Dynamic expression of nerve growth factor and its receptor TrkA after subarachnoid hemorrhage in rat brain
Jin-ning Song, Zun-wei Liu Long Sui, Bin-fei Zhang Yong-lin Zhao Xu-dong Ma Hua Gu Department of Neurosurgery, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi Province, China Department of Neurosurgery, the 5 Hospital of China North Industries Group, Xi’an, Shaanxi Province, China
Dynamic expression of nerve growth factor and its receptor TrkA after subarachnoid hemorrhage in rat brain
Jin-ning Song1,*, Zun-wei Liu1, Long Sui1,2, Bin-fei Zhang1, Yong-lin Zhao1, Xu-dong Ma1, Hua Gu1
1 Department of Neurosurgery, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi Province, China
2 Department of Neurosurgery, the 521 Hospital of China North Industries Group, Xi’an, Shaanxi Province, China
How to cite this article: Song JN, Liu ZW, Sui L, Zhang BF, Zhao YL, Ma XD, Gu H (2016) Dynamic expression of nerve growth factor and its receptor TrkA after subarachnoid hemorrhage in rat brain. Neural Regen Res 11(8)∶1278-1284.
Funding: This study was funded by the National Natural Science Foundation of China, No. 30870844; and the New Century Supporting Program to Excellent Talents in China, No. NCET-05-0831.
Jin-ning Song, M.D.,
jinningsong@126.com.
orcid:
0000-0002-0620-8983
(Jin-ning Song)
Accepted: 2016-07-09
Graphical Abstract

Delayed ischemic neurologic deficit after subarachnoid hemorrhage results from loss of neural cells. Nerve growth factor and its receptor TrkA may promote regeneration of neural cells, but their expression after subarachnoid hemorrhage remains unclear. In the present study, a rat model of subarachnoid hemorrhage was established using two injections of autologous blood into the cistern magna. Immunohistochemical staining suggested that the expression of nerve growth factor and TrkA in the cerebral cortex and brainstem increased at 6 hours, peaked at 12 hours and decreased 1 day after induction of subarachnoid hemorrhage, whereas the expression in the hippocampus increased at 6 hours, peaked on day 1, and decreased 3 days later. Compared with those for the rats in the sham and saline groups, neurobehavioral scores decreased significantly 12 hours and 3 days after subarachnoid hemorrhage (P < 0.05). These results suggest that the expression of nerve growth factor and its receptor TrkA is dynamically changed in the rat brain and may thus participate in neuronal survival and nerve regeneration after subarachnoid hemorrhage.
nerve regeneration; subarachnoid hemorrhage; nerve growth factor; TrkA; intrinsic; dynamic expression; cortex; hippocampus; brainstem; acute phase; neural regeneration
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) is a common but severe disease. The incidence is 6-7 per 100,000 persons every year, and the fatality rate determined in population-based studies is approximately 50% (van Gijn et al., 2007). Most patients die during the acute phase, and survivors generally experience delayed ischemic neurologic deficit and have a low life quality. During the acute phase after SAH, neuronal loss is common and may be the key reason for the delayed ischemic neurologic deficit (Sehba et al., 2012). Hence, maintaining neuronal survival and improving nerve regeneration are good therapeutic strategies.
Nerve growth factor (NGF) is a bioactive neurotrophic factor in the central and peripheral nervous systems (Beglova et al., 1998). NGF plays important roles via interactions with its functional receptor TrkA in neuronal protection, development, regeneration, axonal growth, and reconstruction of synapses (Li et al., 2015). Numerous studies have indicated that NGF can protect damaged nerve cells, reduce nerve cell death, support the survival of neurons, and promote the recovery of neurological function in brain and spinal cord injuries (Davies, 1994; Auld et al., 2001; Chiaretti et al., 2008a, b, d), especially in traumatic brain injuries. This evidence suggests a potential role for NGF in protecting neurons and improving neurogenesis after SAH. However, this role has not been well studied, and even the expression of NGF in brains with SAH remains unclear. Hence, we studied the temporal and regional variations of brain NGF and TrkA expression in an animal model of SAH, especially during the vitally important acute phase. Our results provide new understanding of NGF in SAH and offer a theoretical basis for the therapeutic use of NGF in patients with SAH.
