Secondary parkinsonism induced by hydrocephalus after subarachnoid and intraventricular hemorrhage
IMAGING IN NEURAL REGENERATION
Secondary parkinsonism induced by hydrocephalus after subarachnoid and intraventricular hemorrhage
Hydrocephalus can induce secondary parkinsonism (Shahar et al., 1988; Aggarwal et al., 1997; Racette et al., 2004). Physical factors such as increased ventricular pressure near the upper midbrain and basal ganglia are thought to cause mechanical disruption of dopaminergic systems (i.e., the nigrostriatal pathways from the medial substantia nigra to the caudate nuclei and putamen and its frontal projection fibers from the basal ganglia) (Asamoto et al., 1998; Yomo et al., 2006). Symptoms of parkinsonism are bradykinesia with rigidity, resting tremor, or postural instability, which can deteriorate a patient’s physical function and functional recovery (Racette et al., 2004). Parkinsonian symptoms can be controlled by dopaminergic medication, meaning that the accurate diagnosis of secondary parkinsonism is important for proper management of stroke patients (Shahar et al., 1988; Aggarwal et al., 1997; Racette et al., 2004). Previous studies have investigated dopaminergic system dysfunction induced by hydrocephalus using single photon emission computed tomography (SPECT) and position emission tomography (PET) scanning (Shahar et al., 1988; Aggarwal et al., 1997; Racette et al., 2004; Nakayama et al., 2007). However, these methods are limited in their ability to clearly present the dopaminergic system.
Recently,18F-florinated-N-3-fluoropropyl-2-β-carboxymethoxy-3-β-(4-lodophenyl) nortropane (18F-FP-CIT) PET scanning was introduced and can be used to evaluate dopamine transporter (DAT) binding. It provides more sophisticated attenuation correction and better spatial resolution, compared with SPECT or other PET scanning techniques (Lee et al., 2007). Using18F-FP-CIT PET, regional changes in the striatal DAT can be visualized, thus allowing accurate evaluation of the dopaminergic system. Previous studies have demonstrated the usefulness of18F-FP-CIT PET scanning for the diagnosis of several diseases affecting domaninergic neurons of the brain, including idiopathic Parkinson disease and atypical parkinsonian disorders (Lee et al., 2007; Lim et al., 2013). However, little is known about the usefulness of18F-FP-CIT PET for the diagnosis of parkinsonism induced by hydrocephalus.
In this study, we report a case on the patient who showed parkinsonism and dopaminergic system deterioration induced by hydrocephalus after subarachnoid hemorrhage (SAH) and intraventricular hemorrhage (IVH) as detected on18F-FP-CIT PET.
A 64-year-old right-handed man who does not have any history of neurological or psychiatric illness underwent extraventricular drainage for spontaneous SAH and IVH resulting from right posterior inferior cerebellar artery rupture (Figure 1A). At the initial stage of SAH and IVH, he was drowsy and had quadriparesis (Medical Research Council [MRC] (Jang et al., 2013): 3/5). Two months after onset, on neurologic examination, the patient showed severe rigidity in both the upper and lower extremities, which prevented him from performing any daily activities although he could move all the extremities against gravity (MRC: 3+). Rigidity symptoms in this patient manifested from 1 month after the onset of hemorrhage and gradually became severe. Also, he could walk slowly with small steps using continuous support from another person (Functional Ambulatory Category [FAC] (Cunha et al., 2002)): 1). Additionally, he showed a resting tremor of 3 Hz in both upper extremities. A computed tomography (CT) scan of the brain performed 2 months after onset revealed dilatation of the ventricle (Figure 1B). Written informed consent was obtained from the patient.
For the purpose of evaluating the integrity of the nigrostriatal dopaminergic system, we performed18F-FP-CIT PET scans, at 6 months after SAH and IVH onset, using a Biograph Truepoint 40 (Siemens/CTI, Knoxville, Tennessee, USA) scanner. Images were acquired 3 hours after intravenous administration of 173.9 MBq18F-FP-CIT PET at rest. Emission PET data were collected for 10 minutes in the 3-dimentional mode after brain CT. We performed data acquisition in spiral mode at 120 kVp and 380 mA using Siemens CARE Dose 4D. The reconstruction of18F-FP-CIT PET images was performed using CT data for attenuation correction with the all-pass filtered-True X algorithm using a 336 × 336 pixel matrix.
On18F-FP-CIT PET images, dopamine transporter (DAT) binding in the head portions of the left caudate nuclei and the left posterior putamen were decreased (Figure 2). A neurologist diagnosed our patient as secondary parkinsonism caused by hydrocephalus following SAH and IVH. Carbidopa/levodopa (75/300 mg/d) was administered. After administration of carbidopa/levodopa, the patient showed improvement in rigidity in all the extremities. He could perform daily activities with minimal assistance by another person; he could independently use a spoon with his right hand and hold and release objects (e.g., spoon, pencil, and cell phone) using either his right or left hand. He could also ambulate with verbal supervision (FAC: 3). The shortened steps gradually normalized with the administration of carbidopa/ levodopa and resolved after 3 days of administration of carbidopa/ levodopa. Gait velocity was higher than that before administration of carbidopa/levodopa. The resting tremor completely disappeared immediately after administration of carbidopa/levodopa.
Hydrocephalus is known to induce secondary parkinsonism (Shahar et al., 1988; Aggarwal et al., 1997; Racette et al., 2004). Increased ventricular pressure near the diencephalon and upper midbrain is thought to cause mechanical disruption or ischemia on dopaminergic systems (Asamoto et al., 1998; Yomo et al., 2006). However, to date, clinicians are unable to confirm injury or dysfunction of the dopaminergic system after hydrocephalus due to a lack of tools to visualize such changes.18F-FPCIT PET was recently developed and is suitable for evaluating dysfunction of the dopaminergic system, which allows us to accurately diagnose idiopathic Parkinson disease and atypical parkinsonian disorders (Lee et al., 2007; Lim et al., 2013). In our present patient, we demonstrated decreased DAT binding in the head portions of the left caudate nuclei and the left posterior putamen using18F-FP-CIT PET images. These results indicate that the patient’s parkinsonian symptoms were caused by such decreases in DAT binding. Furthermore, in line with previous studies reporting the development of parkinsonism following hydrocephalus, our patient’s parkinsonism is thought to be secondary to hydrocephalus after SAH and IVH (Shahar et al., 1988; Aggarwal et al., 1997; Racette et al., 2004). Additionally, other than hydrocephalus, any lesions associated with the patient’s parkinsonian symptoms were not observed on a CT image. We believe that the patient’s enlarged ventricle increased the ventricular pressure, which damaged the dopaminergic system including the nigrostriatal pathways, basal ganglia, and/or its frontal projection fibers. In addition, the good response to carbidopa/levodopa indicates that dysfunction of the dopaminergic system following hydrocephalus can be at least partially reversible by treatment with dopaminergic agents.
To date, four SPECT or PET studies have reported damage of the dopaminergic system caused by the hydrocephalus (Shahar et al., 1988; Aggarwal et al., 1997; Racette et al., 2004; Nakayama et al., 2007). In 1988, Shahar et al. (1988) reported a patient with acute parkinsonism that occurred by obstructionof a ventriculoperitoneal shunt with subsequent hydrocephalus. A brain SPECT using99mTc-hexamethylpropylenamin oxime showed decrease of cerebral blood flow in the left caudate and putamen. After shunt revision and levodopa/cardidopa (100/25 mg) administration, the patient’s parkinsonian symptoms almost disappeared. In 1997, Aggarwal et al. (1997) presented a patient with parkinsonian symptoms (akinesia) following hydrocephalus induced by non-tumoral aqueductal stenosis. A brain SPECT using99mTc-hexamethylpropylenamin oxime showed decrease of basal ganglia perfusion. After administration of levodopa/carbidopa (100/25 mg), the patient showed faster movements. In 2004, Racette et al. (2004) described a patient with hydrocephalus induced by aqueductal stenosis and underwent a ventriculoperitoneal shunt. Eighteen years after hydrocephalus onset, the patient developed parkinsonian symptoms, including bradykinesia/akinesia, rigidity, and tremor in both hands. [18F] dopa PET scans showed decreased uptake in both the putamen and caudate nucleus. Shunt revision and Levopa/carbidopa at a dose of 1,500 mg/375 mg/d significantly improved the patient’s parkinsonian symptoms. In 2007, Nakayama et al. (2007) recruited 8 patients with idiopathic normal pressure hydrocephalus, and performed11C-radiopride PET scan twice before and 1 month after ventriculoperitoneal shunt. They revealed that the improvement of gait disturbance after ventriculoperitoneal shunt was correlated with enhanced D2 responsiveness in the striatum. However, SPECT can assess blood perfusion and therefore only indirectly measure basal ganglia function. In addition, the resolution of [18F] dopa or11C-radiopride PET is inferior to that of18F-FP-CIT PET images. To the best of our knowledge, this is the first 18F-FP-CIT PET study to demonstrate that hydrocephalus after SAH or IVH can induce dysfunction of the dopaminergic system.

Figure 1 Brain computed tomography (CT) images of a 64-year-old male patient with parkinsonism induced by hydrocephalus after subarachnoid hemorrhage (SAH) and intraventricular hemorrhage (IVH) at onset and 2 months after onset.
In conclusion, we describe a patient with parkinsonism caused by hydrocephalus following SAH and IVH. Using18F-FP-CIT PET images, we demonstrated dysfunction of the head portions of the left caudate nuclei and the left posterior putamen, which is thought to have caused parkinsonism. Secondary parkinsonism following hydrocephalus tends to be reversible by dopaminergic agents. Thus, clinicians need to give attention to the development of parkinsonism in patients with hydrocephalus. However, this study reported a single case, and we were not able to clearly confirm the etiology of the patient’s symptoms related with parkinsonism. Also, PET scan in our study was performed at a single time point, not multiple and serial time points. Therefore, further studies addressing these limitations are necessary.
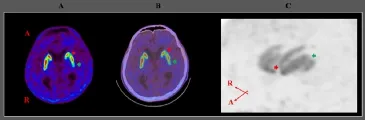
Figure 2 Brain position emission tomography (PET), PET/computed tomography (CT), and maximum intensity projection images of18F-FP-CIT of a 64-year-old male patient with parkinsonism induced by hydrocephalus after subarachnoid hemorrhage (SAH) and intraventricular hemorrhage (IVH) at 6 months after onset.
Min Cheol Chang, Min Ho Chun*
Department of Physical Medicine and Rehabilitation, College of
Medicine, Yeungnam University, Daegu, Republic of Korea (Chang MC) Department of Physical Medicine and Rehabilitation, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea (Chun MH)
*Correspondence to: Min Ho Chun, M.D., mhchun0@gmail.com.
Accepted: 2016-06-08
orcid: 0000-0002-2075-6820 (Min Ho Chun)
How to cite this article: Chang MC, Chun MH (2016) Secondary parkinsonism induced by hydrocephalus after subarachnoid and intraventricular hemorrhage. Neural Regen Res 11(8):1359-1360.
References
Aggarwal S, Childers MK, Jimenez D (1997) Use of carbidopa-levodopa in a patient with hydrocephalus and frozen movement. Brain Inj 11:831-836.
Asamoto S, Sugiyama H, Doi H, Yokochi M, Hirabayashi K, Tanaka S, Sugiura K, Nakama H, Matsumoto K (1998) Levodopa effective parkinsonism associated with aqueductal stenosis: a case report and review of the literature. No Shinkei Geka 26:1089-1092.
Cunha IT, Lim PA, Henson H, Monga T, Qureshy H, Protas EJ (2002) Performance-based gait tests for acute stroke patients. Am J Phys Med Rehabil 81:848-856.
Jang SH, Chang MC (2013) Motor outcomes of patients with a complete middle cerebral artery territory infarct. Neural Regen Res 8:1892-1897.
Lee SJ, Oh SJ, Chi DY, Kang SH, Kil HS, Kim JS, Moon DH (2007) One-step high-radiochemical-yield synthesis of [18F]FP-CIT using a protic solvent system. Nucl Med Biol 34:345-351.
Lim HS, Kim SJ, Noh YH, Lee BC, Jin SJ, Park HS, Kim S, Jang IJ, Kim SE (2013) Exploration of optimal dosing regimens of haloperidol, a D2 Antagonist, via modeling and simulation analysis in a D2 receptor occupancy study. Pharm Res 30:683-693.
Nakayama T, Ouchi Y, Yoshikawa E, Suqihara G, Torizuka T, Tanaka K (2007) Striatal D2 receptor availability after shunting in idiopathic normal pressure hydrocephalus. J Nucl Med 48:1981-1986.
Racette BA, Esper GJ, Antenor J, Black KJ, Burkey A, Moerlein SM, Videen TO, Kotagal V, Ojemann JG, Perlmutter JS (2004) Pathophysiology of parkinsonism due to hydrocephalus. J Neurol Neurosurg Psychiatry 75:1617-1619.
Shahar E, Lambert R, Hwang PA, Hoffman HJ (1998) Obstructive hydrocephalus-induced parkinsonism. I: Decreased basal ganglia regional blood flow. Pediatr Neurol 4:117-119.
Yomo S, Hongo K, Kuroyanagi T, Kobayashi S (2006) Parkinsonism and midbrain dysfunction after shunt placement for obstructive hydrocephalus. J Clin Neurosci 13: 373-378.
10.4103/1673-5374.189203
- 中国神经再生研究(英文版)的其它文章
- Huangqi Guizhi Wuwu Decoction for treating diabetic peripheral neuropathy: a meta-analysis of 16 randomized controlled trials
- Prospects for bone marrow cell therapy in amyotrophic lateral sclerosis: how far are we from a clinical treatment?
- Uncoupling protein 2 in the glial response to stress: implications for neuroprotection
- Selective neuronal PTEN deletion: can we take the brakes off of growth without losing control?
- TRPV1 may increase the effectiveness of estrogen therapy on neuroprotection and neuroregeneration
- Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery