含有双氰基环丙烷甲酰胺类化合物的合成及生物活性
徐高飞, 刘艳红, 杨新玲, 王道全, 袁德凯
(中国农业大学理学院应用化学系, 北京 100193)
含有双氰基环丙烷甲酰胺类化合物的合成及生物活性
徐高飞, 刘艳红, 杨新玲, 王道全, 袁德凯
(中国农业大学理学院应用化学系, 北京 100193)
以含氰基及环丙烷的酰胺类杀菌剂为先导, 设计合成了结构全新的双氰基环丙烷甲酰胺类化合物. 通过Strecker反应获得中间体2-氨基-2-(取代)苯基乙(丙)腈(1a~1n), 以氰乙酸乙酯和1,2-二溴乙烷环经环化、 水解得到1-氰基环丙烷-1-甲酸(3), 由化合物3和1经缩合反应得到目标化合物4a~4n, 其结构均经NMR和HRMS表征. 生物活性测试结果表明, 在离体条件下, 50 μg/mL的化合物4f对瓜果腐霉和稻瘟菌的抑制活性分别为55.3%和67.1%; 盆栽实验中, 400 μg/mL的化合物4f对黄瓜霜霉病和小麦白粉病的抑制活性分别为50%和85%, 化合物4m对玉米锈病的抑制活性达100%; 5 μg/mL的化合物4c, 4d, 4g, 4j和4m对蚊幼虫的致死率均>60%, 600 μg/mL的化合物 4h和4j对粘虫的致死率分别为66.7%和50%.
双氰基环丙烷甲酰胺; Strecker反应; 缩合; 生物活性
环丙烷结构广泛存在于天然产物和农药分子中[1], 可作为抗肿瘤活性杂环化合物的重要药效团[2]. 菊酯类、 腐霉利、 嘧环菌胺、 环丙酰菌胺和环丙嘧啶醇等都是含有环丙烷的农药活性分子[3~7]. 氰基能改善分子的理化性质, 提高其生物活性, 在农药及医药领域中含氰基化合物的合成及生物活性研究受到广泛关注[8,9]. 在商品化农药中, 约10%的分子中含有氰基[10], 这些农药包括氰菊酯、 辛硫磷、 氟虫腈、 氰氟虫腙、 氰虫酰胺、 噻虫啉、 啶虫脒、 氟啶虫胺腈和氟啶虫酰胺等杀虫剂[11~18], 早期的溴菌腈、 百菌清、 霜脲氰和二氰蒽醌[19~22]以及近期开发的嘧菌酯、 氰霜唑、 噻唑菌胺、 氰菌唑、 唑菌腈、 氰菌胺和双氯氰菌胺等杀菌剂[23~29]. 环丙酰菌胺、 氰菌胺和双氯氰菌胺为黑色素生物合成抑制剂(MBIs)[6,28,29], 可通过抑制真菌黑色素合成而降低孢子的侵染性, 用于防治稻瘟病、 霜霉病和晚疫病[28,30]. 噻唑菌胺为噻唑甲酰胺结构, 能抑制菌丝生长和孢子形成, 可防治霜霉病和晚疫病[25,31].
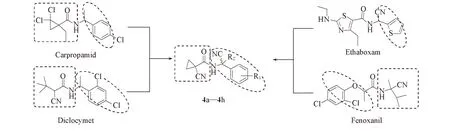
Scheme 1 Design strategy of title compounds
本文参照上述高活性化合物的结构, 通过活性中间体衍生化策略[32], 以氰基乙酸乙酯和1,2-二溴乙烷的环合反应制备1-氰基环丙烷甲酸乙酯, 水解得到1-氰基环丙烷甲酸; 以(取代)苯甲醛(酮)的氰氨化反应得到2-氨基-2-(取代)苯乙腈或2-氨基-2-(取代)苯丙腈, 在二环己基碳二亚胺(DCC)/4-二甲氨基吡啶(DMAP)作用下缩合得14个全新目标化合物(设计思路如Scheme 1所示), 经1H NMR,13C NMR及HRMS确证了其结构. 以平皿法和盆栽法[37]测定了目标化合物的杀菌活性, 以浸叶法和浸液法测定了其杀虫活性.
1 实验部分
1.1 试剂与仪器
氰基三甲基硅烷(TMSCN)、 二环己基碳二亚胺(DCC)、 4-(N,N-二甲氨基)吡啶(DMAP)、 氯化三乙基苄基铵(TEBA)、 聚乙二醇400(PEG400)和1-丁基-3-甲基咪唑六氟磷盐([BMIM]PF6)等试剂均为分析纯, 购自北京偶合科技有限公司; 其它试剂均为分析纯, 购自北京化工厂; 乙腈经CaH2进行无水处理.
Yanaco-300型熔点仪(日本芝山制造所); Bruker DPX 300 MHz核磁共振仪(TMS为内标, 德国Bruker公司); Agilent 1100 Series HPLC/MSD Trap液相色谱-质谱联用仪(ESI, 美国Agilent公司); Varian 7.0T FT-ICR MS HRMS质谱仪(MALDI, 美国Varian公司).
1.2 实验部分
中间体及目标化合物的合成路线见Scheme 2.
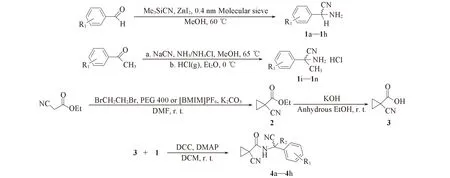
a: R1=H, R2=H; b: R1=4-F, R2=H; c: R1=3-Cl, R2=H; d: R1=4-Br, R2=H; e: R1=4-Me, R2=H; f: R1=4-t-Bu, R2=H; g: R1=4-OMe, R2=H; h: R1=3,4-dioxomethylene, R2=H; i: R1=H, R2=Me; j: R1=4-Cl, R2=Me; k: R1=3-Br, R2=Me; l: R1=4-Br, R2=Me; m: R1=4-Me, R2=Me; n: R1=4-OMe, R2=Me.Scheme 2 General synthetic routes for title compounds 4a—4h
1.2.1 2-氨基-2-(取代)苯乙腈(1a~1h)的合成 参照文献[33]方法合成化合物1a~1h, 用乙酸乙酯/石油醚重结晶, 经真空干燥后直接用于合成目标化合物. 化合物1a: m.p. 52~54 ℃(文献值[34]: 55 ℃); 化合物1b: m.p.72~74 ℃(文献值[34]: 73~74 ℃); 化合物1c: MS(C8H7ClN2计算值), m/z: 167.0(166.0); 化合物1d: MS(C8H7BrN2计算值), m/z: 211.0(210.0); 化合物1e: MS(C9H10N2计算值), m/z: 147.1(146.1); 化合物1f: MS(C12H16N2计算值), m/z: 189.1(188.1); 化合物1g: m.p. 35~37 ℃(文献值[35]: 37~38 ℃); 化合物1h: MS(C12H16N2计算值), m/z: 177.1(176.1).
1.2.2 2-氨基-2-(取代)苯丙腈盐酸盐(1i~1n)的合成 2-氨基-2-(取代)苯丙腈参照文献[36]方法合成. 将经无水Na2SO4干燥后的2-氨基-2-(取代)苯丙腈的乙醚溶液冷却至0 ℃, 搅拌下于0.5 h内通入干燥HCl气体, 生成产物1i~1n. 于0 ℃静置1 h, 抽滤, 真空干燥, 用于合成目标化合物. 化合物1i: m.p. 109~111 ℃(文献值[37]: 110~112 ℃); 化合物1j: MS(C9H9ClN2计算值), m/z: 181.0(180.0); 化合物1k: MS(C9H9BrN2计算值), m/z: 225.0(224.0); 化合物1l: MS(C9H9BrN2计算值), m/z: 225.0(224.0); 化合物1m: MS(C10H12N2计算值), m/z: 161.1(160.1); 化合物1n: MS(C10H12N2O计算值), m/z: 177.1(176.1).
1.2.3 1-氰基环丙烷-1-甲酸(3)的合成 1-氰基环丙烷-1-甲酸乙酯(2)参照文献[38]方法合成, 减压蒸馏, 收集72~77 ℃/6.65 kPa的馏分, 得无色液体, 产率77.2%;1H NMR(CDCl3, 300 MHz), δ: 1.71~1.60(m, 4H), 1.34(t, 3H), 4.27(q, 2H). 将1-氰基环丙烷-1-甲酸乙酯(2, 5 g, 0.036 mol)溶于20 mL无水乙醇, 0 ℃下滴入含KOH粉末(2.62 g, 0.038 mol)的25 mL无水乙醇中, 室温下搅拌2 h, 减压除去溶剂, 加入20 mL水, 用20 mL CHCl3萃取, 将水相酸化至pH=2, 用氯仿萃取(60 mL×3), 减压除去溶剂, 得到1-氰基环丙烷-1-甲酸(3), 白色固体, 产率41.1%;1H NMR(CDCl3, 300 MHz), δ: 1.72~1.82(m, 4H), 10.64(s, 1H).
1.2.4 目标化合物4a~4n的合成 将0.005 mol 2-氨基-2-(取代)苯丙腈盐酸盐溶于15 mL CH2Cl2中, 缓慢滴加15 mL质量分数为10%的NaOH溶液, 滴加完毕后搅拌10 min, 静置分液, 有机相用无水Na2SO4干燥3 h, 得到2-氨基-2-(取代)苯丙腈CH2Cl2溶液, 备用.
冰浴下, 将二环己基碳二亚胺(DCC, 1.02 g, 0.0050 mol)、 4-二甲氨基吡啶(DMAP, 0.60 g, 0.0050 mol)以及2-氨基-2-(取代)苯乙腈或2-氨基-2-(取代)苯丙腈(0.0050 mol)加入到含1-氰基环丙烷-1-甲酸(3, 0.5 g, 0.0045 mol)的20 mL CH2Cl2中后, 于常温下搅拌反应5 h[39], 过滤, 减压除去溶剂, 加入15 mL乙酸乙酯, 于4 ℃静置12 h, 过滤, 经柱层析[V(乙酸乙酯)∶V(石油醚)=5∶1]分离得到白或黄色固体化合物4a~4n. 目标化合物的理化数据见表1和表2, 核磁共振氢谱、 碳谱及高分辨质谱见支持信息图S1~图S21.