Materials and Methods
Animals
Healthy adult male Sprague-Dawley rats (n = 96; weight, 280-300 g) were provided by the Experimental Animal Center of Xi’an Jiaotong University, China (production license number: SCXK [Shaan] 08-018). The rats were housed two per cage, allowed free access to food and water, and maintained at 20-25°C in a humidity-controlled (50-60%) room under a 12-hour light/dark cycle for 1 week prior to experimentation. This study was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals produced by the National Institutes of Health. The protocol was approved by the Biomedical Ethics Committee of Medical College of Xi’an Jiaotong University, China.
Rat models of SAH
The rats were randomly divided into the following four groups: normal (n = 6), sham (n = 30), saline (n = 30; rats received two saline injections), and SAH (n = 30; rats received two autologous blood injections). The saline, sham, and SAH groups were then divided into five subgroups according to the time after operation when the rats were sacrificed, based in part on the results of our previous study (Zhang et al., 2015): 6 hours (n = 6), 12 hours (n = 6), 1 day (n = 6), 3 days (n = 6) and 5 days (n = 6).
A rat model of SAH was established using a method that involved two autologous blood injections into the cisterna magna. This procedure simulates the pathophysiological process in SAH, and, compared with other models, this model has good repeatability and stable results. Rats were anesthetized with 10% chloral hydrate injected intraperitoneally. A total of 0.3 mL of blood was collected from the tail. The cisterna magna was punctured with an 18-G trocar needle (Becton Dickinson Medical Devices, Jiangsu, China) at a site 1 cm below the midpoint of the line between the two external auditory canals. Clear cerebrospinal fluid was detected but not withdrawn. Non-anticoagulated autologous blood was slowly injected into the cisterna magna at 1 mL/min. The puncture point was pressed for 4-5 minutes following this aseptic injection. A headdown position was maintained for at least 30 minutes after surgery. The procedure was performed again 48 hours after the initial blood injection to establish the “double autologous blood injection” SAH model.
The normal control group did not undergo the operation. The sham group underwent the operation, but received no cisterna magna injection. The saline group underwent the same procedure as the SAH model group, but 0.3 mL of saline (37°C) was injected into the cisterna magna, followed by a second saline injection of the same volume, rather than the autologous blood injections.
Neurobehavioral assessment
Neurobehavioral scores were assigned using Loeffler’s 5-point scoring method (Miao et al., 2010; Table 1). This assessment was performed twice: 12 hours and 3 days after the operation to induce SAH.
Histopathological analysis and immunohistochemical assay
Rats were euthanized by intracardiac perfusion with saline and were then perfused intracardiacally with 4% paraformaldehyde. The brains were removed. The cortex, brainstem, and hippocampus were dissected, fixed in 4% paraformaldehyde for 24 hours, and then embedded in paraffin following routine methods. Slices (5 μm thick) were obtained with intermittent serial sectioning. Histopathological analysis was performed using routine hematoxylin and eosin staining procedures. Immunohistochemical staining was then conducted using the streptavidin-peroxidase technique. Briefly, after rehydration, endogenous peroxidase activity was blocked by incubating slices with 3% hydrogen peroxide for 10 minutes. Sections were then placed in 10 mM citrate buffer (pH 6.0), heated in a microwave oven at 95°C for 30 minutes, cooled at room temperature for 20 minutes, and then rinsed in 0.1 M PBS (pH 7.2). Non-specific protein binding was blocked by incubation for 1 hour in 5% goatserum in PBS at room temperature. The primary antibodies were rabbit anti-rat NGF monoclonal antibody (1:200; Beijing Biosynthesis, Beijing, China) and rabbit anti-rat TrkA monoclonal antibody (1:300; Beijing Biosynthesis). The sections were incubated in primary antibody overnight at 4°C. A goat anti-rabbit biotin-labeled secondary antibody (Beijing Biosynthesis; ready to use type) was added dropwise and incubated for 10 minutes at room temperature. The slices were rinsed with PBS three times for 5 minutes each time. The secondary antibody incubation and rinse steps were repeated for the second biotin-labeled antibody (Beijing Biosynthesis; ready to use type). PBS (0.1 M) was used as a negative control. Images were collected with an image analysis and acquisition system (Leica-Q550CW, LEICA, Manheim, Germany). The system detected grayscale values, ranging from 0 to 255. Higher grayscale values indicated lower expression of NGF and TrkA. The same exposure conditions were used to capture the images of all specimens, with a consistent light source intensity and microscope aperture. Three rat brains from each group (except for the normal group) were selected for immunohistochemical staining. Each site of the brain was analyzed in two slices. Five fields were randomly selected in every slice, and mean grayscale values were calculated.
