Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery
Jennifer M. Colón, Jorge D. MirandaDepartment of Physiology, School of Medicine, University of Puerto Rico Medical Sciences Campus, San Juan, PR, USA
Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery
Jennifer M. Colón, Jorge D. Miranda*
Department of Physiology, School of Medicine, University of Puerto Rico Medical Sciences Campus, San Juan, PR, USA
How to cite this article: Colón JM, Miranda JD (2016) Tamoxifen∶ an FDA approved drug with neuroprotective effects for spinal cord injury recovery. Neural Regen Res 11(8)∶1208-1211.
Funding: The project was partially supported by COBRE (P20-GM103642), the MBRS-RISE Program (R25 GM061838), NIH-MARC (5T34GM007821-35) and the RCMI program (5G12MD007600).
Jorge D. Miranda, Ph.D.,
jorge.miranda3@upr.edu.
orcid:
0000-0001-7705-1111
(Jorge D. Miranda)
Accepted: 2016-07-21
Spinal cord injury (SCI) is a condition without a cure, affecting sensory and/or motor functions. The physical trauma to the spinal cord initiates a cascade of molecular and cellular events that generates a non-permissive environment for cell survival and axonal regeneration. Among these complex set of events are damage of the blood-brain barrier, edema formation, inflammation, oxidative stress, demyelination, reactive gliosis and apoptosis. The multiple events activated after SCI require a multi-active drug that could target most of these events and produce a permissive environment for cell survival, regeneration, vascular reorganization and synaptic formation. Tamoxifen, a selective estrogen receptor modulator, is an FDA approved drug with several neuroprotective properties that should be considered for the treatment of this devastating condition. Various investigators using different animal models and injury parameters have demonstrated the beneficial effects of this drug to improve functional locomotor recovery after SCI. Results suggest that the mechanism of action of Tamoxifen administration is to modulate anti-oxidant, anti-inflammatory and anti-gliotic responses. A gap of knowledge exists regarding the sex differences in response to Tamoxifen and the therapeutic window available to administer this treatment. In addition, the effects of Tamoxifen in axonal outgrowth or synapse formation needs to be investigated. This review will address some of the mechanisms activated by Tamoxifen after SCI and the results recently published by investigators in the field.
selective estrogen receptor modulator; trauma; antioxidant; anti-inflammatory; regeneration; reactive gliosis; demyelination, estradiol
Spinal Cord Injury: a Multi-factorial Event
For years, research efforts have focused on the development of therapeutic strategies to revert the damage caused by trauma to the spinal cord. Every year, over 12,000 new cases of spinal cord injury (SCI) are reported, 80% of which are male. The complexity of SCI resides on the initial cellular and molecular chain of events that are critical in the pathophysiology observed weeks to years after the trauma. The initial events have been clearly characterized by necrosis in central nervous system (CNS) cells, axotomy, blood-brain barrier (BBB) disruption, vascular damage, edema, ischemia, and infiltration of cells from the immune system (macrophages, neutrophils, and lymphocytes). A second phase is characterized by apoptosis, demyelination, inflammation, and a gliotic response that leads to the formation of the glial scar. Together with the acute set of events, the CNS cellular and molecular response will lead to the generation of a non-permissive environment for neuronal survival, axonal regeneration, and recovery of sensory-motor functions after the injury (Figure 1). The initial trauma should be considered a changing entity that influences signaling molecules in the cells present at the lesion epicenter, cells in the surrounding areas (rostral and caudal to the injury), and cells from the immune system that penetrate the area. Therapeutic interventions to treat SCI should consider the dynamic influence on endothelial, neuronal, astroglial, microglial, and oligodendroglial cells locally and proximally to the lesion epicenter but also on inflammatory cells.
Since SCI is a multi-factorial event, there is a need for a multi-active drug to treat this condition; one that will target several of the events activated by SCI (Figure 2). Estradiol is a potent multi-active neuroprotective modulator in traumatic conditions and the hormone with the most estrogenic activity among the estrogens (estradiol, estriol, and estrone). Published data demonstrated that estradiol reduces lesion volume, white matter loss, exerts anti-oxidant effects, and improves behavioral outcomes in animals with SCI (Mosquera et al., 2014; Letaif et al., 2015). However, long-term treatment with estradiol is associated with abnormal cell proliferation in the breast, ovary, and uterus. Therefore, a multi-active compound similar to estradiol, which retains the neuroprotective effects but lack the adverse side-effects would be useful for the treatment of SCI.
