CD40 siRNA对 MRL/Lpr狼疮小鼠炎症反应的影响
王志华,张 伟,张艳清,庞春艳,王永福△
(1.包头医学院第一附属医院风湿免疫科,内蒙古自治区包头 014010;2. 内蒙古自治区自体免疫学重点实验室,内蒙古自治区包头 014010)
·论著·
CD40 siRNA对 MRL/Lpr狼疮小鼠炎症反应的影响
王志华1,张 伟1,张艳清1,庞春艳2△,王永福1△
(1.包头医学院第一附属医院风湿免疫科,内蒙古自治区包头 014010;2. 内蒙古自治区自体免疫学重点实验室,内蒙古自治区包头 014010)
目的:观察CD40的小干扰RNA(small interfering RNA, siRNA)对系统性红斑狼疮(systemic lupus erythematosus, SLE)动物模型MRL/Lpr小鼠细胞因子IFN-γ、IL-17、IL-4和抗双链DNA(anti-double strand DNA,anti-ds-DNA)抗体表达水平的影响,探讨CD40 siRNA治疗MRL/Lpr小鼠的可能性。方法:16只MRL/Lpr小鼠随机分为对照组、空载体组、CD40-siRNA1组及CD40-siRNA2组,每组4只,将成功构建的CD40 siRNA表达载体尾静脉注射MRL/Lpr小鼠,对照组和空载体组小鼠分别注射等剂量、等比例的磷酸盐缓冲液(PBS)和pGFP-V-RS空载体,每隔1天1次,共6次,14 d处死小鼠,称其脾的质量,组织切片在荧光显微镜下观察小鼠脾是否有CD40 siRNA的表达,注射前及注射后第2、5、8、11和14 天小鼠尾部采血,ELISA法检测4组小鼠血清中IFN-γ、IL-17、IL-4和anti-dsDNA抗体的表达水平变化, RT-PCR法检测小鼠脾组织中CD40 mRNA的表达水平,免疫组织化学法检测小鼠脾组织中CD40蛋白的表达水平。结果:注射后的siRNA表达载体可以在小鼠脾中表达;注射CD40 siRNA组的小鼠脾质量减轻,由对照组(141.88±7.81) mg和空载体组(153.10±7.60) mg降低至CD40-siRNA1组(78.85 ±5.61) mg和CD40-siRNA2组(80.25±4.07) mg,注射前4组小鼠血清中IFN-γ、IL-17、IL-4和anti-dsDNA抗体的(质量)浓度比较,差异无统计学意义;而注射后第2、5和8天 CD40-siRNA1组和CD40-siRNA2组MRL/Lpr小鼠血清中IFN-γ、IL-17和(质量)浓度明显下降,CD40-siRNA1组的IFN-γ的(质量)浓度依次为(118.74±10.32) ng/L(pg/mL)、 (115.24±8.26) ng/L、(113.71±5.02) ng/L ,CD40-siRNA2组依次为(117.83±6.83) ng/L 、(114.07±0.97) ng/L、(112.67±9.66) ng/L; CD40-siRNA1组的IL-17的(质量)浓度依次为(7.05±0.41) ng/L、(6.34±0.76) ng/L、(5.83±0.43) ng/L,CD40-siRNA2组依次为(7.07±0.22) ng/L、(6.35±0.49) ng/L、(6.12±0.80) ng/L;CD40-siRNA1组的anti-dsDNA的浓度依次为(7.51±0.29) ng/L、(6.74±0.45) ng/L、(6.32±0.39) ng/L;CD40-siRNA2组依次为(8.19±0.38) ng/L、(7.14±0.50) ng/L、(6.48±0.29) ng/L。