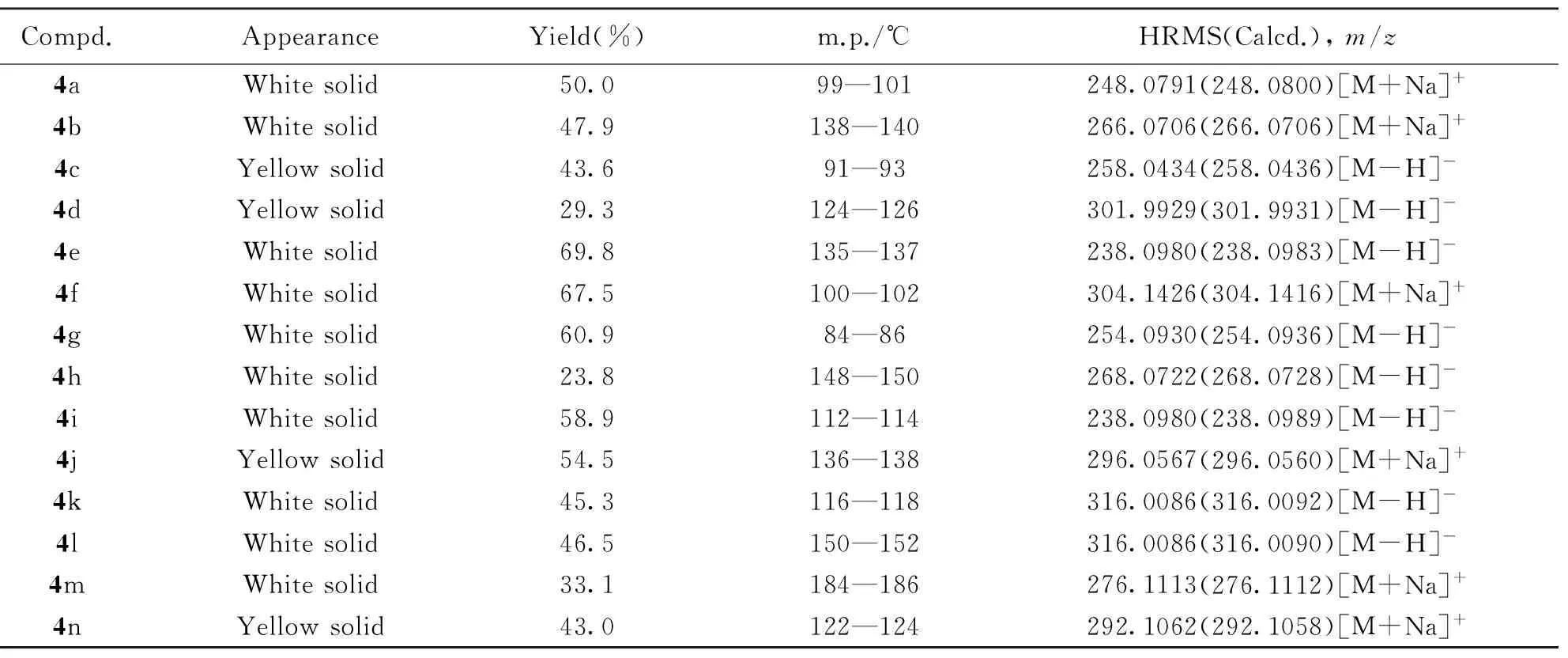
Table 1 Appearance, yield, melting points and HRMS data of compounds 4a—4h
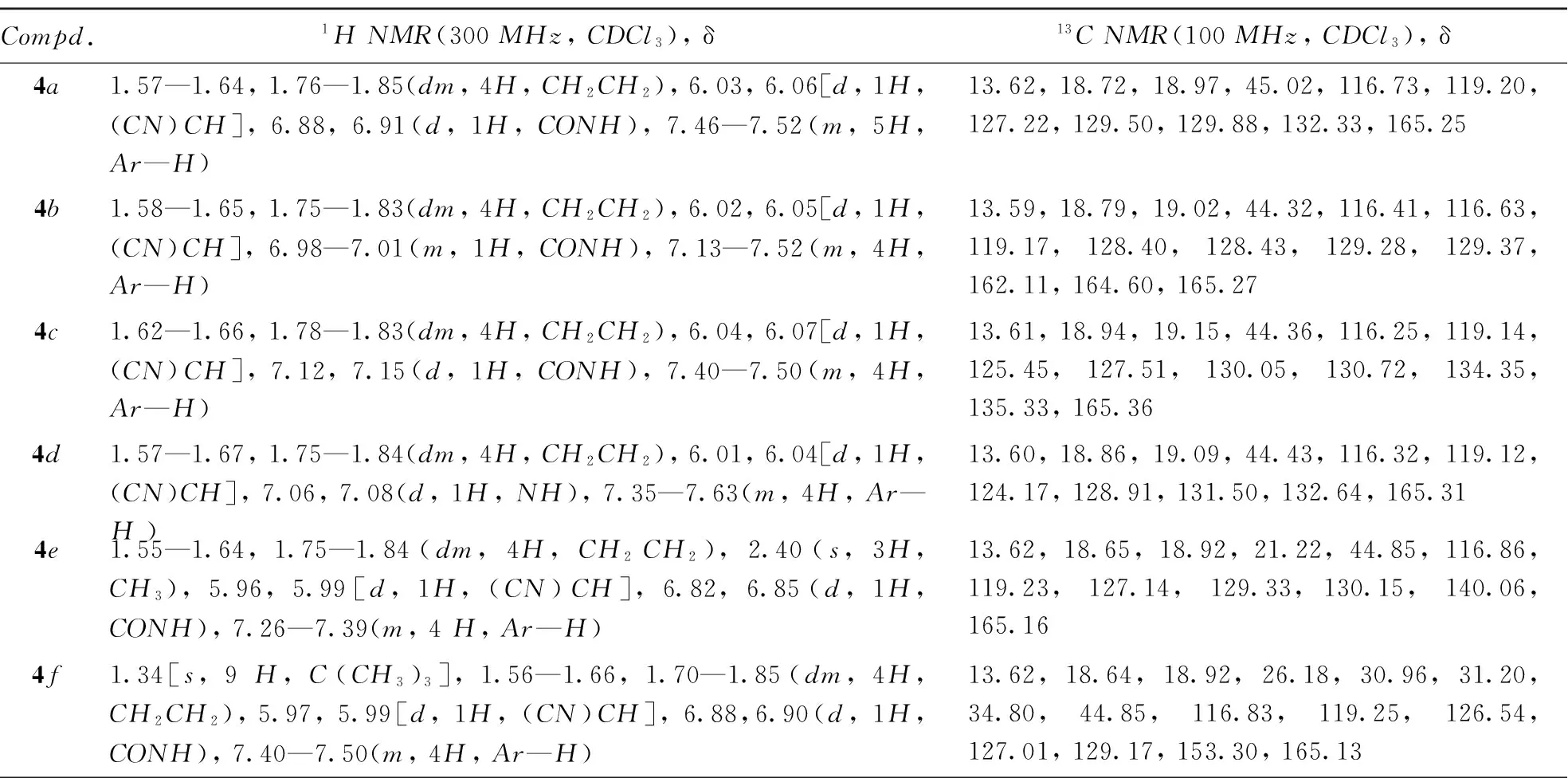
Table 2 1H NMR data of compounds 4a—4h
Continued
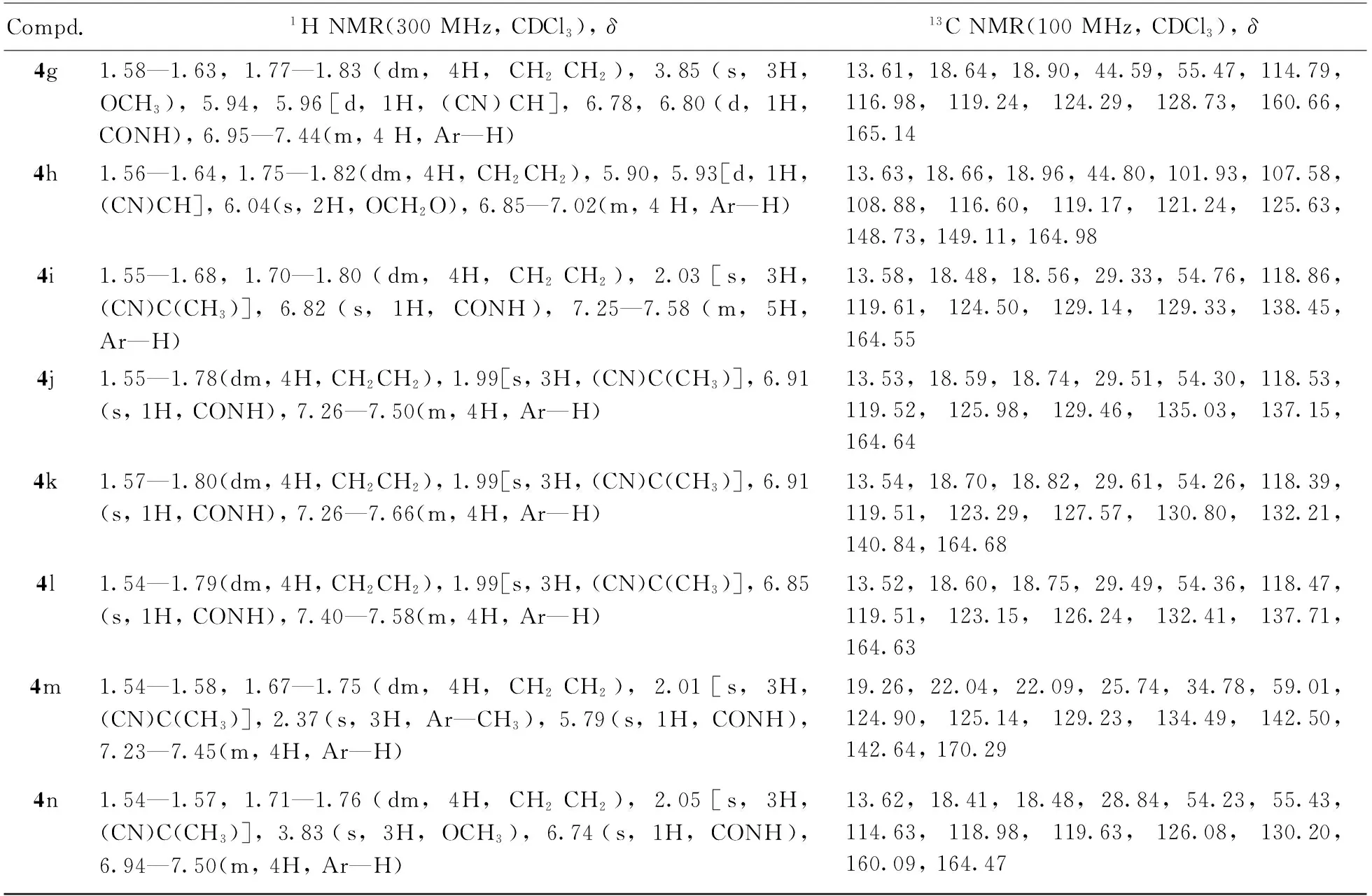
Compd.1HNMR(300MHz,CDCl3),δ13CNMR(100MHz,CDCl3),δ4g1.58—1.63,1.77—1.83(dm,4H,CH2CH2),3.85(s,3H,OCH3),5.94,5.96[d,1H,(CN)CH],6.78,6.80(d,1H,CONH),6.95—7.44(m,4H,Ar—H)13.61,18.64,18.90,44.59,55.47,114.79,116.98,119.24,124.29,128.73,160.66,165.144h1.56—1.64,1.75—1.82(dm,4H,CH2CH2),5.90,5.93[d,1H,(CN)CH],6.04(s,2H,OCH2O),6.85—7.02(m,4H,Ar—H)13.63,18.66,18.96,44.80,101.93,107.58,108.88,116.60,119.17,121.24,125.63,148.73,149.11,164.984i1.55—1.68,1.70—1.80(dm,4H,CH2CH2),2.03[s,3H,(CN)C(CH3)],6.82(s,1H,CONH),7.25—7.58(m,5H,Ar—H) 13.58,18.48,18.56,29.33,54.76,118.86,119.61,124.50,129.14,129.33,138.45,164.554j1.55—1.78(dm,4H,CH2CH2),1.99[s,3H,(CN)C(CH3)],6.91(s,1H,CONH),7.26—7.50(m,4H,Ar—H)13.53,18.59,18.74,29.51,54.30,118.53,119.52,125.98,129.46,135.03,137.15,164.644k1.57—1.80(dm,4H,CH2CH2),1.99[s,3H,(CN)C(CH3)],6.91(s,1H,CONH),7.26—7.66(m,4H,Ar—H)13.54,18.70,18.82,29.61,54.26,118.39,119.51,123.29,127.57,130.80,132.21,140.84,164.684l1.54—1.79(dm,4H,CH2CH2),1.99[s,3H,(CN)C(CH3)],6.85(s,1H,CONH),7.40—7.58(m,4H,Ar—H)13.52,18.60,18.75,29.49,54.36,118.47,119.51,123.15,126.24,132.41,137.71,164.634m1.54—1.58,1.67—1.75(dm,4H,CH2CH2),2.01[s,3H,(CN)C(CH3)],2.37(s,3H,Ar—CH3),5.79(s,1H,CONH),7.23—7.45(m,4H,Ar—H)19.26,22.04,22.09,25.74,34.78,59.01,124.90,125.14,129.23,134.49,142.50,142.64,170.294n1.54—1.57,1.71—1.76(dm,4H,CH2CH2),2.05[s,3H,(CN)C(CH3)],3.83(s,3H,OCH3),6.74(s,1H,CONH),6.94—7.50(m,4H,Ar—H)13.62,18.41,18.48,28.84,54.23,55.43,114.63,118.98,119.63,126.08,130.20,160.09,164.47
1.3 目标化合物的生物活性测定
采用菌丝生长法[40]测试了目标化合物对油菜菌核菌(Sclerotinia sclerotiorum)、 黄瓜灰霉菌(Botrytis cinerea)、 瓜果腐霉菌(Pythium aphanidermatum)、 稻瘟梨孢菌(Pyricularia oryzae)、 芦笋茎枯菌(Phomopsis asparagi)和棉花立枯菌(Rhizoctonia solani)的离体杀菌活性, 结果见表3.
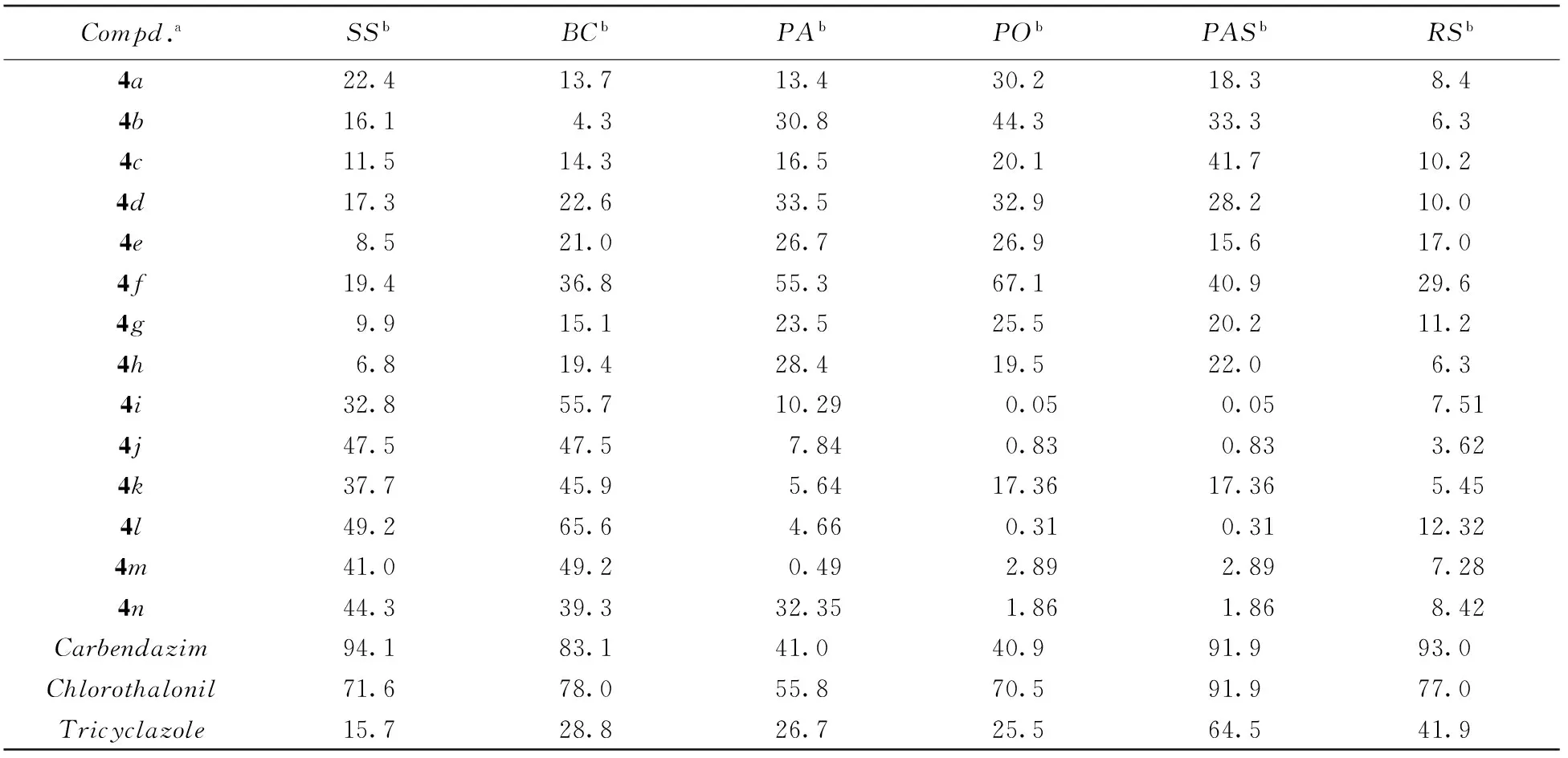
Table 3 In vitro fungicidal activity of title compounds
a. Test concentration was 50 μg/mL; b. SS: Sclerotinia sclerotiorum; BC: Botrytiscinerea; PA: Pythium aphanidermatum; PO: Pyricularia oryzae; PAS: Phomopsis asparagi; RS: Rhizoctonia solani.
采用盆栽法[41], 以烯肟菌胺(SYP-1620)为阳性对照测定了目标化合物对黄瓜霜霉病(Pseudoperonospora cubensis)、 小麦白粉病(Erysiphe graminis)和玉米锈病(Puccinia sorghi)的保护活性, 结果见表4.
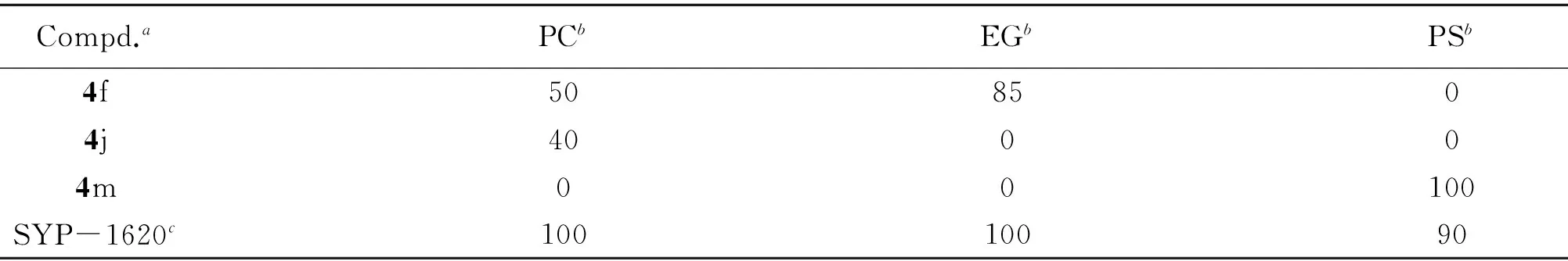
Table 4 In vivo fungicidal activity of some title compounds
a. Test concentration was 400 μg/mL, compounds 4i, 4k, 4l and 4n showed no activity against the above fungi; b. PC: Pseudoperonospora cubensis; EG: Erysiphe graminis; PS: Puccinia sorghi; c. positive control.
采用浸叶法[42]测定了目标化合物对东方粘虫(Mythimma separata Walker)的杀虫活性, 采用浸液法[43]测定了目标化合物对尖音库蚊淡色亚种(Culex pipiens pallens) 的杀虫活性, 结果见表5.
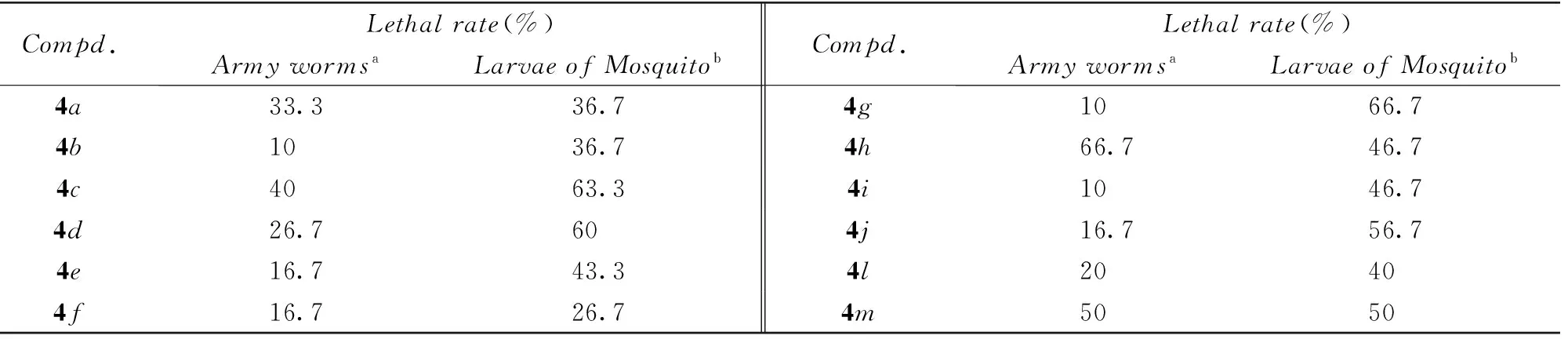
Table 5 Larvicidal activity of title compounds
a. Test concentration was 600 μg/mL; b. test concentration was 5 μg/mL.
2 结果与讨论
2.1 中间体及目标化合物的合成
在化合物1a~1h的合成中使用低毒的氰基三甲基硅烷(TMSCN)代替金属氰化物, 产物可通过重结晶或柱层析纯化; 中间体1i~1n使用氰化钠制备, 为便于后续反应的准确计量, 于0 ℃下向其干燥后的乙醚粗提溶液中通入干燥的氯化氢气体, 制得其盐酸盐沉淀. 将该沉淀经真空干燥后, 低温保存.
在1-氰基环丙烷-1-甲酸乙酯(2)的合成中尝试使用氯化三乙基苄基铵(TEBA)、 PEG400和1-丁基-3-甲基咪唑六氟磷盐([BMIM]PF6)分别作为相转移催化剂, 发现[BMIM]PF6催化的产物收率接近80%, 高于TEBA和PEG400催化的收率(约60%); 使用NaOH水溶液水解1-氰基环丙烷-1-甲酸乙酯(2)无法得到1-氰基环丙烷-1-甲酸(3), 需在低温下于无水乙醇中用KOH水解后酸化才能获得产物.
2.2 目标化合物的生物活性
在离体活性测试中, 化合物4j, 4l和4n对油菜菌核菌的抑制率接近50%; 化合物4i, 4j, 4k, 4l和4m对黄瓜灰霉菌的抑制率为45.9%~65.6%; 化合物4f对瓜果腐霉菌的抑制率为55.3%; 化合物4b和4f对稻瘟菌的抑制率为44.3%和67.1%, 与对照药剂百菌清相当; 化合物4c和4f对芦笋茎枯菌的抑制率约40%. 在盆栽实验中, 化合物4f, 4j和4m对黄瓜霜霉病、 小麦白粉病及玉米锈病有较好的抑制活性, 其中以化合物4f对白粉病的抑制率(85%)和化合物4m对玉米锈病的抑制率(100%)较突出. 由以上结果可知: (1) 目标化合物对油菜菌核菌、 黄瓜灰霉菌、 瓜果腐霉菌和稻瘟菌具有一定的抑制活性; (2) 酰胺α位有甲基取代的目标化合物(4i~4m)对油菜菌核菌和黄瓜灰霉病的抑制活性较好, 无甲基取代的化合物(4a~4j)对稻瘟菌、 芦笋茎枯菌和棉花立枯菌的抑制活性较好, 表明酰胺α位的甲基对化合物的抑制活性具有重要影响; (3) 化合物4f对黄瓜灰霉菌、 瓜果腐霉菌、 稻瘟菌和芦笋茎枯菌均具有显著抑制活性, 其典型特征为苯环的C4位有叔丁基取代, 表明苯环上的C4空间体积较大的取代基对活性有利;(4) 活体活性测试结果表明, 化合物4f和4m具有确切的杀菌活性, 与离体测试中化合物4f和4m的杀菌活性特征一致, 另外二者苯环C4位均有较大的给电子取代基.
此外, 目标化合物具有一定的杀虫活性, 化合物4h和4m对粘虫的致死率分别为66.7%和50%; 化合物4c, 4d, 4g, 4j和4m等对蚊幼虫有一定的杀虫活性, 可归因于其与菊酯的结构相似性.
3 结 论
合成了14个全新化合物, 通过1H NMR,13C NMR和HRMS确证了其结构. 制备了2-氨基-2-(取代)苯丙腈盐酸盐以对其提纯和准确计量; 使用1-丁基-3-甲基咪唑六氟磷盐([BMIM]PF6)催化提高了1-氰基环丙烷-1-甲酸乙酯的收率. 研究发现, 目标化合物对稻瘟菌和黄瓜霜霉菌具有一定的抑制活性, 化合物4f和4m对小麦白粉病和玉米锈病具有活体保护活性; 此外, 目标化合物还具有一定的杀虫活性. 该工作对杀菌剂的创制研究具有一定的指导意义.
感谢中国农业科学院植保所闫晓静副研究员和南开大学元素所赵卫光研究员在活性测试工作中的大力支持.
支持信息见http://www.cjcu.jlu.edu.cn/CN/10.7503/cjcu20150729.
[1] Zhu L.T., A Collection of Fine Chemicals, Agrichemicals, Zhejiang Science and Technology Press, Hangzhou, 2000, 10, 11, 22, 23, 163—239(朱良天. 精细化学品大全—农药卷, 杭州: 浙江科学技术出版社, 2000, 10, 11, 22, 23, 163—239)
[2] Zhai X., Wang L. M., Shi J.Y., Gong P., Chem. Res. Chinese Universities, 2015, 31(3), 372—380
[3] Zhao S. H., Plant Chemical Protection, China Agricultural Press, Beijing, 2000, 83—90(赵善欢. 植物化学保护, 北京: 中国农业出版社, 2000, 83—90)
[4] Fujinami A., Ozaki T., Ooba S., Yamamoto S., Nodera K., Tanaka K., Akiba K., Ooishi T., Kameda N., Antimicrobial N-(3,5-dihalophenyl)imide Compounds, ZA 7001624, 1970-10-05
[5] Baettig W., Hanreich R. G., Crystal Modification of (4-Cyclopropyl-6-methyl-pyrimidin-2-yl)-phenyl-amine, Process for Its Preparation and Its Use as Fungicide, EP 655441, 1995-05-31
[6] Kurahashi Y., Shiokawa K., Kagabu S., Sakawa S., Moriya K., N-Benzylcyclopropane-carboxamide Derivatives, Intermediates for Their Preparation, and Fungicides for Agriculture and Horticulture, EP 170842, 1986-02-12
[7] Snel M., Gramlich J. V., Med. van de Facult. Landbouw., Univ. Gent., 1973, 38(3), 1033—1041
[8] Wang D. J., Zhang E. S., Xu T. L., Li J., Huang T. K., Zou Y., Chem. J. Chinese Universities, 2015, 36(2), 267—273(王德建, 张恩生, 徐田龙, 李军, 黄桐堃, 邹永. 高等学校化学学报, 2015, 36(2), 267—273)
[9] Liao H. M., Chong L. E., Tan L., Chen X. D., You R., Gong P., Chem. Res. Chinese Universities, 2014, 30(5), 759—763
[10] Zhang Y. B., World Pesticides, 2013, 35(Supplement), 38—42(张一宾. 世界农药, 2013, 35(增刊), 38—42)
[11] Walter L., Wilheim S., Ingeborg H., Christa F., Manfred F., Winfride F., Phosphorus Containing Alpha Oximino Acetic Acid Nitriles, US 3591662, 1966-05-26
[12] Moffat A. S., Science, 1993, 261, 550—551
[13] Takagi K., Ohtani T., Nishida T., Hamaguchi H., Nishimatsu T., Kanaoka A., Hydrazinecarboxamide Derivatives, A Process for Production Thereof, and Uses Thereof, EP 462456, 1991-12-27
[14] Hughes K. A., Lahm G. P., Selby T. P., Stevenson T. M., Preparation of Cyano Anthranilamide Insecticides, WO 2004067528, 2004-08-12
[15] Shiokawa K., Tsuboi S., Kagabu S., Sasaki S., Moriya K., Hattori Y., Heterocyclic Compounds, EP 235725, 1987-09-09
[16] Ishimitsu K., Suzuki J., Ohishi H., Yamada T., Hatano R., Takakusa N., Mitsui J., Amine Derivatives, WO 9104965, 1991-04-18
[17] Loso M. R., Nugent B. M., Huang J. X., Rogers R. B., Zhu Y., Renga J. M., Hegde V. B., Demark J. J., Inseticidal N-Substituted(6-Haloalkylpyridin-3-yl)Alkyl Sulfoximines, WO 2007095229, 2007-08-23
[18] Toki T., Koyanagi T., Morita M., Yoneda T., Kagimoto C., Okada H., Pyridine Amides and Their Salts, Processes for Their Production and Pesticidal Compositions Containing Them, EP 580374, 1994-01-26
[19] Grier N., Lederer S. J., Dihalomethylglutaronitriles Used as Antibacterical and Antifungal Agents, US 3833731, 1974-09-03
[20] Ugi I., Polyisonitriles and A Process for Their Production, DE 1158500B, 1963-12-05
[21] Klopping H. L., Delp C. J., J. Agric. Food Chem., 1980, 28(2), 467—468
[22] Van Schoor A., Mohr G., 2,3-Dicyano-1,4-dithiaanthrahydroquinone and Anthraquinone, DE 1156821, 1963-11-07
[23] Clough J. M., Godfrey C. R. A., Streeting I. T., Cheetham R., Fungicides, EP 382375, 1990-08-16
[24] Nasu R., Komyoji T., Suzuki K., Nakajima T., Ito K., Ohshima T., Yoshimura H., Imidazole Compounds and Biocidal Compositions Comprising the Same, BR 8801098, 1988-10-18
[25] Rew Y. S., Cho J. H., Ra C. S., Ahn S. C., Kim S. K., Lee Y. H., Jung B. Y., Choi W. B., Rhee Y. H., Yoon M. Y., Chun S. W., 2-Aminothiazolecarboxamide Derivatives, Processes for Their Preparation and Their Use for Controlling Phytopathogenic Organisms, EP 639574, 1995-02-22
[26] Fujimoto T. T., Substituted Triazoles, Processes for Making Them, Their Use as Fungicides and Fungicidal Compositions Containing Them, EP 145294, 1985-06-19
[27] Shaber S. H., Flynn K. E., Weinstein B., Alpha-aryl-alpha-phenylethyl-1H-1,2,4- triazole-1-propanenitriles, DE 3721786, 1988-01-07
[28] Sieverding E., Hirooka T., Nishiguchi T., Yamamoto Y., Spadafora V. J., Hasui H., Brighton Crop Protection Conference Pests and Diseases, 1998, 2, 359—366
[29] Enomoto M., Magara O., Yamada Y., Production of Lower Alkyl 2-Cyano-3-metyl-2-butenoate, JP H08143528, 1996-06-04
[30] Manabe A., Enomoto M., Yamada Y., Oguri Y., Sasaki M., Pesticide Science, 1999, 55(6), 649—650
[31] Ra C. S., Rew Y. S., Choi W. B., Korean J. Med. Chem., 1995, 5(2), 72—75
[32] Guan A.Y., Liu C. L., Yang X. P., Dekeyser M., Chem. Rev., 2014, 114, 7079—7107
[33] Khuong M., Ghanshyam P., Tetrahedron Lett., 1984, 25(41), 4583—4586
[34] Sakulsombat M., Vongvilai P., Amstrom O., Chemistry—A European Journal, 2014, 20(36), 11322—11325
[35] Morris G. F., Hauser C. R., J. Org. Chem., 1961, 26(11), 4741—4743
[36] Wang B. Y., Zhang W., Zhang L. L., Du D. M., Liu G., Xu J. X., Eur. J. Org., 2008, (2), 350—355
[37] Allen D. J., Ruso E. R., Frankel S. A., α-Caboxamidomethylaminocarboxamides, US 3293294, 1966-12-20
[38] Muthusamy S., Gnanaprakasam B., Tetrahedron Lett., 2005, 46(4), 635—638
[39] Li Q. R., Gu C. Z., Yin H., Zhang Y., Chin J. Org. Chem., 2005, 25(11), 1416—1419 (李前荣, 顾承志, 尹浩, 张毅. 有机化学, 2005, 25(11), 1416—1419)
[40] Xiao Y. S., Zhang J. J., Yan X. J., Dong Y. H., Yuan H. Z., Liang X. M., Wang D. Q., Chin. J. Org. Chem., 2014, 34(12), 2493—2498(肖炎双, 张建军, 闫晓静, 董燕红, 袁会珠, 梁晓梅, 王道全. 有机化学, 2014, 34(12), 2493—2498)
[41] Wang L. Z., Sun X. F., Li Z. N., Guan A. Y., Proceedings of the 16th Annual Meeting of CIESC, Pesticide Professional Committee, Guiyang, 2014, 49—51(王立增, 孙旭峰, 李志念, 关爱莹. 中国化工学会农药专业委员会第十六届年会论文集, 贵阳, 2014年9月, 49—51)
[42] Mao C. H., Zhao Y., Li Y. Q., Huang R. Q., Bi F. C., Wang Q. M., Chin. J. Org. Chem., 2009, 29(6), 929—935(毛春晖, 赵毓, 李永强, 黄润秋, 毕富春, 汪清民. 有机化学, 2009, 29(6), 929—935)
[43] Raymond M., Marquine M., J. Evol. Biol., 1994, 7(3), 315—337
(Ed.: P, H, S, K)
Synthesis and Biological Activity of Novel Dicyano-containning Cyclopropane-1-carboxamides†
XU Gaofei, LIU Yanhong, YANG Xinling, WANG Daoquan, YUAN Dekai*
(Department of Applied Chemistry, Science College, China Agricultural University, Beijing 100193, China)
A series of novel dicyano-contained cyclopropanecarboxamide derivatives were designed and synthesized using fungicides containing cyano or cyclopropyl as leading structures. Intermediates 2-amino-2-(substituted) phenylacetonitriles or propionitriles 1a—1n were prepared via Strecker reaction. 1-Cyano-cyclopro-pyl-1-carboxylic(3) was obtained from ethyl cyanacetate and 1,2-dibrimoethane via cyclization and hydrolysis. 14 title compounds were obtained via the condensation of intermediates 1 and 3. The structures of all title compounds were confirmed by1H NMR and HRMS. Compound 4f showed good fungicidal activity against Pythium aphanidermatum and Pyricularia oryzae with inhibiton rates of 55.3% and 67.1% at 50 μg/mL in vitro and against Pseudoperonospora cubensis and Erysiphe graminis with inhibiton rates of 50% and 85% at 400 μg/mL in vivo. Compound 4m could give total control against Puccinia sorghi at 400 μg/mL in vivo. In addition, compounds 4c, 4d, 4g, 4j and 4m showed good larvicidal activity against mosquitoes(Culex pipiens pallens) at 5 μg/mL with the lethal rate above 60%; compounds 4h and 4j possessed larvicidal activity against armyworms(Mythimna separata) at 600 μg/mL with the lethal rate of 66.7% and 50%.
Dicyano-containning cyclopropane-1-carboxamide; Strecker reaction; Condensation; Biological activity
10.7503/cjcu20150729
2015-09-17.
日期: 2016-01-24.
国家自然科学基金(批准号: 20902107)资助.
O624.5; O625.4
A
联系人简介: 袁德凯, 男, 博士, 副教授, 主要从事有机合成及新农药创制研究. E-mail: yuandekai@aliyun.com
† Supported by the National Natural Science Foundation of China(No.20902107).