Tamoxifen, a Multi-active Drug to Target Spinal Cord Injury
Tamoxifen (TAM) is a selective estrogen receptor modulator (SERM) with a biochemical structure similar to estradiol.This compound has been proposed to exert its beneficial activity on target tissues by an estrogen receptor (ER) dependent and independent mechanisms. Tamoxifen is metabolized by the hepatic enzyme CYP2D6 into its active metabolites 4-hydroxytamoxifen, N-desmethyl tamoxifen, and endoxifen (Maximov et al., 2013). The current hypothesis is that this SERM and its metabolites will interact with ERs and exert agonist or antagonist activity depending on the expression of co-activators and co-repressors in specific target cell (Maximov et al., 2013). By this mechanism, TAM may exert its beneficial effects in all cells of the CNS that express ERs (alpha or beta). However, the involvement of the estrogenic plasma membrane receptor (GPER-1) in TAM mediated neuroprotection is still a subject to be studied.
Our recent report on the use of TAM after SCI in female rats, suggests that this Federal Drug Administration (FDA) approved drug exerts neuroprotective effects by favoring neuronal survival, myelin spared tissue, axonal preservation and functional locomotor recovery (Colón et al., 2016). In addition, the results obtained by other investigators have shown that TAM may exert anti-oxidant, anti-apoptotic, anti-gliotic, and anti-inflammatory effects while reducing brain and spinal cord barrier permeability and increasing white matter spared tissue in various models of SCI (Zhang et al., 2007; Tian et al., 2009; Ismailoğlu et al., 2010; Guptarak et al., 2014; Mosquera et al., 2014; Wei and Ma, 2014; Colón et al., 2016; De la Torre Valdovinos et al., 2016). The promising results of these experiments for CNS conditions, suggest a beneficial use for TAM, which is currently used as an adjuvant for breast cancer treatment. Moreover, the multiple actions of TAM support this drug as an ideal pharmacological agent to treat SCI (Figure 2).
Sex Differences and Therapy Delay Influence Recovery after Spinal Cord Injury
Most studies in the SCI field focus on the use of male animals since most of the cases reported are male subjects. Studies by others have shown that sex differences exist regarding locomotor recovery in rats after SCI (Hauben et al., 2002). This suggests that a therapeutic intervention for SCI should consider the effects of therapy associated to sex differences, together with the set of events triggered by the trauma, and how these extend to the rostral and caudal penumbra. An important factor to consider is the amount of time it will take the patient to reach clinical aid after suffering from SCI. Since sex differences influence SCI outcome, there is a need to characterize the therapeutic window available to administer treatment and the sex differences associated in response to this or any therapy. For the past years, methylprednisolone was the standard care for patients that suffered SCI and it was administered up to 8 hours after the traumatic event. However, the use of this treatment in humans is considered highly controversial (Bydon et al., 2013). Our recent report on the effects of TAM after SCI in female rats revealed that TAM exerts beneficial effects when administered up to 24 hours after the injury without apparent toxic effects (Colón et al., 2016). We demonstrated, with three different behavioral assays, that TAM administration up to 24 hours after SCI produced significant behavioral recovery. The locomotor improvement was correlated with an increase in the amount of white matter spared tissue and in NeuN positive cells. If translated to humans, this scenario would allow for reasonable time for patient stabilization and proper health care. The scenario where a prolonged therapeutic window exists in adult male vertebrates after SCI still remains to be studied. Nonetheless, the scenario where a drug exerts neuroprotective effects in both sexes would be ideal, but it also conveys important considerations. First, the initial impact causes spatial and temporal chain of events that develop from the acute (days) to the chronic (weeks to months) stages and require coordinated signaling mechanisms (Figure 1). The robust effect observed with TAM suggest that this drug is acting upon several cellular mechanisms. Moreover, the multi-active effects are observed in multiple cell types and various anatomical areas. The question still remains as to, how this drug is exerting numerous effects on multiple cell types and anatomical areas and if these effects are sex dependent.
Physiological Effects of Chronic Spinal Cord Injury and Therapy Intervention
Major focus on the SCI field resides on stimulating specific cellular pathways in order to favor functional locomotor recovery. Yet, another aspect that has been under special consideration for the past few years is the development of chronic neuropathy after SCI and how it could be diminished. Characterizing the effects of specific therapeutic agents is of great importance for new patients arriving to the clinic, but it creates an important consideration: the need to characterize the effects of therapy during long term administration in male and female vertebrates. This will require the design of systematic studies where age, hormonal status, sex, therapeutic dose, length of administration, and therapeutic window are considered. The endpoints should consider the effects in functional locomotor recovery and in the recovery of sensation. Since most patients that suffer SCI develop chronic neuropathy during chronic stages, the effects of therapy on the development of neuropathy should also be evaluated. A special consideration for the use of a therapeutic intervention with TAM in male and female rats should be the effects of this drug on the regenerative machinery after an insult to the spinal cord. A follow up of current hypothesis on TAM effects in ERs, deserves some emphasis, particularly, on the effects of these receptors on axonal regeneration after an insult to the CNS and the modulation of TAM in the assembly of regenerative machinery. Moreover, since TAM may exert genomic modulation by an ER-dependent mechanism, studies should focus on the changes in expression of regeneration associated genes. The study of this mechanism and its time-dependent activation is important due to the effects these may have in the development of neuropathic pain due to non-specificsynapse formation.

Figure 1 Acute and chronic events initiated after spinal cord injury: anatomical and temporal events.
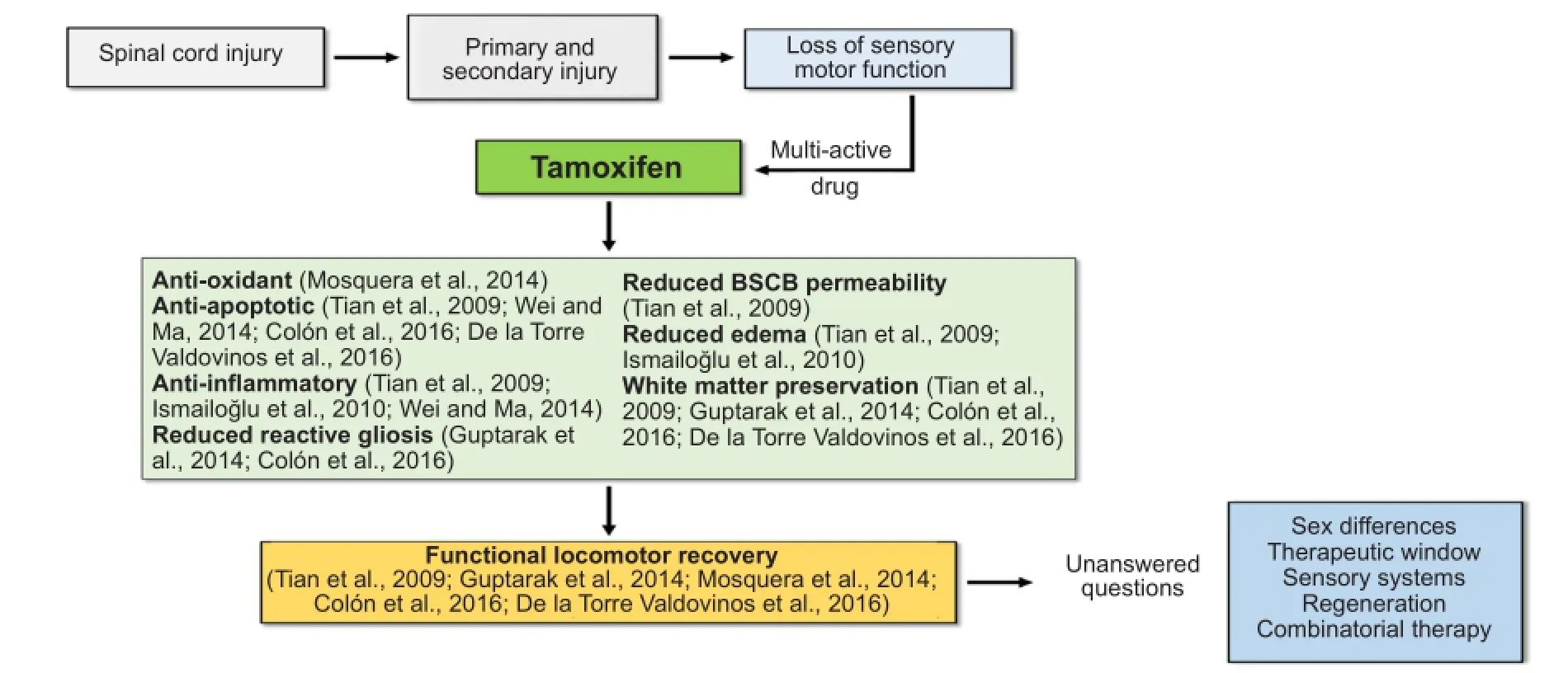
Figure 2 Multi-active properties of Tamoxifen administration after spinal cord injury.