IL-4的(质量)浓度明显升高,CD40-si-RNA1组IL-4的(质量)浓度依次为(26.51±1.81) ng/L、(27.80±1.72) ng/L、(28.08±2.21) ng/L,CD40-siRNA2组依次为(26.28±2.03) ng/L、(28.15±2.95) ng/L、(28.37±1.71) ng/L。 CD40-siRNA1组和CD40-siRNA2组与对照组和空载体组比较差异有统计学意义(P<0.05), 注射后第11和14天 CD40-siRNA1组和CD40-siRNA2组中这4个指标的(质量)浓度逐渐恢复至对照组和空载体组的(质量)浓度水平,IFN-γ、 IL-17、IL-4的(质量)浓度4组小鼠比较,差异无统计学意义,注射后第11天 CD40-siRNA1组和CD40-siRNA2组anti-dsDNA抗体的水平尽管与对照组和空载体组比较有所提高,4组比较差异有统计学意义(P<0.05),而注射后第14天 anti-dsDNA抗体的水平4组小鼠比较,差异无统计学意义。注射后第14天 CD40-siRNA1组和CD40-siRNA2组 MRL/Lpr小鼠脾组织中CD40 mRNA和蛋白的表达水平明显低于对照组和空载体组,差异有统计学意义(P<0.05)。结论: MRL/Lpr小鼠注射CD40 siRNA载体后,血清中IFN-γ、IL-17和anti-dsDNA抗体水平降低,IL-4的浓度升高,CD40 mRNA和蛋白的表达水平下降,说明CD40 siRNA抑制其mRNA和蛋白后,MRL/Lpr小鼠炎症反应减轻,疾病活动度下降,提示其对SLE动物模型MRL/Lpr小鼠有治疗作用。
抗原,CD40;RNA,小分子干扰;红斑狼疮,系统性;细胞因子类;小鼠,近交MRL Lpr
系统性红斑狼疮(systemic lupus erythematosus, SLE)是一种多发于青年女性,并可累积多系统、多器官的自身免疫性疾病,严重危害育龄期妇女的健康,SLE的确切病因不清楚,可能由多种因素综合引起,由于异常的免疫激活和自身耐受性丧失可引起免疫活性过高,从而导致该病的发生。Th1细胞、Th2细胞、Th17细胞及其分泌的多种细胞因子(IFN-γ 、IL-4、 IL-17等)和自身抗体,以及CD40与其配体CD40L形成信号通路在SLE的病理过程中起重要作用[1-2],其中抗双链DNA(anti-double strand DNA, anti-dsDNA)抗体在SLE诊断和指导治疗方面具有重要的意义[3-4]。目前,SLE的治疗主要是应用糖皮质激素和免疫抑制剂,其毒副作用较大,而且治疗周期较长。小干扰RNA(small interfering RNA, siRNA)是一种特定基因沉默技术,它具有高效性、特异性等优点。因此,本研究采用siRNA技术沉默MRL/Lpr小鼠中CD40 基因的表达,探讨CD40基因的siRNA对MRL/Lpr小鼠的可能治疗作用。
1 材料与方法
主要试剂及仪器:载体pGFP-V-RS 购自美国Origene公司,AxyPrep质粒DNA大量抽提试剂盒购自美国Axygen公司,TRIzol购自日本Takara公司,M-MLV逆转录酶和脂质体lipofectamineTM2000购自美国Invitrogen公司, ELISA试剂盒为美国RD公司国内分装试剂盒,购自上海生工生物工程有限公司。
siRNA的设计和CD40-siRNA是否在小鼠脾中表达:针对CD40基因的cDNA序列设计2段siRNA,第1段序列为CTTGGAGGTCCTACAGAAA,第2段序列为GTGTTATCCCTGGACAAGC,将上述序列的模板插入质粒载体pGFP-V-RS中,构建其真核表达载体。将构建好的60 μg CD40 siRNA1和脂质体lipofectamine 2000TM的混合物200 μL [DNA(μg) ∶lipofectamineTM2000 (μL)为1 ∶2] 分别自尾静脉注射MRL/Lpr小鼠,对照组小鼠注射等剂量、等比例的磷酸盐缓冲液(PBS)和lipofectamineTM2000的混合物,6 h后处死其中一只小鼠取脾,快速冰冻切片,荧光显微镜下观察是否有绿色荧光。
实验动物分组及给药:十二周龄MRL/Lpr雌性小鼠16只,体质量18~23 g,购自南京大学动物研究所。按照简单随机化分组方法将小鼠分为对照组、空载体组、CD40 siRNA1组和CD40 siRNA2 组4组,每组4只。注射方法同上,空载体组小鼠注射和空载体与lipofectamineTM2000的混合物,每隔1天1次,共6次。注射前一天和第6次注射后的第2、5、8、11和14天小鼠尾部采血,取血清-80 ℃保存。
各项指标的检测:小鼠血清50倍稀释后ELISA检测 IFN-γ、 IL-17、IL-4和anti-dsDNA抗体的水平,450 nm处检测其光密度(D)值;根据标准曲线计算出IFN-γ、IL-17、IL-4和anti-dsDNA抗体的(质量)浓度。注射后第14天处死所有组MRL/Lpr小鼠,取小鼠脾,分成两份,其中一份TRIzol提RNA,实时荧光定量PCR检测小鼠脾中CD40 mRNA的表达水平,根据ct值计算2-ΔΔct。引物由上海生工生物工程有限公司合成,CD40上游引物5′-GAGAAGACCCAATGCCACC-3′,下游引物5′-GCACATGCCTCGCAATCC-3′;内参β-actin上游引物5′-CTGTCCCTGTATGCCTCTG-3′,下游引物5′-ATGTCACGCACGA-TTTCC-3′。另一份脾放入4%(体积分数)甲醛溶液固定,石蜡包埋,切片,然后脱蜡,TrionX-100通透,5%(质量分数)牛血清白蛋白(bovine serum albumin, BSA) 37 ℃封闭,滴加兔抗小鼠的一抗4℃ 过夜,PBS洗涤后加Alexa FluorRR 546标记山羊抗兔的IgG二抗,封片,荧光显微镜观察红色荧光强度,同时采集图片,图片采用IPP6软件分析平均D值。
统计学分析:采用SPSS 17. 0 统计软件进行数据分析,以均数±标准差表示,组间的比较采用单因素方差分析,P<0.05认为差异有统计学意义。
2 结果
2.1 MRL/Lpr小鼠脾质量的改变
4组小鼠脾质量比较,对照组和空载体组小鼠的脾质量明显比siRNA1组和siRNA2组小鼠的脾质量增加,对照组和空载体组比较差异无统计学意义,siRNA1组和siRNA2组比较差异也无统计学意义。对照组和空载体组与siRNA1组和siRNA2组比较,差异有统计学意义(P<0.05,表1)。
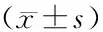
表1 4组小鼠脾质量Table 1
*P<0.05, campared with control group.
2.2 CD40 siRNA真核表达载体可以在MRL/Lpr小鼠脾中的表达
由于本实验用到的质粒载体pGFP-V-RS含有绿色荧光蛋白,因此荧光显微镜下观察冰冻切片,结果发现:对照组小鼠脾中看不到绿色荧光,而CD40-siRNA1组可看到明显的绿色荧光,即CD40 siRNA真核表达载体可以在MRL/Lpr小鼠脾中表达(图1)。
A, control group; B, CD40-siRNAL group.
图1 CD40 siRNA表达载体在小鼠脾中的表达
Figure 1 CD40 siRNA expression vector in spleen of mice
2.3 4组小鼠CD40 siRNA注射前后IFN-γ的表达
注射前1天对照组IFN-γ的(质量)浓度为(132.51±8.50) ng/L,空载体组为(130.72±8.05) ng/L, CD40-siRNA1组和CD40-siRNA2组分别为(129.70±4.42) ng/L和(131.97±2.98) ng/L,4组比较差异无统计学意义,但注射后第2、5、8天 CD40-siRNA1组和CD40-siRNA2组MRL/Lpr小鼠血清中IFN-γ的(质量)浓度逐渐下降,而对照组和空载体组的(质量)浓度并未明显下降,CD40-siRNA1组和CD40-siRNA2组与对照组和空载体组比较差异有统计学意义(P<0.05), 注射后第11和14天 siRNA1组和siRNA2组MRL/Lpr小鼠血清中IFN-γ的浓度逐渐升高,略低于对照组和空载体组的(质量)浓度,4组比较差异没有统计学差异(P>0. 05,图2)。
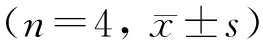
*P<0.05, compared with control group and emply vector group.图2 4组小鼠血清IFN-γ表达水平Figure 2 IFN-γ expression levels in serum of MRL/Lpr mice
2.4 4组小鼠CD40 siRNA注射前后IL-4的表达水平
注射前一天对照组IL-4的(质量)浓度为(21.83±0.67) ng/L,空载体组为(21.30±4.29)ng/L, CD40-siRNA1组和CD40-siRNA2组分别为(20.17±1.70) ng/L和(20.94±1.35) ng/L,4组比较差异无统计学意义,但注射后第2、5、8和11天 CD40-siRNA1组和CD40-siRNA2组MRL/Lpr小鼠血清中IL-4的(质量)浓度逐渐升高,而对照组和空载体组IL-4的(质量)浓度没有明显变化,CD40-siRNA1组和CD40-siRNA2组与对照组和空载体组比较差异有统计学意义(P<0. 05),注射后第14天 siRNA1组和siRNA2组MRL/Lpr小鼠血清中IL-4的浓度逐渐下降,略高于对照组和空载体组中的浓度,4组比较差异没有统计学意义(P>0. 05,图3)。
2.5 4组小鼠CD40 siRNA注射前后IL-17的表达
注射前一天对照组IL-17的(质量)浓度为(7.16±0.23) ng/L,空载体组为(7.14±0.60) ng/L, CD40-siRNA1组和CD40-siRNA2组分别为(7.00±0.46) ng/L和(7.08±0.55) ng/L,4组比较差异无统计学意义,但注射后第2、5和8天 CD40-siRNA1组和CD40-siRNA2组MRL/Lpr小鼠血清中IL-17的浓度逐渐下降,而对照组和空载体组IL-17的浓度略有上升,CD40-siRNA1组和CD40-siRNA2组与对照组和空载体组比较差异有统计学意义(P<0. 05),注射后第11和14天 siRNA1组和siRNA2组MRL/Lpr小鼠血清中IL-17的(质量)浓度逐渐升高,略低于对照组和空载体组中的(质量)浓度,4组比较差异没有统计学意义(P>0.05,图4)。
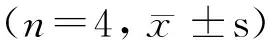
*P<0.05, compared with control group and emply vector group.图3 4组小鼠血清IL-4 表达水平Figure 3 IL-4 expression levels in serum of MRL/Lpr mice
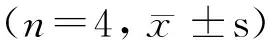
*P<0.05, compared with control group and emply vector group.图4 4组小鼠血清IL-17 表达水平Figure 4 IL-17 expression levels in serum of MRL/Lpr mice
2.6 4组小鼠CD40 siRNA注射前后anti-dsDNA抗体的变化
注射前一天 anti-dsDNA抗体对照组的(质量)浓度为(9.71±0.33) ng/L,空载体组为(9.54±0.32) ng/L, CD40-siRNA1组为(9.62±0.67) ng/L,CD40-siRNA2组为(9.96±0.45) ng/L,4组比较差异无统计学意义,注射后第2、5、8和11天 CD40-siRNA1组和CD40-siRNA2组MRL/Lpr小鼠血清anti-dsDNA抗体的(质量)浓度逐渐下降,但在注射后第14天 CD40- siRNA1组和CD40-siRNA2组anti-dsDNA抗体的浓度开始升高,而对照组和空载体组小鼠血清中anti-dsDNA抗体的浓度随着时间的延长略有上升,CD40-siRNA1组和CD40-siRNA2组与对照组和空载体组比较差异有统计学意义(P<0.05,图5)。
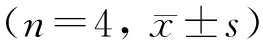
*P<0.05, compared with control group and emply vector group.图5 4组小鼠anti-dsDNA抗体的表达水平Figure 5 anti-dsDNA antibody expression levels in serum of
2.7 4组小鼠CD40 siRNA注射后CD40 mRNA的表达
注射后第14天处死小鼠, CD40-siRNA1组和CD40-siRNA2组小鼠脾中CD40 mRNA的表达明显低于对照组和空载体组,差异有统计学意义(P<0.05),对照组和空载体组比较差异无统计学意义(P>0.05),CD40-siRNA1组和CD40-siRNA2组比较差异有统计学意义(P<0.05,表2)。
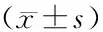
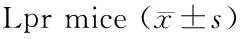

Group2-ΔΔCtControlgroup1.00±0.00Emptyvectorgroup1.00±0.12siRNA1group0.19±0.08*siRNA2group0.33±0.06*
*P<0.05, compared with control group.
2.8 4组小鼠CD40 siRNA注射后CD40 蛋白的表达
注射后第14天处死小鼠, 检测4组小鼠脾中CD40蛋白的表达,发现对照组和空载体组有很强的红色荧光,而CD40-siRNA1组和CD40-siRNA2组的红色荧光量明显减弱,即CD40-siRNA1组和CD40-siRNA2组CD40的表达明显低于对照组和空载体组,对照组和空载体组比较差异不明显,siRNA1组和siRNA2组比较也无明显差异(图6),上述应用的为荧光免疫组织化学法,无需染色,放大倍数为10倍。
3 讨论
系统性红斑狼疮是一种常见的自身免疫性疾病,好发于育龄期女性,Th1分泌的细胞因子IFN-γ在其发病过程中发挥重要作用,并与疾病活动度呈正相关,Th17和Treg之间的失衡在SLE的病理发展中起关键作用[5]。MRL/Lpr小鼠作为一种SLE的动物模型,被广泛用于SLE 的研究。RNA干扰(RNA interference, RNAi)技术作为一种疾病基因治疗手段被广泛用于自身免疫性疾病和肿瘤等的治疗[6-8]。
A, control group; B, empty vector group; C, CD40-siRNA1 group; D, CD40-siRNA2 group.
图6 4组小鼠脾中CD40蛋白的表达水平
Figure 6 the expression levels of CD40 protein in spleen from MRL/Lpr mice
CD40是与T细胞和B细胞功能有关的一种表面抗原,为Ⅰ型跨膜糖蛋白。研究发现CD40在SLE患者骨髓造血祖细胞中高表达,SLE患者的骨髓细胞和健康对照者相比,CD40L在其培养的上清液中和贴壁层细胞中表达水平明显升高,因此,CD40与其配体CD40L形成信号通路在SLE中可能起重要作用[9],同时,CD40还可激活SLE患者B细胞,使其增殖和分化能力增强,免疫球蛋白(immunoglobulin, IgS)分泌增多[10],因此,针对CD40的基因治疗是治疗SLE的策略之一。本课题组的前期体外研究发现,CD40的siRNA可抑制小鼠巨噬细胞J774.1细胞中CD40 mRNA和蛋白的表达水平。用本研究成功构建的2段siRNA真核表达载体注射MRL/Lpr小鼠尾静脉后发现,MRL/Lpr小鼠脾重量减轻,同时,C40的siRNA可以在小鼠脾中表达, siRNA1组和siRNA2组MRL/Lpr小鼠脾中CD40 mRNA和蛋白的表达与对照组和空载体组比较明显下降,充分说明CD40的特异性siRNA能在基因和蛋白水平上沉默其表达,同时它对免疫器官有一定的保护作用。
Harigai等[11]检测SLE患者外周血T细胞中IFN-γ的表达,结果发现IFN-γ的表达明显升高,它通过诱导可溶性B淋巴细胞刺激因子(soluble B lymphocyte stimulator,sBLyS)的表达而参与SLE的免疫病理过程,从而在SLE 的发病过程中起非常重要的作用。Amarilyo等[12]研究发现2,6,10,14-四甲基十五烷(pristane)不能诱导IL-17缺失的小鼠形产生狼疮相关的自身抗体和狼疮肾炎的形成,原因是IL-17的缺失可减少CD3+CD4-CD8-T 的产生,同时增加CD4+ 调节性 T细胞的产生。本研究针对基因CD40成功构建2段siRNA真核表达载体,并经尾静脉注射MRL/Lpr小鼠,结果显示CD40-siRNA真核表达载体可以靶向到MRL/Lpr小鼠脾,从而发挥作用。ELISA的结果显示注射后第2、5和8天 CD40-siRNA1组和CD40-siRNA2组MRL/Lpr小鼠血清中IFN-γ和 IL-17的(质量)浓度随时间的延长而逐渐下降,与治疗前比较差异均有统计学意义,而对照组和空载体组的浓度与治疗前比较并未明显下降,3个时间段CD40-siRNA1组和CD40-siRNA2组与对照组和空载体组比较差异均有统计学意义,提示CD40的siRNA可减弱MRL/Lpr小鼠的Th1和Th17相关炎症因子的表达水平。Robak等[13]研究发现SLE患者血清中IL-4的表达水平降低,与健康对照者相比差异有统计学意义。本研究发现注射后第2、5和8天 CD40-siRNA1组和CD40-siRNA2组MRL/Lpr小鼠血清中IL-4的(质量)浓度随时间的延长而逐渐升高,与治疗前比较差异均有统计学意义,提示CD40的siRNA可升高MRL/Lpr小鼠的Th2相关炎症因子的表达水平,而注射后第11和14天 CD40-siRNA1组和CD40-siRNA2组MRL/Lpr小鼠血清中IFN-γ和 IL-17的浓度开始逐渐升高,IL-4的浓度逐渐降低,接近于对照组和空载体组中的浓度,4组比较差异没有统计学意义,这可能与CD40 的siRNA 载体在小鼠体内代谢及产生的免疫反应有关。
anti-dsDNA抗体是判断SLE活动度的重要标记,在诊断SLE时有很好的灵敏度和特异性。ZHOU等[7]研究发现B淋巴细胞诱导成熟蛋白1(B-lymphocyte-induced maturation protein 1,Blimp-1)的siRNA可以清除产生anti-dsDNA抗体的浆细胞和降低血清中anti-dsDNA抗体的浓度,从而延缓狼疮的进程。本研究结果发现CD40的siRNA真核表达载体注射MRL/Lpr小鼠第2、5、8和11天后anti-dsDNA抗体的浓度逐渐下降,与治疗前比较差异有统计学意义,而对照组和空载体组的浓度比治疗前略有上升,4个时间段CD40-siRNA1组和CD40-siRNA2组与对照组和空载体组比较差异均有统计学意义,但在注射后第14天 CD40-siRNA1组和CD40-siRNA2组anti-dsDNA抗体的浓度开始升高,但还是要远低于对照组和空载体组,CD40-siRNA1组和CD40-siRNA2组与对照组和空载体组比较差异有统计学意义。因此,CD40的siRNA载体注射MRL/Lpr小鼠后anti-dsDNA抗体的表达明显减弱,即CD40的siRNA可减轻SLE的疾病活动程度。
综上所述,CD40 siRNA可抑制CD40 mRNA和蛋白的表达水平,降低血清中IFN-γ和 IL-17的表达,提升IL-4的表达水平,说明CD40 siRNA可使MRL/Lpr小鼠炎症反应减轻,疾病活动度下降,提示其对SLE动物模型MRL/Lpr小鼠有治疗作用,为SLE的基因治疗提供了实验依据。
[1]Mok MY, Wu HJ, Lo Y, et al. The relation of interleukin 17 (IL-17) and IL-23 to Th1/Th2 cytokines and disease activity in systemic lupus erythematosus [J]. J Rheumatol, 2010, 37(10): 2046-2052.
[2]Shah K, Lee WW, Lee SH, et al. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus [J]. Arthritis Res Ther, 2010, 12(2): 53.
[3]Wichainun R, Kasitanon N, Wangkaew S, et al. Sensitivity and specificity of ANA and anti-dsDNA in the diagnosis of systemic lupus erythematosus: a comparison using control sera obtained from healthy individuals and patients with multiple medical problems [J]. Asian Pac J Allergy Immunol, 2013, 31(4): 292-298.
[4]Villalta D, Bizzaro N, Bassi N, et al. Anti-dsDNA antibody isotypes in systemic lupus erythematosus: IgA in addition to IgG anti-dsDNA help to identify glomerulonephritis and active disease [J]. PLoS One, 2013, 8(8): e71458.
[5]Szmyrka-Kaczmarek M, Kosmaczewska A, Ciszak L, et al. Peripheral blood Th17/Treg imbalance in patients with low-active systemic lupus erythematosus [J]. Postepy Hig Med Dosw (Online), 2014, 68: 893-898.
[6]Huang ZY, Kim MK, Kim-Han TH, et al. Effect of locally administered Syk siRNA on allergen-induced arthritis and asthma [J]. Mol Immunol, 2013, 53(1/2): 52-59.
[7]Zhou Z, Ren Y, Hu Z. Blimp-1 siRNA inhibits B cell differentiation and prevents the development of lupus in mice [J]. Hum Immunol, 2013, 74(3): 297-301.
[8]Patil SP, Kim SH, Jadhav JR, et al. Cancer-specific gene silencing through therapeutic siRNA delivery with B vitamin-based nanoassembled low-molecular-weight hydrogelators [J]. Bioconjug Chem, 2014, 25(8): 1517-1525.
[9]Pyrovolaki K, Mavroudi I, Sidiropoulos P, et al. Increased expression of CD40 on bone marrow CD34+ hematopoietic progenitor cells in patients with systemic lupus erythematosus: contribution to Fas-mediated apoptosis[J]. Arthritis Rheum, 2009, 60(2): 543-552.
[10]Néron S, Boire G, Dussault N, et al. CD40-activated B cells from patients with systemic lupus erythematosus can be modulated by therapeutic immunoglobulinsinvitro[J]. Arch Immunol Ther Exp (Warsz), 2009, 57(6): 447-458.
[11]Harigai M, Kawamoto M, Hara M, et al. Excessive production of IFN-gamma in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B[J]. J Immunol, 2008, 181(3): 2211-2219.
[12]Amarilyo G, Lourenço EV, Shi FD, et al. IL-17 promotes murine lupus [J]. J Immunol, 2014, 193(2): 540-543.
[13]Robak E, Smolewski P, Wozniacka A, et al. Relationship between peripheral blood dendritic cells and cytokines involved in the pathogenesis of systemic lupus erythematosus [J]. Eur Cytokine Netw, 2004, 15(3): 222-230.
(2015-02-04收稿)
(本文编辑:王 蕾)
Effect of CD40 siRNA on inflammatory response of MRL/Lpr mice
WANG Zhi-hua1, ZHANG Wei1, ZHANG Yan-qing1, PANG Chun-yan2Δ,WANG Yong-fu1Δ
(1.Department of Rheumatology, the First Affiliated Hospital of Baotou Medical College, Baotou 014010, Inner Mogolia, China; 2. Key Autoimmunity Lab of Inner Mongolia, Baotou 014010, Inner Mogolia, China)
Objective:To observe the effect of CD40 siRNA on expression of IFN-γ, IL-17, IL-4 and anti-dsDNA antibody of systemic lupus erythematosus (SLE) animal model MRL/Lpr mice and to discuss its therapy on MRL/Lpr mice. Methods: In the study, 16 female MRL/Lpr mice were randomly divided into control group (n=4), empty vector group (n=4), CD40-siRNA1 group (n=4) and CD40-siRNA2 group (n=4). The vectors expressing siRNA against CD40 were injected by tail veil into MRL/Lpr mice, while MRL/Lpr mice in control group and empty vector group were injected with the same dose of PBS and pGFP-V-RS vector respectively. The injection was given six times and every one day. The mice were sacrificed 14 d after injection, and the spleen tissue was weighed. The pGFP-V-RS was labeled by green fluorescent protein(GFP) and the tissue sections were observed whether siRNA expressed in the spleen. The expression levels of IFN-γ, IL-17, IL-4 and anti-dsDNA antibody in the sera were detected by ELISA method on the 1st day before the first time and the 2nd, 5th, 8th, 11th, and 14th days after last injection, and the expression levels of CD40 mRNA in spleen tissue of MRL/Lpr mice were detected by RT-PCR and the expression levels of CD40 protein in spleen tissue of MRL/Lpr mice were detected by immunohistochemistry method. Results: The expression vector of CD40-siRNA could express in the spleen of MRL/Lpr. The spleens in CD40-siRNA1 group [(78.85 ±5.61) mg] and CD40-siRNA2 group [(80.25±4.07) mg] were lower than those in control [(141.88±7.81) mg] and empty vector group [(153.10±7.60) mg]. The levels of IL-17, IFN-γ and anti-dsDNA antibody were lower and the levels of IL-4 was higher in CD40-siRNA1 group and CD40-siRNA2 group on the 2nd, 5th and 8th days after last injection than on the 1st day before the first time (P<0.05). The levels of IFN-γ in CD40-siRNA1 group were (118.74±10.32) ng/L, (115.24±8.26) ng/L and (113.71±5.02) ng/L in turn, the levels of IFN-γ in CD40-siRNA2 group were (117.83±6.83) ng/L, (114.07±0.97) ng/L and (112.67±9.66) ng/L in turn. The levels of IL-17 in CD40-siRNA1 group were (7.05±0.41) ng/L, (6.34±0.76) ng/L and (5.83±0.43) ng/L in turn, the levels of IL-17 in CD40-siRNA2 group were (7.07±0.22) ng/L, (6.35±0.49) ng/L and (6.12±0.80) ng/L in turn. The levels of anti-dsDNA antibody in CD40-siRNA1 group were (7.51±0.29) ng/L, (6.74±0.45) ng/L and (6.32±0.39) ng/L in turn, the levels of anti-dsDNA antibody in CD40-siRNA2 group were (8.19±0.38) ng/L, (7.14±0.50) ng/L and (6.48±0.29) ng/L in turn. The levels of IL-4 in CD40-siRNA1 group were (26.51±1.81)ng/L (27.80±1.72) ng/L and (28.08±2.21) ng/L in turn, the level of IL-4 in CD40-siRNA2 group were (26.28±2.03) ng/L, (28.15±2.95) ng/L and (28.37±1.71) ng/L in turn. The expression levels of IL-17 and IFN-γ antibody increased gradually and the levels of IL-4 decreased gradually in CD40-siRNA1 group and CD40-siRNA2 group on the 11th and 14th days after last injection, then reached to the levels of control group and empty vector group (P>0.05). Though the levels of anti-dsDNA antibody in CD40-siRNA1 group and CD40-siRNA2 group on the 11th day was higher than on the 8th day, there was more significance than those in control group and empty vector group (P<0.05). There was no significance between the 4 groups on the 14th day. The levels of CD40 mRNA and protein were lower in CD40-siRNA1 group and CD40-siRNA2 group than in control group and empty vector group on the 14th day after last injection (P<0.05). Conclusion: CD-40 si-RNA can reduce the concentration of IL-17, IFN-γ and of anti-dsDNA antibody in serum, and at the same time, it can elevate the concentration of IL-4 and suppress CD40 mRNA and protein of spleen in MRL/Lpr. Meanwhile after suppressing CD40 mRNA and protein, it can reduce inflammatory response of the mice and the disease activity of MRL/Lpr, suggesting that CD-40 siRNA has therapy effect on SLE.
Antigens, CD40; RNA, small interfering; Lupus erythematosus, systemic; Cytokines; Mice, inbred MRL Lpr
国家自然科学基金(30960354)资助Supported by the National Natural Science Foundation of China (30960354)
时间:2016-9-5 15:56:42
http://www.cnki.net/kcms/detail/11.4691.R.20160915.1556.050.html
R593.2
A
1671-167X(2016)05-0771-06
10.3969/j.issn.1671-167X.2016.05.004
△ Corresponding authors’ e-mail, pchy_fighting@163.com, wyf5168@hotmail.com

