[J].Proc Natl Acad Sci USA,2010,107(49):20980-20985.
[34]Ernst T,Chase AJ,Score J,et al.Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders[J].Nat Genet,2010,42(8):722-726.
[35]Nikoloski G,Langemeijer SM,Kuiper RP,et al.Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes[J].Nat Genet,2010,42(8):665-667.
[36]Ntziachristos P,Tsirigos A,Van Vlierberghe P,et al.Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia[J].Nat Med,2012,18 (2):298-301.
[37]Abdel-Wahab O,Pardanani A,Patel J,et al.Concomit antanalysis of EZH2 and ASXL1 mutations in myelofibrosis,chronic myelomonocytic leukemia and blast-phase myeloproliferative neoplasms[J].Leukemia,2011,25(7):1200-1202.
[38]Bejar R,Stevenson K,Abdel-Wahab O,et al.Clinical effect of point mutations in myelodysplastic syndromes[J].N Engl J Med,2011,364(26):2496-2506.
[39]Patel JP,Gönen M,Figueroa ME,et al.Prognostic relevance of integrated genetic profiling in acute myeloid leukemia[J].N Engl J Med,2012,366(12):1079-1089.
[40]Muto T,Sashida G,Oshima M,et al.Concurrent loss of Ezh2 and Tet2 cooperates in the pathogenesis of myelodysplastic disorders[J].J Exp Med,2013,210(12):2627-2639.
[41]Yang Y,Zhou L,Lu L,et al.A novel miR-193a-5p-YY1-APC regulatory axis in human endometrioid endometrial adenocarcinoma[J].Oncogene,2013,32(29):3432-3442.
[42]Yoo KH,Hennighausen L.EZH2 methyltransferase and H3K27 methylation in breast cancer[J].Int J Biol Sci,2012,8(1): 59-65.
[43]Toll AD,Dasgupta A,Potoczek M,et al.Implications of enhancer of zeste homologue 2 expression in pancreatic ductal adenocarcinoma[J].Hum Pathol,2010,41(9):1205-1209.
[44]Lee ST,Li Z,Wu Z,et al.Context-specific regulation of NF-κB target gene expression by EZH2 in breast cancers [J].Mol Cell,2011,43(5):798-810.
[45]Shi B,Liang J,Yang X,et al.Integration of estrogen and Wnt signaling circuits by the polycomb group protein EZH2 in breast cancer cells[J].Mol Cell Biol,2007,27(14): 5105-5119.
[46]Jung HY,Jun S,Lee M,et al.PAF and EZH2 induce Wnt/ β-catenin signaling hyperactivation[J].Mol Cell,2013,52 (2):193-205.
[47]Tan J,Yang X,Zhuang L,et al.Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells[J].Genes Dev,2007,21(9):1050-1063.
[48]Chase A,Cross NC.Aberrations of EZH2 in cancer[J]. Clin Cancer Res,2011,17(9):2613-2618.
[49]Suvà ML,Riggi N,Janiszewska M,et al.EZH2 is essential for glioblastoma cancer stem cell maintenance[J].Cancer Res,2009,69(24):9211-9218.
[50]Wu Z,Lee ST,Qiao Y,et al.Polycomb protein EZH2 regulates cancer cell fat edecision in response to DNA damage [J].Cell Death Differ,2011,18(11):1771-1779.
[51]Sun F,Chan E,Wu Z,et al.Combinatorial pharmacologic approaches target EZH2-mediated gene repression in breast cancer cells[J].Mol Cancer Ther,2009,8(12):3191-3202.
[52]Puppe J,Drost R,Liu X,et al.BRCA1-deficient mammary tumor cells are dependent on EZH2 expression and sensitive to Polycomb Repressive Complex 2-inhibitor 3-deazaneplanocin A[J].Breast Cancer Res,2009,11(4):R63.
[53]McCabe MT,Ott HM,Ganji G,et al.EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations[J].Nature,2012,492(7427):108-112.
[54]Knutson SK,Wigle TJ,Warholic NM,et al.A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphomacells[J].Nat Chem Biol,2012,8(11):890-896.
[55]Knutson SK,Kawano S,Minoshima Y,et al.Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-Hodgkin lymphoma[J].Mol Cancer Ther,2014,13(4):842-854.
[56]Qi W,Chan H,Teng L,et al.Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation [J].Proc Natl Acad Sci USA,2012,109(52):21360-21365.
[57]Dimri M,Bommi PV,Sahasrabuddhe AA,et al.Dietary omega-3 polyunsaturated fatty acids suppress expression of EZH2 in breast cancer cells[J].Carcinogenesis,2010,31 (3):489-495.
[58]Hua WF,Fu YS,Liao YJ,et al.Curcumin induces downregulation of EZH2 expression through the MAPK pathway in MDA-MB-435 human breast cancer cells[J].Eur J Pharmacol,2010,637(1/3):16-21.
[59]Nandakumar V,Vaid M,Katiyar SK.(-)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes,Cip1/p21 and p16INK4a,by reducing DNA methylation and increasing histones acetylation in human skin cance rcells[J].Carcinogenesis,2011,32(4):537-544.
Progress on enhancer of zeste homolog 2 gene and tumor development
HUANG Yuan1,WANG Xiaochun2
(1.Department of Clinical Laboratory,the First Hospital of Xi’an,Xi’an Shanxi 710002,China;2.Department of Laboratory,Medical School of Xiangya,Central South University,Changsha Hunan 410013,China)
Polycomb repressive complex 2(PRC2)is the epigenetic regulator that induces histone H3 lysine 27 methylation(H3K27me3)and silences specific gene transcription.Enhancer of zeste homolog 2(EZH2)is an enzymatic subunit of PRC2,and evidence shows that EZH2 plays an essential role in cancer initiation,development,progression,metastasis,and drug resistance.The EZH2 histone methyltransferase usually cooperates with other epigenetic silencing enzymes.In solid tumors,overexpression of EZH2 is associated with aggressive biology,metastasis,and poor clinical outcome.EZH2 expression is indeed regulated by various oncogenic transcription factors.EZH2 activity and stability are regulated by various phosphorylated state of each phosphorylation site.EZH2 gene mutations occur frequently in hematological malignances.In addition to its role as a transcriptional repressor,several studies have shown that EZH2 may also function in target gene activation. As a result,EZH2 is considered a potential drug target.Currently,its highly selective small molecule inhibitors EPZ-6438 is being tested in phaseⅠ/Ⅱclinical trials.
Enhancer of zeste homolog 2(EZH2);Polycomb repressive complex 2 (PRC2);Neoplasms;Transcriptional repression;Gene activation
Q71;R73
A
2095-3097(2016)06-0370-06
10.3969/j.issn.2095-3097.2016.06.014
2016-01-19 本文编辑:徐海琴)
湖南省科学技术厅科技计划一般项目社会发展支撑计划(2014SK3102)
710002陕西西安,西安市第一医院检验科(黄 嫄);410013湖南长沙,中南大学湘雅医学院检验系(王晓春)