The complexity of SCI suggests the need for a drug that targets the multiple set of events once they are initiated. In our study, we evaluated the effects of TAM treatment (t = 0 and 24 hours) in locomotor improvement using the BBB Open Field Test. In this test, we observed that both immediate and delayed treatment resulted in a locomotor improvement that started beyond 2 days post injury (DPI) and reached a plateau at 28 DPI. Reaching a plateau at 28 DPI may have some implications on the TAM-mediated recovery. In our study we used a continuous drug delivery pellet 15 mg per day for 21 days. Therefore, we have limited/reduced drug released beyond 21 DPI. Therefore, this raises the question if we could maximize the behavioral locomotor improvement either by increasing the length of release of TAM for 35 days (instead of 21) or by increasing the daily dose delivered from day 0 after the injury. Based on the current hypothesis, this means that TAM may act upon the ERs in order to provide multiple behavioral, anatomical, and cellular effects. This suggests the possibility of enhancing the stimulation to the ERs machinery by testing the effects of a combinatorial treatment to maximize effects. Ideally, a combinatorial treatment should act synergistically and potentiate cellular beneficial effects in order to favor locomotor recovery after the insult. The continuous use of a drug for therapy must be managed carefully due to the long-term physiological effects. Breast cancer patients who use TAM as an adjuvant are subjected to a maximum of 20 mg of this drug per day, which suggest the dose used in this study remains in the range currently used for patients. Reports have shown that continuous administration of TAM at this dose, may increase risk for endometrial cancer and thromboembolic events during chronic administration (used for several years) in post-menopausal women (Maximov et al., 2013). At this dose, we do not expect detrimental effects in our animal model since TAM will be administered for a short period of time, from the moment of the injury until the locomotor recovery is achieved (approximately 21 days). At this moment, the use of this therapy for long-term periods is highly unlikely since the rationale for TAM use after SCI is to target some of the primary and early-secondary phases initiated by the physical trauma (Figures 1, 2). We expect short periods of drug treatment (at most, a few months) after SCI in order to obtain the beneficial effects and eventually combine it with another therapy like exercise (Osuna-Carrasco et al., 2016), electrical activity, or stem cells transplantation, among others.
Conclusions
In summary, the multiple signaling events activated by injury to the spinal cord suggest the need for a multi-active drug that targets these events in order to favor locomotor recovery. The use of TAM has been characterized at some extent with favorable results in traumatic brain injury and SCI animal models. This FDA approved drug shows promising beneficial effects in traumatic conditions to the CNS with limited side effects when administered for a short period of time (several months). At this moment, we need to investigate the mechanisms of TAM-mediated neuroprotection that could maximize its clinical use for CNS trauma and pathologies.
Author contributions: JMC prepared the figures with the data obtained in her thesis project and the review of the literature. She also prepared the first draft of the manuscript and JDM reviewed the document and was responsible for the final version of the article.
Conflicts of interest: None declared.
References
Bydon M, Lin J, Macki M, Gokaslan ZL, Bydon A (2013) The current role of steroids in acute spinal cord injury. World Neurosurg 82:1-7.
Colón JM, Torrado AI, Cajigas A, Santiago JM, Salgado IK, Arroyo Y, Miranda JD (2016) Tamoxifen administration immediately or 24 hours after spinal cord injury improves locomotor recovery and reduces secondary damage in female rats. J Neurotrauma doi:10.1089/ neu.2015.4111.
De la Torre Valdovinos B, Dueñas Jiménez JM, Jimenez Estada I, Banuelos Pineda J, Franco Rodríguez NE, Lopez Ruiz J, Osuna Carrasco L, Candanedo Arellano A, Dueñas Jiménez SH (2016) Tamoxifen promotes axonal preservation and gait locomotion recovery after spinal cord injury in cats. J Vet Med:1-16.
Guptarak J, Wiktorowicz JE, Sadygov RG, Zivadinovic D, Paulucci-Holthauzen AA, Vergara L, Nesic O (2014) The cancer drug tamoxifen: a potential therapeutic treatment for spinal cord injury. J Neurotrauma 31:268-283.
Hauben E, Mizrahi T, Agranov E, Schwartz M (2002) Sexual dimorphism in the spontaneous recovery from spinal cord injury: a gender gap in beneficial autoimmunity? Eur J Neurosci 16:1731-1740.
Ismailoğlu O, Oral B, Görgülü A, Sütçü R, Demir N (2010) Neuroprotective effects of tamoxifen on experimental spinal cord injury in rats. J Clin Neurosci 17:1306-1310.
Letaif O, Cristante A, Barros Filho T, Ferreira R, Santos G, Rocha I, Marcon R (2015) Effects of estrogen on functional and neurological recovery after spinal cord injury: An experimental study with rats. Clinics 70:700-705.
Maximov PY, Lee TM, Jordan VC (2013) The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol 8:135-155.
Mosquera L, Colón JM, Santiago JM, Torrado AI, Meléndez M, Segarra AC, Rodríguez-Orengo JF, Miranda JD (2014) Tamoxifen and estradiol improved locomotor function and increased spared tissue in rats after spinal cord injury: their antioxidant effect and role of estrogen receptor alpha. Brain Res 1561:11-22.
Osuna-Carrasco LP, López-Ruiz JR, Mendizabal-Ruiz EG, De la Torre-Valdovinos B, Bañuelos-Pineda J, Jiménez-Estrada I, Dueñas-Jiménez SH (2016) Quantitative analysis of hindlimbs locomotion kinematics in spinalized rats treated with Tamoxifen plus treadmill exercise. Neuroscience 333:151-161.
Tian D shi, Liu JL, Xie MJ, Zhan Y, Qu WS, Yu ZY, Tang ZP, Pan DJ, Wang W (2009) Tamoxifen attenuates inflammatory-mediated damage and improves functional outcome after spinal cord injury in rats. J Neurochem 109:1658-1667.
Wei HY, Ma X (2014) Tamoxifen reduces infiltration of inflammatory cells, apoptosis and inhibits IKK/NF-kB pathway after spinal cord injury in rats. Neurol Sci 35:1763-1768.
Zhang Y, Milatovic D, Aschner M, Feustel PJ, Kimelberg HK (2007) Neuroprotection by tamoxifen in focal cerebral ischemia is not mediated by an agonist action at estrogen receptors but is associated with antioxidant activity. Exp Neurol 204:819-827.
10.4103/1673-5374.189164
*Correspondence to:
- 中国神经再生研究(英文版)的其它文章
- Secondary parkinsonism induced by hydrocephalus after subarachnoid and intraventricular hemorrhage
- Volume transmission and receptor-receptor interactions in heteroreceptor complexes: understanding the role of new concepts for brain communication
- Uncoupling protein 2 in the glial response to stress: implications for neuroprotection
- Selective neuronal PTEN deletion: can we take the brakes off of growth without losing control?
- TRPV1 may increase the effectiveness of estrogen therapy on neuroprotection and neuroregeneration
- Automatic counting of microglial cell activation and its applications