过表达A53T突变型α-synuclein细胞毒性及对损伤性刺激的影响
东惟玲 方琪 王达鹏 周赟 单青婷 惠国桢 单立冬
(苏州大学: 1附属第一医院神经内科; 2附属第一医院神经外科; 3基础医学部神经生物学教研室,江苏 苏州 215123)
·论著·
过表达A53T突变型α-synuclein细胞毒性及对损伤性刺激的影响
东惟玲1方琪1王达鹏1周赟1单青婷1惠国桢2单立冬3*
(苏州大学:1附属第一医院神经内科;2附属第一医院神经外科;3基础医学部神经生物学教研室,江苏 苏州 215123)
目的通过比较过表达A53T(Ala53Thr)突变型α-突触核蛋白(α- synuclein)的人神经母细胞瘤SH-SY5Y细胞与正常细胞的特性及对鱼藤酮引起细胞损伤的影响,探索α-synuclein在帕金森病发病机制中的作用,为寻找治疗药物潜在靶点提供依据。方法通过比较过表达A53T突变型α-synuclein的SH-SY5Y细胞与正常细胞的细胞形态及生长曲线观察突变型α-synuclein对细胞毒性的影响;用呼吸链抑制剂鱼藤酮刺激细胞,用3-(4,5-二甲基噻唑-2)-2,5-二苯基四氮唑溴盐(MTT)法观察细胞的存活率,免疫印迹法观察自噬相关蛋白的变化。结果正常细胞形态上接近于梭形,过表达A53T α-synuclein的细胞胞体变的肿胀,小的突起增多,甚至出现多种变异的形态;过表达A53Tα-synuclein 的细胞生长较正常细胞缓慢,培养相同时间达到的细胞总数相对较少;4 μM 鱼藤酮处理两种细胞,过表达A53Tα-synuclein 细胞损伤较正常细胞大,正常细胞随鱼藤酮处理时间的延长自噬相关蛋微管相关蛋白1轻链3 Ⅱ ( LC3-Ⅱ)的水平出现先下降后上升的趋势(Plt;0.05),A53T细胞出现先上升后下降的趋势。结论过表达A53T突变型α-synuclein具有细胞毒性,且与正常细胞相比更容易受到损伤,可能与自噬敏感性增高,加速自噬耗竭相关。因此增加突变型α-synuclein的清除或增加自噬合成的相关蛋白诱导自噬可能会成为PD潜在的治疗靶点。
突变型α-synuclein; 细胞毒性; 细胞损伤
帕金森病(Parkinson disease, PD)是常见的神经退行性疾病,严重影响老年人的生活质量,但是到目前为止PD的发病机制并不清楚,相应的治疗措施只是改善症状及延缓病情进展,不能从根本上阻止和逆转疾病。α-突触核蛋白(α-synuclein)是第一个被发现的PD相关基因,其突变体A53T(Ala53Thr)、A30P(Ala30Pro)是最常见的被证实与家族性PD的发病密切相关基因型;路易小体(lewy bodies, LB)是PD患者残存神经元中特征性的包涵体,其主要成分为α-synuclein[1],所以α-synuclein与PD密切相关,但是这种蛋白从哪些方面影响到LB的形成,进而影响到PD的发生还需进一步研究。许多研究表明A53T突变型α-synuclein较野生型(wild type, WT)及A30P突变型更容易聚集形成蛋白聚集体[2],所以本文选用A53T突变型α-synuclein作为实验对象与正常细胞进行比较,力求以α-synuclein为突破点寻找PD潜在的治疗靶点。
材料与方法
一、材料
人神经母细胞瘤SH-SY5Y细胞、过表达A53T α-synuclein的SH-SY5Y细胞为本实验室所有,胎牛血清及DMEM培养基购自Gibco公司,MTT,LC3抗体及鱼藤酮购自美国Sigma公司。
二、方法
1.细胞培养及生长曲线的绘制:过表达A53T突变型α-synuclein的SH-SY5Y细胞与正常细胞培养在含10%胎牛血清的DMEM中,培养箱设置为37℃,5% CO2,95%湿度。在细胞生长至80%~90%融合时用0.25%胰酶进行消化1 min,离心后装入新培养基,以初始细胞数为500个/孔种到24孔板中,补足培养基至200 μl。每天观察细胞形态,并收集4孔的细胞计数取平均数,共收集6 d,绘制两种细胞的生长曲线,并在显微镜下拍摄细胞形态。
2.MTT法测定两种细胞的存活率:将两种细胞种到96孔板中至细胞生长至总面积的80%左右时,用鱼藤酮(4 μM)处理两种细胞,0 h、2 h、4 h、8 h后分别利用3-(4,5-二甲基噻唑-2)-2,5-二苯基四氮唑溴盐溶液(3-4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H -tetrazolium bromide, MTT)的方法测定细胞活性,即在每个孔中加入10 μl MTT(5 mg/ml),37℃培养箱中继续培养3 h后加入100 μl 二甲基亚砜(dimethyl sulfoxide, DMSO)使紫色结晶溶解,然后利用分光光度计测定每孔的光密度值(optical density, OD)值,波长设定为570 nm,细胞存活率为加药孔的细胞OD值/正常细胞的OD值。
3.免疫印迹法测定鱼藤酮处理后两种细胞自噬水平的变化:鱼藤酮(4 μM)处理两种细胞0 h、2 h、4 h、8 h后用0.25%胰酶消化,磷酸盐缓冲液(phosphate-buffered solution, PBS)洗两遍后加入细胞裂解液超声裂解,冰上放置1 h后4℃ 12 000×g离心10 min,取上清适量用于蛋白浓度测定,其余蛋白-80℃保存。根据测定的蛋白浓度,用裂解液将蛋白稀释至相同浓度,加入上样缓冲液后98℃ 5 min使蛋白变性,12%的分离胶进行电泳,转到聚偏二氟(polyvinylidene -fluoride, PVDF)膜上,5%脱脂奶粉封闭2 h,4 ℃孵育LC3b抗体(Sigma,1 ∶1 000)过夜。洗膜后用辣根过氧化物酶标记的二抗孵育2 h,凝胶成像仪显色。利用Image Lab 3.0软件计算各条带的灰度值,GraphPad PRISM5软件进行统计。
4.统计学处理:所有实验均重复三次以上,数据均以Mean±SD表示,数据采用SPSS 13.0软件进行统计分析,组间比较采用One-Way ANOVA 方法分析,Plt;0.05 认为有统计学意义。
结 果
一、正常SH-SY5Y细胞与过表达A53T α-synuclein 细胞形态及生长曲线的差异
如图1所示,正常细胞形态上接近于梭形,过表达α-synuclein的A53T细胞胞体变的肿胀,小的突起增多,甚至出现多种变异的形态(E、F、G、H图);从图2中可以看出过表达A53T α-synuclein 的细胞生长较正常细胞缓慢,培养相同时间达到的细胞总数相对较少。
二、鱼藤酮对正常SH-SY5Y细胞与过表达A53Tα-synuclein 细胞毒性的差异
鱼藤酮(4 μM)处理两种细胞0 h、2 h、4 h、8 h后分别利用MTT的方法测定细胞活性,如图3,可以看到随着鱼藤酮处理的时间延长,两种细胞活性均降低,但是过表达A53Tα-synuclein 细胞损伤较正常细胞大,组间比较差异显著(Plt;0.05)。
三、鱼藤酮对两种细胞自噬水平的影响
鱼藤酮(4 μM)处理两种细胞0 h、2 h、4 h、8 h后提取细胞总蛋白,利用免疫印迹的方法获得两种细胞蛋白表达水平,图A、B为正常细胞随鱼藤酮处理时间的延长LC3水平出现先下降后上升 的趋势,图C、D为A53T细胞的变化, LC3水平出现先上升后下降的趋势。
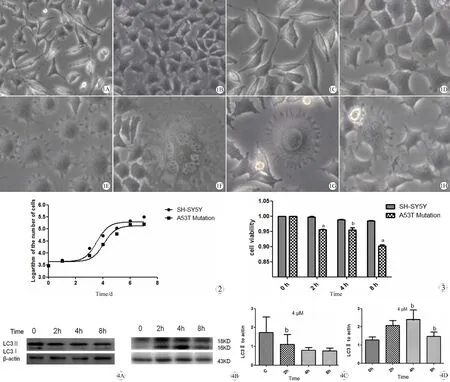
图1 正常SH-SY5Y细胞与过表达A53Tα-synuclein 细胞形态学差异(A, B×250, C~H, ×400)
Fig 1 Morphological differences of SH-SY5Y cells and A53T mutation
A: The morphology of SH-SY5Y cells in low magnification (×250); B; The morphology of A53T mutation in low magnification (×250); C: The morphology of SH-SY5Y cells in high magnification (×400); D: The morphology of A53T mutation in high magnification (×400); E: The small projection increased (×400); F: The flower shape (×400); G: The sun shape (×400); H: The size difference among cells (×400).
图2 正常SH-SY5Y细胞与过表达A53T α-synuclein 细胞生长曲线的差异
Fig 2 Differences of cell growth curve of SH-SY5Y cells and A53T mutation
图3 鱼藤酮对正常SH-SY5Y细胞与过表达A53T α-synuclein 细胞毒性的差异
Fig 3 Rotenone-induced injury in SH-SY5Y cells and A53T mutation cells measured by MTT analysis
aPlt;0.01,vsnormal SH-SY5Y cells;bPlt;0.05,vsnormal SH-SY5Y cells.
图4 鱼藤酮对两种细胞自噬水平的影响
Fig 4 Effects of rontenone on autophagy in SH-SY5Y cells and A53T mutation
A: The blot image of LC3-Ⅱ of normal SH-SY5Y cells; B: The blot image of LC3-Ⅱ of A53T cells ; C: The column chart of LC3-Ⅱ of normal SH-SY5Y cells; D: The column chart of LC3-Ⅱ of A53T cells.
bPlt;0.05,vscells without rotenone (0 h).
讨 论
PD作为老年人中第二大常见的神经退行性疾病,近年来受到广泛关注。尽管对PD的发病过程有很多研究,但是大部分的发病机制仍不清楚,以往研究表明,PD 是遗传和环境因素相互作用的结果,是多种因素导致的疾病。本文我们将环境因素与遗传因素联合起来,以正常细胞作为对照,研究过表达A53T突变型α-synuclein(遗传因素)的SH-SY5Y细胞在呼吸链抑制剂鱼藤酮(环境因素)作用下细胞活性及自噬的变化。结果表明过表达A53T突变型α-synuclein本身对细胞有毒性作用,引起细胞形态上肿胀及突起增多,生长缓慢;在受到鱼藤酮刺激时更容易受到损伤。
α-synuclein基因是最早发现的与家族性PD相关的基因,大量研究表明α-synuclein过表达引起蛋白聚集,进而导致蛋白包涵体的形成,引起细胞毒性[3],α-synuclein基因的突变,例如碱基的替换(A53T、A30P等)、碱基重复等更加速了蛋白聚集[4],增加细胞毒性[5]。有关α-synuclein过表达引起细胞毒性的机制研究有很多,主要包括促进氧化应激[6]、蛋白聚集[7]和线粒体功能紊乱[8],也有研究表明α-synuclein在神经递质的释放方面有重要作用[9]。以往有研究表明突变型A53T α-synuclein可以破坏自噬引起细胞损伤[10],与之相似,我们的研究表明A53T突变型α-synuclein会引起细胞形态上肿胀及突起增多,生长缓慢,可能与α-synuclein过表达诱导自噬,自噬的过载可能会耗竭胞浆内基本物质导致细胞大分子循环失调,导致细胞毒性;在受到鱼藤酮刺激时更容易受到损伤,可能与加快自噬相关分子的耗竭相关。
流行病学显示有机农药鱼藤酮和农村偶发PD发生率高有密切关系,鱼藤酮因此受到广泛关注,被广泛用来制作PD模型。鱼藤酮被广泛使用的原因主要是它可以更准确的复制PD患者的病理特征,以蛋白聚集体LB形成为主[11]。作为氧化呼吸链抑制剂,鱼藤酮与线粒体氧化呼吸链复合体I结合,抑制其氧化磷酸化,使ATP减少,生成的大量活性氧进而导致细胞死亡[12]。我们的研究表明鱼藤酮可以引起两种细胞活性降低,但是过表达A53Tα-synuclein 细胞损伤较正常细胞大。为了探究其中的原因,我们提取了细胞蛋白利用Western blot 观察细胞自噬蛋白的变化,研究表明正常细胞随鱼藤酮处理时间的延长LC3水平出现先下降后上升的趋势,A53T细胞恰好相反,LC3水平出现先上升后下降的趋势。 向正常细胞中加入鱼藤酮后,细胞自噬可能会应激性下降,自噬不能及时作出反应,自噬在初期表现为抑制,随着时间延长,鱼藤酮引起的ROS增多进而诱导自噬,过表达A53Tα-synuclein 细胞对鱼藤酮更加敏感,且α-突触蛋白本身可以诱导自噬,加入鱼藤酮后细胞自噬的反应时间缩短,未出现下降就直接上升,当自噬耗竭后出现下降,细胞死亡。所以当细胞自噬耗竭时细胞活性急骤下降。
由此我们可以看出,过表达A53Tα-synuclein可以引起细胞活性降低,原因可能与增加自噬的敏感性,加速自噬相关分子耗竭相关。增加α-synuclein的清除或增加自噬合成的相关分子诱导自噬可能会成为未来PD治疗的新靶点。
致谢:感谢苏州大学唐仲英血液病学研究中心张秀艳老师对本实验的帮助与支持,感谢苏州科技计划局对本项目的资金支持。
1Intervertebral disc degeneration. Proceedings of a workshop organized by the American Academy of Orthopaedic Surgeons, the National Institutes of Health, and the Orthopaedic Research Society. September 15-18, 2005 [J]. J Bone Joint Surg Am, 2006, 88 Suppl 2: 1-114.
2Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease [J]. Nat Med, 1998, 4(11): 1318-1320.
3Burre J, Sharma M, Tsetsenis T, et al. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro [J]. Science, 2010, 329(5999): 1663-1667.
4Coskuner O, Wise-Scira O. Structures and free energy landscapes of the A53T mutant-type alpha-synuclein protein and impact of A53T mutation on the structures of the wild-type alpha-synuclein protein with dynamics [J]. ACS Chem Neurosci, 2013, 4(7): 1101-1113.
5Schwach G, Tschemmernegg M, Pfragner R, et al. Establishment of stably transfected rat neuronal cell lines expressing alpha-synuclein GFP fusion proteins [J]. J Mol Neurosci, 2010, 41(1): 80-88.
6Siddiqui A, Chinta SJ, Mallajosyula JK, et al. Selective binding of nuclear alpha-synuclein to the PGC1alpha promoter under conditions of oxidative stress may contribute to losses in mitochondrial function: implications for Parkinson's disease [J]. Free Radic Biol Med, 2012, 53(4): 993-1003.
7Lastres-Becker I, Ulusoy A, Innamorato NG, et al. alpha-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson's disease [J]. Hum Mol Genet, 2012, 21(14): 3173-3192.
8Devi L, Anandatheerthavarada HK. Mitochondrial trafficking of APP and alpha synuclein: Relevance to mitochondrial dysfunction in Alzheimer's and Parkinson's diseases [J]. Biochim Biophys Acta, 2010, 1802(1): 11-19.
9Yin S, Na Q, Chen J, et al. Contribution of MRI to detect further anomalies in fetal ventriculomegaly [J]. Fetal Diagn Ther, 2010, 27(1): 20-24.
10Jiang TF, Zhang YJ, Zhou HY, et al. Curcumin ameliorates the neurodegenerative pathology in A53T alpha-synuclein cell model of Parkinson's disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy [J]. J Neuroimmune Pharmacol, 2013, 8(1): 356-369.
11Cannon JR, Tapias V, Na HM, et al. A highly reproducible rotenone model of Parkinson's disease [J]. Neurobiol Dis, 2009, 34(2): 279-290.
12Sindhu KM, Banerjee R, Senthilkumar KS, et al. Rats with unilateral median forebrain bundle, but not striatal or nigral, lesions by the neurotoxins MPP+ or rotenone display differential sensitivity to amphetamine and apomorphine [J]. Pharmacol Biochem Behav, 2006, 84(2): 321-329.
CytotoxicityofA53Tmutantα-synucleinover-expressionandtheeffectsoninjurystimulus
DONGWeiling1,FANGQi1,WANGDapeng1,ZHOUYun1,SHANQingting1,HUIGuozhen2,SHANLidong3
1DepartmentofNeurology,TheFirstAffiliatedHospitalofSoochowUniversity;2DepartmentofNeurosurgery;3DepartmentofNeurobiology,SchoolofBasicMedicine;SoochowUniversity,Suzhou215123, China
ObjectiveThe characteristics of A53T (Ala53Thr) mutant α-synuclein over-expression SH-SY5Y neuroblastoma cells and the normal cells were compared and its impact on rotenone caused cell damage was discussed, which might reveal the possible pathogenesis of Parkinson disease (PD) and provide the novel targets for drug research of PD therapy.MethodsCytotoxicity of A53T mutant α-synuclein over-expression was observed through the cell morphology and growth curve, and then cell viability was detected by 3-(4,5-dimethyl-2-thiazolyl)- 2,5- diphenyl-2-H-tetrazolium bromide (MTT) method, and the autophagy-related protein was observed by immunoblotting with respiratory chain inhibitor rotenone stimulation.ResultsIn morphology, normal cell was close to spindle, while A53T cell body became swollen with increased small protrusions, or even a variety of morphological variation. In cell growth, normal cells grew faster and had relatively less time to reach the same total number of cells; MTT results revealed that A53T mutant α-synuclein cells were more vulnerable to rotenone stimulation. In normal cells, autophagy-related protein microtubule-associated protein 1 light chain 3(LC3-Ⅱ) protein level was decreased firstly, and then increased, while A53T mutant cells demonstrated a different change with LC3-Ⅱ protein, which increased firstly, and then decreased(Plt;0.05).ConclusionA53T mutant α-synuclein have cytotoxicity and make cells more susceptible to injury induced by rotenone, which may related to increased autophagy sensitivity and accelerated the depletion of autophagy. Therefore, increasing mutant α-synuclein clearance or inducing autophagy may become novel targets for drug research of PD therapy.
Mutant α-synuclein; Cytotoxicity; Injury
1671-2897(2016)15-029-04
R 739
A
苏州市科技计划项目基金资助项目(SYS201103)
东惟玲,硕士, E-mail: dongweilingqjf@126.com
*通讯作者:单立冬,讲师, E-mail: danlidong@suda.edu.cn
2015-01-18;
2015-03-04)