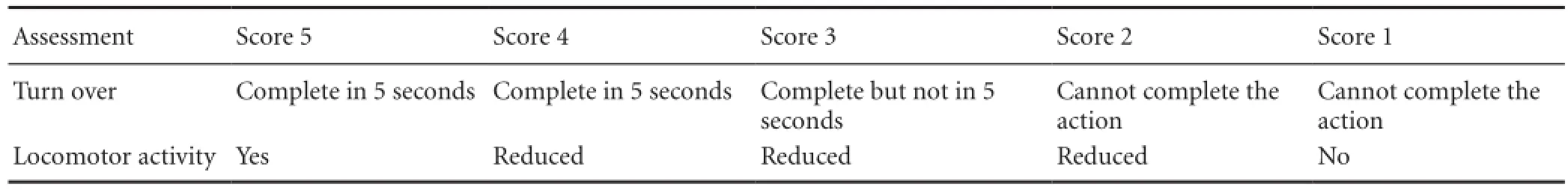
Table 1 Loeffler's 5-point scoring method

Table 2 Neurobehavioral scores of all groups
Statistical analysis
All data were analyzed using SPSS 15.0 software (SPSS, Chicago, IL, USA). Neurobehavioral scores and grayscale values of NGF and TrkA immunoreactivity are presented as the mean ± SD. All data followed a normal distribution. Comparisons among different groups were performed using one-way analysis of variance. The least significant difference test was used to compare the difference between two groups. P-values less than 0.05 were considered statistically significant.
Results
General observations
All rats in the SAH and saline groups displayed transient intracranial hypertension symptoms, such as limb stiffness, neck rigidity, and muscle tremor, during the injections. Behaviors and time of awakening from the anesthesia varied among the groups. The sham group showed normal behaviors at 4 hours after the operation. Rats in the saline group showed a reduction in proactive aggression and were drinking water, eating, or active 4-5 hours after the operation. Rats in the SAH group took longer than those in saline group (6-8 hours) to show these same behaviors. Additionally, rats in the SAH group had severe conjunctival hyperemia and weight loss (lasting 3 days, 20 g lighter on average) and had a longer recovery time (3-5 days) than those in the saline group (1 day).
Neurobehavioral changes
Neurobehavioral scores were assessed at 12 hours and on the third day after autologous blood injections (Table 2). Compared with the normal, sham, and saline groups, the SAH group had a significantly lower neurobehavioral score (P < 0.05) 12 hours after the operation to induce the animal model of SAH. The SAH group score remained significantly lower (P < 0.05) than that of the other groups 3 days after the operation.
Gross observations of brain tissue
The basal cistern and cisterna magna were exposed during the removal of the brain. Rats in the 6-hour SAH group presented with a wide range of subarachnoid hemorrhages and few blood clots. 12-hour, 1-day, 3-day, and 5-day SAH groups displayed significantly more blood clots and fewer hemorrhages than the 6-hour group. The 1-day SAH group had the biggest blood clots, with the other SAH groups showing smaller and faded clots. The normal, sham, and saline groups had no subarachnoid hemorrhages or blood clots.
Changes in hematoxylin and eosin staining under a light microscope
The neurons and glia of rats in the normal group were observed under a light microscope and found to have a normal distribution with distinct and complete structures. In the 6-hour SAH group, evidence of nerve fiber damage began to appear. In the 12-hour SAH group, the space surrounding the vessels had widened and marked brain edema was evident (Figure 1). Nuclear condensation, fragmentation,and cell disintegration were present in the 1-day, 3-day, and 5-day SAH groups in addition to edema. By contrast, rat brains in the sham and saline groups showed few pathological changes.
Alterations in NGF and TrkA immunoreactivity
In the normal, sham, and saline groups, immunostaining of NGF in the cerebral cortex, brainstem, and hippocampus was weak and stable. In all SAH rat groups, NGF was mainly expressed in the cytoplasm of both neurons and glia. The most apparent staining in the cerebral cortex and brainstem appeared 12 hours after SAH (Figure 2), and in the hippocampus on day 1 after SAH.
Expression of TrkA immunoreactivity was similar to that for NGF. Thus, in the normal, sham, and saline groups, TrkA immunostaining was weak and stable in the cerebral cortex, brainstem, and hippocampus. In the SAH group, TrkA immunoreactivity was observed in both neurons and glia. The most apparent staining varied in these subgroups depending on the brain region: cerebral cortex and brainstem, 12 hours; hippocampus, 1 day (Figure 3).
Variations in intensity of NGF and TrkA immunoreactivity in the cerebral cortex, brainstem, and hippocampus among different groups
NGF
Temporal variation: The temporal variation in the intensity of NGF immunostaining was the same in the sham and saline groups (Figure 4). No significant differences were observed in expression for NGF immunostaining in the cortex, brainstem and hippocampus among normal, sham, and saline groups (P > 0.05). By contrast, in the SAH group, NGF expression began to increase 6 hours after SAH. The expression peaked at 12 hours, but NGF expression after the peak was still greater than that observed in normal, sham, and saline groups on day 5 (P < 0.05). Expression was significantly higher in the 12-hour SAH group than in the other SAH groups in the cerebral cortex and brainstem (P <0.05), whereas in the hippocampus, the highest value was in the 1-day subgroup (P < 0.05).
Group variation: NGF expression was significantly higher in all the SAH subgroups than in the normal, sham, and saline groups (P < 0.05).
Regional variation: The highest expression for NGF immunoreactivity was found earlier in the cortex and brainstem than in the hippocampus in the SAH groups.
TrkA
Temporal variation: Similar to that for NGF, the temporal variation in the intensity of TrkA immunostaining was the same in the sham and saline groups (Figure 4). No significant differences were observed in expression for TrkA immunoreactivity among normal, sham, and saline groups (P > 0.05). In SAH subgroups, the variation in TrkA expression was consistent with that for NGF, that is, increasing 6 hours after SAH, reaching a peak, and then decreasing. TrkA expression was still higher compared with that of normal, sham, and saline groups on day 5 (P < 0.05). Multiple comparisons showed that expression for the 12-hour group was significantly higher than those for the other SAH groups in the cerebral cortex and brainstem (P < 0.05). However, the highest expression for the 1-day group was in the hippocampus (P < 0.05).
Group variation: TrkA expression was significantly higher in the SAH groups than in normal, sham, and saline groups at all time points (P < 0.05).
Regional variation: The highest expression for TrkA immunoreactivity in the SAH groups was observed earlier in the cortex and brainstem than in the hippocampus.
Discussion
Neuronal loss during the acute phase of SAH plays an important role. NGF is known for its functions in neuronal survival and growth effects during numerous nerve injuries (Zhao et al., 2015). In our study, NGF expression increased at 6 hours and peaked at 12 hours after SAH in the cerebral cortex and brainstem. We speculate that this increase in NGF, an intrinsic growth factor, during the early phase is helpful for reducing neuronal death and improving neurobehavior, which has been shown in other studies. For example, continuous injection of NGF into the ventricles of infants with severe traumatic brain injury reduces brain lesions and improves neurological function (Chiaretti et al., 2008c). Mashayekhi (2008) injected NGF antibody into the ventricles of rats and found increased neuronal death in the cortex and significant cognitive impairment. In another study, crush injury of the mental nerve was treated with NGF, and a reduction of neuronal apoptosis and apparent neurogenesis were observed (Savignat et al., 2007). Our study indicated that the peak expression of NGF immunoreactivity was later in the hippocampus (1 day after SAH) than in the cortex and brainstem. A plausible explanation is that hemorrhage causes blood clots to directly attach to or stimulate the cortex and brainstem, but not the hippocampus. On day 5, expression for NGF in the SAH group remained higher than those for normal, sham, and saline groups in all specimens. This result suggests that NGF may also play an important role in the recovery of neurological function, such as neurogenesis.
NGF functions via its receptors: p75 with low-affinity and TrkA with high-affinity (Eibl et al., 2012). When the signal TrkA transduces is “positive” (such as maintaining neuronal survival and growth) (Liebl et al., 2001), p75 often presents a “negative” signal (such as apoptosis) (Zagrebelsky et al., 2005). For instance, evidence indicates that TrkA participates in improving neuronal survival and synaptic reconstruction (Holtmaat et al., 1997). In the present study, we focused on TrkA, rather than on p75, and our results showed similar inverted U-shaped temporal variations for both TrkA and NGF expression. The significant overexpression of TrkA may reflect a demand for the relatively insufficient NGF, similar to what has been shown previously. During an intraventricular injection of NGF in rats, effective TrkA messengerRNA-expressing cells increase (Lapchak et al., 1993). Particularly during the acute phase, numerous injured neurons are competing for limited NGF. Most of them will not receive sufficient growth factors, so sequentially delayed neuronal death and delayed neurological deficit will occur. Therefore, timely administration of exogenous NGF may strengthen the protection and repair of damaged neurons and may be considered for treatment of patients with SAH to improve their prognosis.

Figure 1 Histological changes in the cerebral cortex, brainstem, and hippocampus in normal, sham, and 6-hour and 12-hour SAH groups (hematoxylin and eosin staining; 200× magnification; scale bars: 100 µm).
In this study, we established a rat model of SAH using the method that employs two autologous blood injections into the cisterna magna. General observations, pathological changes in hematoxylin and eosin stained specimens, and low neurobehavioral scores indicated that the model successfully reproduced changes similar to those observed during the acute phase of SAH, which is the baseline for evaluating the role of NGF and TrkA.
There are some limitations to this study. First, no direct evidence indicated an effect of NGF on neurogenesis. Although we observed a significant difference in NGF immunohistochemical staining, this does not fully reflect neurogenesis. Thus, in our future studies, we will use intraventricular injection of NGF and compare neurogenesis in this group to that of a sham-injected group. Second, the mechanism for the effect on NGF and TrkA was not studied. Future research may focus on the mechanism underlying the NGF-TrkA signaling pathway. Phosphatidylinositol 3-kinase/Akt and mitogen activated protein kinase have been reported to play important roles in the “positive” effects of NGF, but not in an animal model of SAH (Eibl et al., 2012). Additionally, an intriguing report indicated that NGF levels in the cerebrospinal fluid may be a predictor for severity and prognosis of traumatic brain injury (Chiaretti et al., 2008a). It is unknown whether this predictor may also be applied to SAH. Moreover, further study will be needed before it is determined whether NGF may be clinically useful in SAH. The safe dose, adverse effects, and route of administration may be obstacles.

Figure 2 NGF immunoreactivity in the cerebral cortex, brainstem and hippocampus in normal and SAH groups (immunohistochemical staining observed under the light microscope at 200× magnification; scale bars: 50 µm).
Yellow arrows indicate NGF-positive cells. The most apparent staining appeared 12 hours after SAH in the cerebral cortex and brainstem, and 1 day after SAH in the hippocampus. NGF: Nerve growth factor; SAH: subarachnoid hemorrhage.
In conclusion, our findings suggest that the expression of NGF and TrkA showed obvious dynamic alterations in rat brain tissue after SAH. Thus, NGF may play an important role in maintaining neuronal survival and improving nerve regeneration.
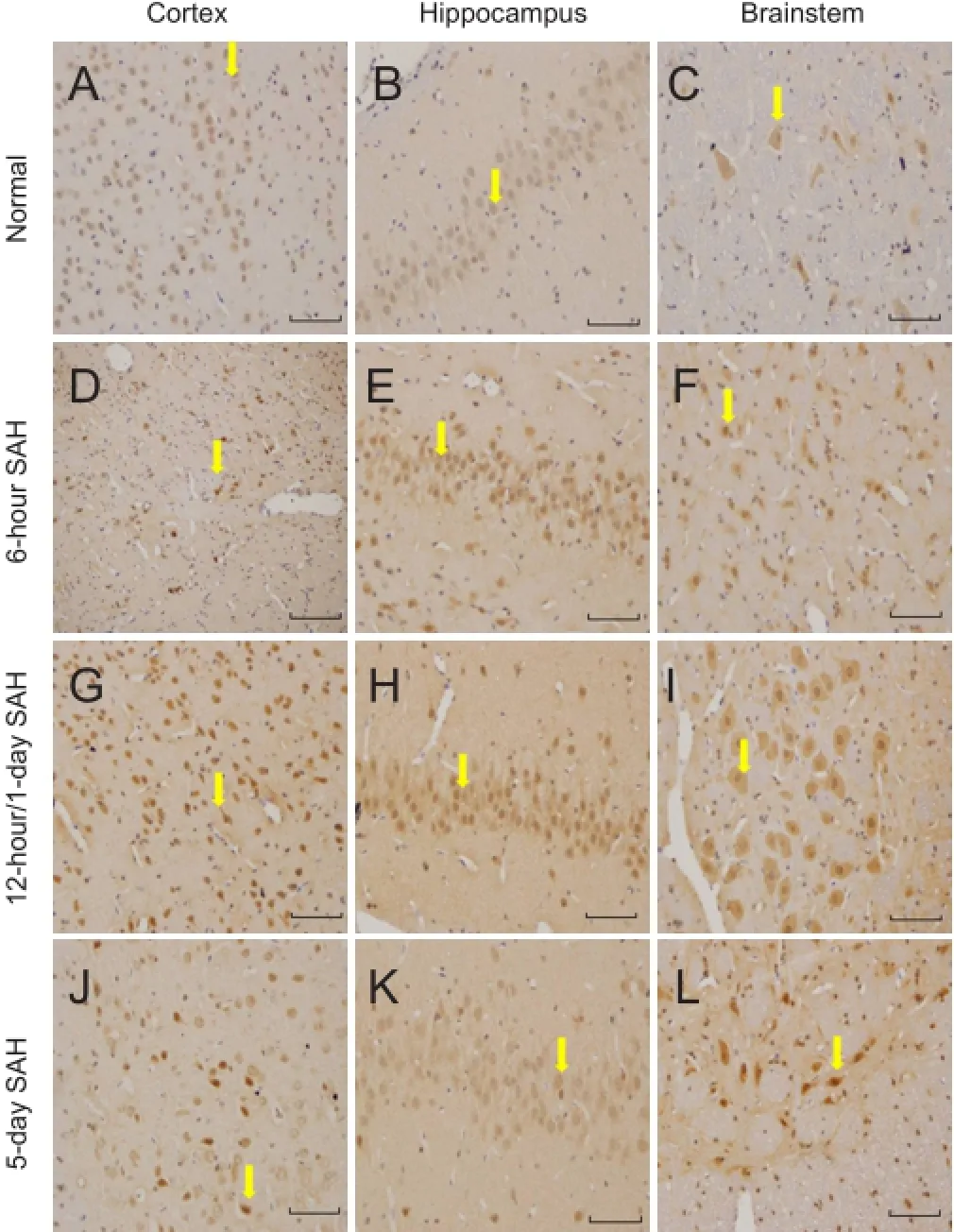
Figure 3 TrkA immunoreactivity in the cerebral cortex, brainstem and hippocampus in normal and SAH groups (immunohistochemical staining observed under the light microscope at 200× magnification; scale bars: 50 µm).

Figure 4 Temporal variations in mean grayscale values of brain NGF and TrkA immunoreactivity in SAH rats.
Author contributions: JNS contributed to definition of intellectual content of this topic and paper review. LS did literature searching and designed the experiment. ZWL prepared and edited the paper. LS, ZWL, BFZ and YLZ made the model. XDM contributed to data acquisition and data analysis. HG did statistical analysis. All authors approved the final version of the paper. Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
Auld DS, Mennicken F, Day JC, Quirion R (2001) Neurotrophins differentially enhance acetylcholine release, acetylcholine content and choline acetyltransferase activity in basal forebrain neurons. J Neurochem 77:253-262.
Beglova N, LeSauteur L, Ekiel I, Saragovi HU, Gehring K (1998) Solution structure and internal motion of a bioactive peptide derived from nerve growth factor. J Biol Chem 273:23652-23658.
Chiaretti A, Antonelli A, Genovese O, Pezzotti P, Rocco CD, Viola L, Riccardi R (2008a) Nerve growth factor and doublecortin expression correlates with improved outcome in children with severe traumatic brain injury. J Trauma 65:80-85.
Chiaretti A, Antonelli A, Mastrangelo A, Pezzotti P, Tortorolo L, Tosi F, Genovese O (2008b) Interleukin-6 and nerve growth factor upregulation correlates with improved outcome in children with severe traumatic brain injury. J Neurotraum 25:225-234.
Chiaretti A, Antonelli A, Genovese O, Fernandez E, Giuda D, Mariotti P, Riccardi R (2008c) Intraventricular nerve growth factor infusion improves cerebral blood flow and stimulates doublecortin expression in two infants with hypoxic-ischemic brain injury. Neurol Res 30:223-228.
Chiaretti A, Antonelli A, Riccardi R, Genovese O, Pezzotti P, Di Rocco C, Tortorolo L, Piedimonte G (2008d) Nerve growth factor expression correlates with severity and outcome of traumatic brain injury in children. Eur J Paediatr Neurol 12:195-204.
Davies AM (1994) Neural development. Chemoattractants for navigating axons. Curr Biol 4:1142-1145.
Eibl JK, Strasser BC, Ross GM (2012) Structural, biological, and pharmacological strategies for the inhibition of nerve growth factor. Neurochem Int 61:1266-1275.
Holtmaat AJ, Hermens WT, Sonnemans MA, Giger RJ, Van Leeuwen FW, Kaplitt MG, Oestreicher AB, Gispen WH, Verhaagen J (1997) Adenoviral vector-mediated expression of B-50/GAP-43 induces alterations in the membrane organization of olfactory axon terminals in vivo. J Neurosci 17:6575-6586.
Lapchak PA, Araujo DM, Carswell S, Hefti F (1993) Distribution of [125I]nerve growth factor in the rat brain following a single intraventricular injection: correlation with the topographical distribution of trkA messenger RNA-expressing cells. Neuroscience 54:445-460.
Li HF, Wang YR, Huo HP, Wang YX, Tang J (2015) Neuroprotective effects of ultrasound-guided nerve growth factor injections after sciatic nerve injury. Neural Regen Res 10:1846-1855.
Liebl DJ, Huang W, Young W, Parada LF (2001) Regulation of Trk receptors following contusion of the rat spinal cord. Exp Neurol 167:15-26.
Mashayekhi F (2008) Neural cell death is induced by neutralizing antibody to nerve growth factor: an in vivo study. Brain Dev 30:112-117.
Miao CM, Luo Q, Wang WW, Kang JS, Shi GY, Li HY, Zhang Y (2010) Significance and expression of FKHR and AKT after subarachnoid hemorrhage in rat brain cortex. Zhonghua Yi Xue Za Zhi 90:1507-1509.
Savignat M, De-Doncker L, Vodouhe C, Garza JM, Lavalle P, Libersa P (2007) Rat nerve regeneration with the use of a polymeric membrane loaded with NGF. J Dent Res 86:1051-1056.
Sehba FA, Hou J, Pluta RM, Zhang JH (2012) The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol 97:14-37.
van Gijn J, Kerr RS, Rinkel GJE (2007) Subarachnoid haemorrhage. Lancet 369:306-318.
Zagrebelsky M, Holz A, Dechant G, Barde YA, Bonhoeffer T, Korte M (2005) The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J Neurosci 25:9989-9999.
Zhang BF, Song JN, Ma XD, Zhao YL, Liu ZW, Li Y, Sun P, Li DD, Pang HG, Huang TQ (2015) Etanercept alleviates early brain injury following experimental subarachnoid hemorrhage and the possible role of tumor necrosis factor-alpha and c-Jun N-terminal kinase pathway. Neurochem Res 40:591-599.
Zhao M, Li XY, Xu CY, Zou LP (2015) Efficacy and safety of nerve growth factor for the treatment of neurological diseases: a meta-analysis of 64 randomized controlled trials involving 6,297 patients. Neural Regen Res 10:819-828.
Copyedited by Smith T, Frenchman B, Wang J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.189193
*Correspondence to:
- 中国神经再生研究(英文版)的其它文章
- Secondary parkinsonism induced by hydrocephalus after subarachnoid and intraventricular hemorrhage
- Prospects for bone marrow cell therapy in amyotrophic lateral sclerosis: how far are we from a clinical treatment?
- Uncoupling protein 2 in the glial response to stress: implications for neuroprotection
- Selective neuronal PTEN deletion: can we take the brakes off of growth without losing control?
- TRPV1 may increase the effectiveness of estrogen therapy on neuroprotection and neuroregeneration
- Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery