葱叶一步法裂解制备多孔炭及其电容性能研究
于 晶, 高利珍, 李雪莲, 吴 超, 高丽丽,3, 李长明
(1.太原理工大学 环境科学与工程学院,山西 太原030024;2.西南大学 清洁能源与先进材料研究所,重庆400715;3.太原理工大学 绿色能源材料与储能系统实验室,山西 太原030024)
葱叶一步法裂解制备多孔炭及其电容性能研究
于 晶1, 高利珍1, 李雪莲1, 吴 超2, 高丽丽1,3, 李长明2
(1.太原理工大学 环境科学与工程学院,山西 太原030024;2.西南大学 清洁能源与先进材料研究所,重庆400715;3.太原理工大学 绿色能源材料与储能系统实验室,山西 太原030024)
以葱叶为炭前驱体,在不添加任何活化剂的条件下,炭化活化同时进行,制备了孔径分布主要集中于0.6~1.2 nm和3~5nm之间的葱基多孔炭材料,并对其电容性能进行研究。分别采用扫描电子显微镜(SEM)、场发射扫描电子显微镜(FE-SEM)、能量弥散X射线光谱(EDX)、火焰原子吸收光谱(FAAS)、X射线衍射(XRD)、热重分析(TGA)和氮气吸脱附曲线等方法表征了葱基炭的形貌、成分、比表面积及孔径分布等性能;通过循环伏安(CV)、交流阻抗(EIS)、恒流充放电(GCD)等电化学方法考察了材料的比电容和循环寿命等电化学性能。结果表明,葱叶中本身含有的微量矿物质如钙、钾等在其炭化的过程中同时起到了活化的作用。研究了不同温度下(600~800 ℃)制备的多孔炭的性能,发现800 ℃条件下制得的样品性能最佳,以微孔为主,介孔辅之,孔径为0.6~1.2 nm的微分孔隙体积达2.608 cm-3/g/nm,3~5 nm的微分孔隙体积有0.144 cm-3g/nm,BET比表面积为551.7 m2/g,质量比电容为158.6 F/g,有效面积电容可高达28.8 μF/cm2。这表明孔径分布情况对多孔炭的电荷存储能力有很重要的影响,此法也为提高“有效面积电容”提供了思路。
多孔炭; 葱叶; 一步炭化活化法; 有效面积电容
1 Introduction
With the increase of the environmental pollution and the scarcity of fossil fuels, the demand for clean energy sources is growing rapidly all around the world. Supercapacitor, as a kind of clean energy conversion and storage device, has attracted much attention owing to its high power density, long cycle life and high dynamic of charge propagation, which bridges the power/energy gap between traditional dielectric capacitor and battery[1-6]. Especially, electrical double-layer supercapacitors (EDLSs), draw much more attention owing to their simple charging mechanism, long cycling life and short charging time. Since pure physical charge accumulation occurs at the electrochemical interface between electrode and electrolyte during the charge/discharge process, EDLS is able to store and deliver energy at a relatively high rate[7-10]. Compared to batteries, supercapacitors have the advantages of high power density, long life expectancy, long shelf life, high efficiency, wide range of operating temperatures, environmental friendliness and safety. However, they also face challenges at the current stage of technology, such as low energy density, high cost and high self-discharging rate. Among the components of a supercapacitor, electrode materials dominate the performance of supercapacitors[11]. Therefore, developing new materials with improved performance is important to improve the property of supercapacitors[12]. In general, electrode materials of supercapacitors include three types[13,14]: carbon materials, conducting polymers, and metal oxides. Porous carbons have large surface areas, relatively good electrical conducting properties and the 3D porous network structure that ensures fast electronic and ionic conduction through charge/discharge process. Furthermore, porous carbons are considered as the most promising candidate materials for supercapacitors in industry owing to their moderate cost[3, 7, 15]. Generally, the synthesis of porous carbons includes two steps: carbonization and activation. Among various precursors, cheap and renewable biomass such as agricultural byproducts have attracted much attention owing to their low cost and environmental friendly properties[16-18]. Activation is a crucial procedure, which include physical and chemical activation. For these two methods, either high temperature or large amount of chemical agent is used, which require expensive equipments or bring about difficulty in post-treatment[19-25]. Though various porous carbons have been tried as electrode materials in supercapacitors, their applications are still limited owing to their complicated production processes[26]. As reported, natural constituents such as mineral substances in some kinds of leaves may replace the additional pore generators to create micropores, thereby simplifying the process[27,28]. Green onions are widely planted in China and could be stored in winter. However, during the storage, the leaves of green onions are usually withered and need to be discarded. Therefore, we reported a facile, cost-effective approach to synthesize porous carbon via one-step pyrolysis of the discarded green onion leaves without any additive. The reason might be that green onion leaves contain Ca and K that act as pore generators[27,28]. The pore sizes are mainly centered around 0.6-1.2 and 3-5 nm. Although the specific surface area and the mass specific capacitance for the green onion leave-derived carbons (GOLCs) are not so high, their “effective areal capacitance” is high, indicating that the proportion of their effective pores in GOLCs is high.
2 Experimental
2.1 Chemicals
The green onions used in this study were directly obtained from the local farm. Nafion solution was purchased from Sigma. All other chemical reagents, such as hydrochloric acid (HCl, 36%), nitric acid (HNO3, 65%), perchloric acid (HClO4, 70%), hydrogen peroxide (H2O2, 30%) and potassium hydroxide (KOH, 98%), were purchased from Sinopharm Chemical Reagent Co. Ltd and used as received without any further purification. All the aqueous solutions were prepared with Millipore water having a resistivity of 18.2 MΩ (Purelab Classic Corp., USA).
2.2 Synthesis of porous carbons
The synthesis process of green onion leave-derived carbons (GOLCs) is shown in Fig. 1.

Fig. 1 Schematic diagram for the synthesis of porous carbons from green onion leaves.
The leaves of green onion were separated from the white stem, washed thoroughly with deionized water and dried at 60 ℃ in an oven over night. The dried leaves were crushed into powder. The carbonization and activation processes were carried out at one step. The dried leave powder was heated at 600-800 ℃ under the protection of argon for 2 h in a tubular furnace. The heating rate was 10 ℃/min. After cooled down to room temperature under argon, the green powder was totally turned into black color. The obtained products were washed thoroughly by deionized water and then dried in an oven over night. For comparison, some products were rinsed by a diluted hydrochloric solution (0.1 M).
2.3 Electrochemical measurements
Electrochemical characterizations were carried out in a three-electrode electrochemical system using Hg/HgO electrode and platinum foil as the reference and counter electrode, respectively. The GOLC powder was dispersed in water by sonication. Then the suspension was dripped on a glassy carbon electrode and coated by Nafion solution.
All the electrochemical measurements were carried out on a CHI 660D electrochemical workstation (Shanghai Chenhua Co. Ltd, China) in 3 M KOH aqueous electrolyte solution at room temperature. Cyclic voltammetry (CV) curves were obtained between a potential range of -1.0-0.1 V at different scanning rates. The electrochemical impedance spectroscopy (EIS) was performed in a three-electrode system at 5 mV-alternating current-disturbance around the open circuit potential vs Hg/HgO. The scanning frequency was from 0.01 to 100 kHZ. The galvanostatic charge/discharges (GCD) were carried out under different current densities.
The mass specific capacitance is calculated from GCD curves through equation (1) :
(1)
where “Cs” is the specific capacitance, “I” is the current, “m” is the active mass and “dv/dt” is the slope obtained from the discharge curve.
Effective areal capacitance (Cea, μF/cm2) means the ratio of “mass specific capacitance (Cms, F/g)” and “BET surface area (A, m2/g)”, which is calculated by the equation (2).
(2)
2.4 Characterizations
The morphology of GOLCs was observed by a JSM-6510LV (Japan) scanning electron microscope (SEM) and a JSM-7800F field-emission scanning electron microscope (FE-SEM, Japan). Elemental composition analysis was qualitatively measured by JSM-6510LV (Japan) energy dispersive X-ray spectroscopy (EDX) and quantitatively determined by WFX-110 flame atomic absorption spectrometry (FAAS). The samples were pretreated before FAAS measurement. Firstly, they were ground into powder and poured into an acid mixture of HNO3and HClO4, followed by heating and dissolving at a hot plate until most of water evaporated. Then H2O2was added to get rid of the residual acid. Through the treatment, minerals such as K and Ca could be totally dissolved from the samples, which could be used for FAAS measurements. The nitrogen adsorption and desorption isotherms at 77 K were measured using a Quantachrome Instruments (USA) Inc. Nova 1200e surface area and pore size analysis system. The specific surface area was calculated from the N2adsorption isotherm by applying the Brunauer-Emmett-Teller (BET) equation. In order to reflect the pore size distribution exactly, both Barrett-Joyner-Halenda (BJH) and Density functional theory (DFT) models were applied. BJH model is more suitable to mesopore analysis while DFT for micropore analysis. XRD patterns were obtained by a XRD-7000 (Japan). Thermogravimetric analysis (TGA) and differential thermogravimetric (DTG) analysis were carried out using with a Thermogravimetric Analyzer Q50 (USA).
3 Results and discussion
The morphology of all GOLCs prepared at different temperatures is shown in Fig. 2. From the SEM images (from 2a to 2f), all the samples prepared at different temperatures show similar fiber structure as the original leaves, implying that the macroscopical structure haven’t been changed during carbonization. However, mesopores and micropores could not be clearly observed under SEM, which might be caused by the low magnification and resolution of SEM. The GOLC prepared at 800 ℃ (GOLC-800) under FE-SEM is shown in Fig. 2g-h, which reveals that more tiny pores can be observed, but still not quite clear. This might be because some of the pores may be hidden by the original mineral substances that are uniformly distributed in green onion leaves.
The pore structure could be further verified by nitrogen adsorption-desorption isotherms as shown in Fig. 3. An obvious hysteresis loop can be observed in the isotherms in Fig. 3a at the relative pressure from 0.4 to 0.9. The hysteresis loop can be categorized as H4 type, revealing that mesopores exist in the samples[28,29]. The specific surface areas for different GOLCs prepared at 600, 700 and 800 ℃, abbreviated as GOLC-600, GOLC-700 and GOLC-700, are calculated with standard BET method to be and respectively 230.5,348.4 and 551.7 m2/g, respectively. Fig. 3b-d depict the pore size distributions of GOLCs with the two models, which show bimodal distribution of micropores and mesopores. Through calculation, the differential pore volumes of micropores (0.6-1.2 nm) are 1.432, 1.449 and 2.608 cm-3/g/nm for GOLC-600, GOLC-700 and GOLC-800, respectively. Furthermore, most of the micropores are centered around 0.6-0.8 nm. Micropores have a high surface area to volume ratio and contribute more to surface area when present in significant amounts. Some studies have reported that pore sizes around 0.7 nm may be a suitable dimension for aqueous electrolyte,which could match the dimension of the aqueous ion[2, 32,33]. And the corresponding differential pore volumes of mesopores (3 to 5 nm) are 0.016, 0.071 and 0.144 cm-3/g/nm for GOLC-600, GOLC-700 and GOLC-800, respectively. As reported[30], mesopores play a significantly important role to obtain an ideal capacitor behavior, because they can not only contribute to the surface area but also provide wide transport channels for adsorbate accessibility[31]. Both the differential micropore volume and differential mesopore volume for GOLC-800 are the highest among the three samples, implying that high activation temperature is favorable for the generation of pores. Therefore, GOLC-800 is the most excellent material among the three, followed by GOLC-700 and then GOLC-600, if it is judged merely from the pore size distributions and BET surface areas.

Fig. 2 (a-f) SEM and (g-h) FESEM images of green onion leave-derived carbons prepared at different temperatures: (a-b) 600 ℃, (c-d) 700 ℃ and (e-h) 800 ℃.
The elements and their relative contents in the GOLC-800 were also determined by EDX as shown in Fig. 4a. It is seen that carbon (C) is the most prominent ingredient, implying that the green onion has been well carbonized. Trace of inorganic elements such as oxygen (O), sulphur (S), chlorine (Cl) and phosphorus (P) can be observed as shown in Fig. 4a. The existing of oxygen (O) implies that there are lots of oxygen-groups on the surface of the carbon. Furthermore, some mineral substances can be as well detected, such as calcium (Ca) and potassium (K). Since no element addition was involved during the carbonization of GOLC-800, it can be inferred that all the mineral substances originate directly from the green onion leaves.

Fig. 3 (a) Nitrogen adsorption-desorption isotherms for green onion leave-derived carbons prepared at different temperatures; (b-d) pore size distributions with the BJH and DFT models.

Fig. 4 (a) Images of EDX analysis and (b) XRD patterns for green onion leave-derived carbon at 800 ℃.
To further verify the content of these mineral substances, TGA measurement of original green onion leaves was also carried out as shown in Fig. 5.
Stage I from 25 to approx. 200 ℃ might correspond to the elimination water including free and bonded water, and the total content of water in green onion is 15 wt%. The main pyrolysis of green onion occurs at Stage II (200-300 ℃) and Stage III (300-500 ℃), which show highest weight loss. Stage II may be correlated to the decomposition of carbohydrates and proteins[27]while stage III to cellulose and hemicellulose[34]. The weight loss for stage II and III is approximately 55% in general. When the temperature is higher than 500 ℃ (stage IV), only a 5%-8% weight reduction happens until 800 ℃,which might be caused by the decomposition of the small amount of lignin contained in green onion[34]. The residual content after Stage IV is above 20%, part of which may be due to the large amount of minerals such as Ca, K originally present in green onion leaves.
The XRD patterns of GOLCs in Fig. 4b could further confirm the existence of mineral substances. The upper line in Fig. 4b represents the GOLC-800 that was washed only with pure water, from which, two sharp peaks near 28° and 33° could be seen obviously; however, after the GOLC-800 was rinsed by diluted HCl solution, these two peaks disappeared as shown in the lower line. Through comparison to the standard spectrum diagrams, the sharp peaks might be attributed to CaC2. After rinsing with HCl, CaC2might reacts with in water. Furthermore, a broad peak near 2θ=25°can be seen in both lines, corresponding to the crystalline graphite. As reported[27,28], Ca and K salts can be acted as pore generators to create pores during the synthesis. Nakagawa[35]reported that more mesopores and micropores could be obtained in the porous carbons by adding some calcium compound into the raw material before activation. Raymundo also illustrated that the presence of K derivatives in carbon precursor played the same role as additives of chemical pore generators during the activation[27].

Fig. 5 TGA and (DTG) analysis of green onion leaves under a nitrogen atmosphere (heating rate: 10 ℃/min).
To quantitatively analyze the contents of mineral substances (K, Ca), FAAS was applied. Three different samples were measured, dried green onion leaves prepared by drying green onion leaves under 60 ℃ at vacuum oven for 12 h, GOLC-800 and GOLC-800 rinsed by HCl solution. The results are listed in Table 1, which reveal that the original contents of K and Ca in dried green onion leaves are 20.5 and 3.5 mg/g, respectively, which are similar to the reported results[28]. After the carbonization at 800 ℃, the contents of K and Ca increase to 42.7 and 7.3 mg/g, respectively. The increase of their relative contents in the samples might be attributed to pyrolysis of carbohydrates and proteins, namely, the loss of H, O and other elements. These results agree well with the TGA conclusions as shown in Fig. 5. Compared with the amount of the activating agents added in chemical activation, the contents of K and Ca are very low. However, as reported by Biswal[28], natural constituents such as mineral substances in biomass are distributed uniformly. So despite the very few amounts, they are very effective to create pores in activation. In this work, the total content of K and Ca in GOLC-800 is 50 mg/g, so they could play an important role to generate pores in carbonization as activating agents. This is why no more external activating agents are needed. After the GOLC samples were thoroughly rinsed in HCl solution, the K and Ca were removed to an extent too little to be detected.
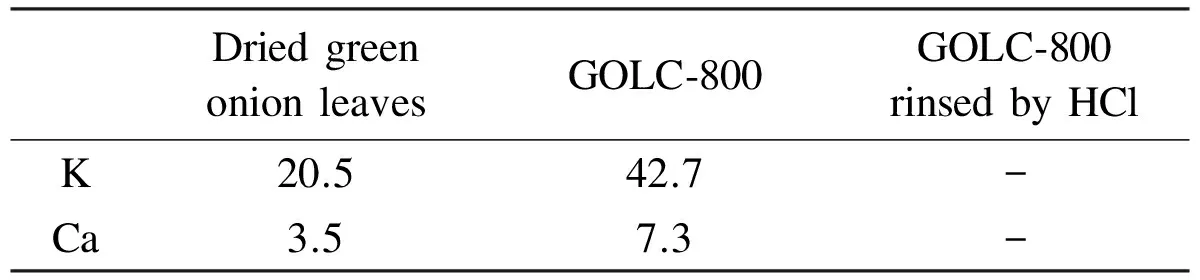
Table 1 Contents of K and Ca in dried green onion leaves, GOLC-800 and GOLC-800 rinsed by HCl.
Electrochemical behaviors of GOLCs prepared under different temperatures were measured in 3 M KOH aqueous electrolyte, as shown in Fig. 6 and Fig. 7. To measure whether the residual K and Ca in GOLC-800 have great effect on capacitance, the GOLC-800 samples were thoroughly rinsed by HCl, as shown in Fig 6a. The XRD results in Fig 4b have shown that materials such as Ca could be gotten rid of through rinsing with HCl. However, it could be obviously seen that CV curves of GOLC-800 and GOLC-800 rinsed by HCl are similar, implying that the mineral substances as K and Ca in GOLC have little effects. Thus, GOLCs were just washed by deionized water and measured in the following samples.
Fig. 6b is the galvanostatic charge/discharge curve at 0.2 A/g of GOLCs, linear and nearly symmetrical curves could be seen in all samples, confirming that the product has excellent electrochemical reversibility and charge/discharge properties. Comparison of the three samples at the same charge/discharge current density of 0.2 A/g, discharge time of GOLC-800 is nearly 870 s, and GOLC-700 and GOLC-600 is 570 and 520 s, respectively, implying that GOLC-800 has better electrochemical performance than GOLC-600 and GOLC-700. The mass specific capacitances for GOLC-800, GOLC-700 and GOLC-600 at a current density of 0.2 A/g calculated from equation (1) are 158.6,104.2 and 94.8 F/g, respectively. The higher mass specific capacitance for GOLC-800 may be ascribed to its larger specific surface area and higher differential pore volume[36]. Actually, this capacitance value is relatively higher than those of other electrode materials for supercapacitor application from biomass precursor[8,37]. Fig 6c is the galvanostatic charge/discharge curves of GOLC-800 at different current densities. It can be seen that the capacitances drastically change for GOLC-600, GOLC-700 and GOLC-800 when the current density increases from 0.2 to 5.0 A/g as shown in Fig. 6d. This can be explained as follows[38]. At lower current densities, ions can be transported and diffused into the pores easily, which results in higher capacitance. However, when the current density increases, ions cannot be easily diffused into the pores so that the effective double layers are formed at the surface of the electrode. Hence, the capacitance at high current densities are low.

Fig. 6 Measurements of GOLCs’ electrochemical behavior.
Fig. 7a and Fig. 7b depict the cyclic voltammetry curves of GOLC-800 at different scanning rates. At lower scanning rate such as 2 mV/s, a redox hump could be observed betwwen -0.15-0.25 V, which might be casued by oxygen-groups reaction at the carbon surface[39]. This Faradaic redox reaction also contributes to the capacitance. However, in the whole scaning rang from -1.0 to 0.1 V, the CV curves represent nearly rectangular shape, revealing an ideal capacitance behavior and the charge/discharge process is nearly reversible[23,40].With the increasing of scanning rate, there is almost no deviation from rectangular shape in CV curves, implying the low ohmic polarization and high electrolyte ion transfer rate. At the same time, when the direction of the scanning rate changes, current responses quickly, implying the fast kinetics of the double layer formation.
Electrochemical impedance spectrometry (EIS) is a steady state technique with small potential variation, which is more reliable for measuring the capacitance. The sloping line in the range of low frequency corresponds to the diffusive resistance. In Fig. 7c, the Nyquist plots for all the samples are dominated by nearly vertical trend capacitive lines in the range of low frequency which indicate capacitive behavior according to the equivalent circuit theory and could be attributed to the capacitive properties. However, the sloping line for GOLC-800 is more vertical than that for GOLC-700 and GOLC-600, revealing that GOLC-800 represents low diffusive resistance and high capacitance. In the range of high frequency, no obvious semicircle could be observed, implying that the intrinsic resistance of the active material is relatively small, which agree well with the results in Fig. 7a, b.
Furthermore, the GOLC-800 shows an excellent cycling stability as shown in Fig.7d. The mass specific capacitance still remains 96% of the initial after 5 000 galvanostatic charge/discharge cycles at a current density of 10 A/g.
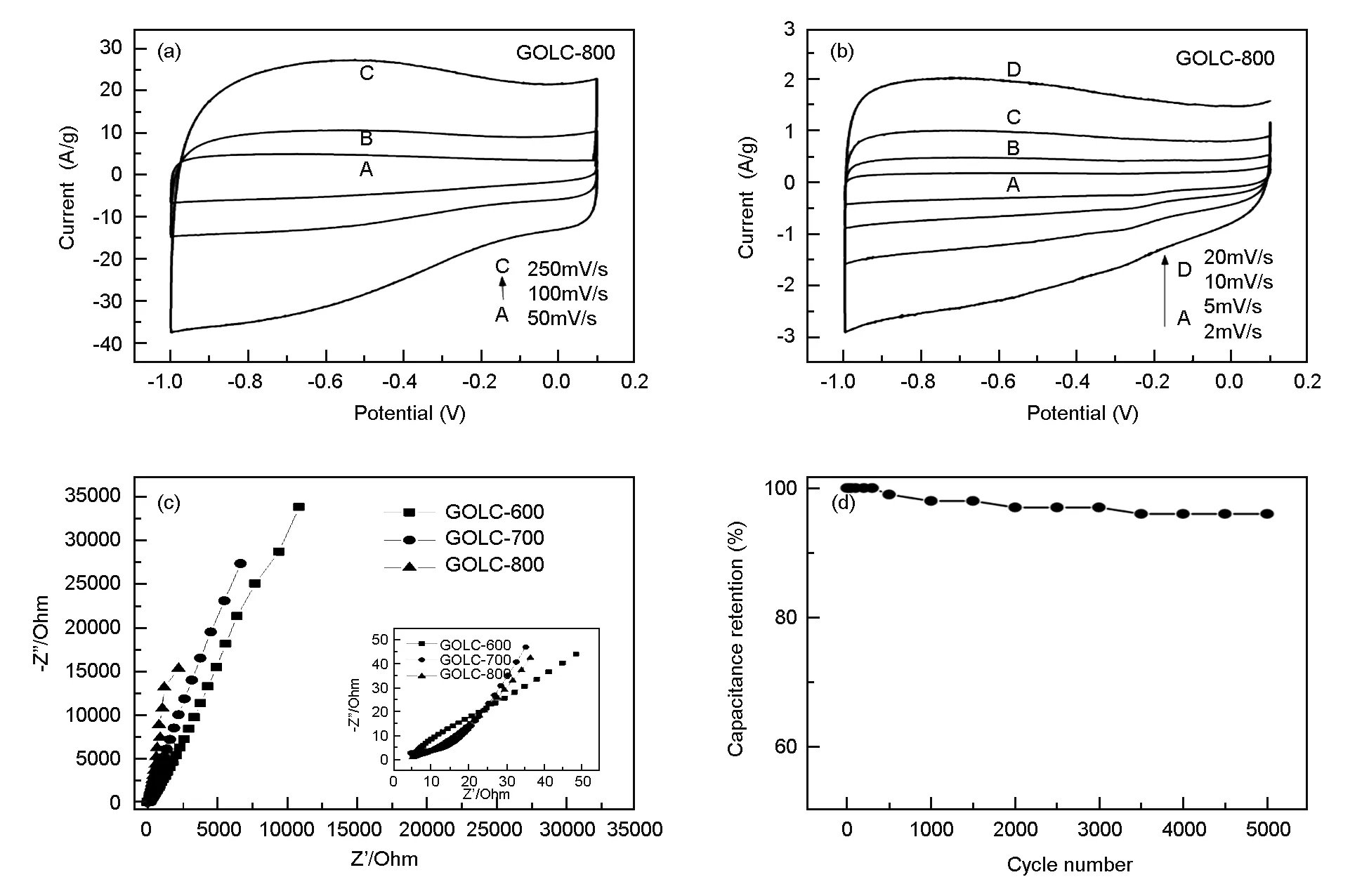
Fig. 7 Measurements of GOLCs’ electrochemical behavior.
Some other carbons synthesized from biomass materials are compared with ours as shown in Table 2. Rice husk[41], firewood[25], bamboo[42], bean dregs[43]and many other biomass materials were applied as precursor. Mass specific capacitance is an important factor that should be considered in practical application. However, for some small electronic devices, effective areal capacitance is very important in supercapacitor applications[44,45]. Compared with other biomass derived carbons, BET surface area and mass specific capacitance of GOLC prepared in this work might not be that high, but the effective areal capacitance is much high, reaching 28.8 μF/cm2at 0.2 A/g.

Table 2 Comparison of carbon synthesized from biomass materials.
4 Conclusions
Green onion leaves derived carbons (GOLCs) were prepared by a simple carbonization without any external additives. Three kinds of GOLCs were prepared at different carbonization temperatures: GOLC-600, GOLC-700 and GOLC-800. All the carbons have a bimodal pore distribution of micropores and mesopores, and GOLC-800 has highest differential pore volume in both micropore and mesopore range. GOLC-800 shows the highest mass specific capacitance and specific surface area among the three.More importantly, the effective areal capacitance of GOLC-800 could reach 28.8 μF /cm2at 0.2 A/g,which is the highest among the samples reported. This is mainly due to the suitable pore distribution GOLC-800 has. In addition, the surface functional groups, especially oxygen groups on the surface of GOLC-800 induce pseudocapacitance, which could contribute to the capacitance. From XRD, EDX, TGA and FAAS analysis, Ca and K could be detected. These original mineral substances in green onion leaves act as pore-generator during the carbonization. The porous carbons derived from green onion leaves are promising electrode materials for supercapacitors, especially for small devices, in which a high areal capacitance of the electrode material is required.
[1] Kandalkar S G, Dhawale D S, Kim C K, et al. Chemical synthesis of cobalt oxide thin film electrode for supercapacitor application[J]. Synthetic Metals, 2010, 160(11): 1299-1302.
[2] Largeot C, Portet C, Chmiola J, et al. Relation between the ion size and pore size for an electric double-layer capacitor[J]. Journal of the American Chemical Society, 2008, 130(9): 2730-2731.
[3] Zhang L L, Zhao X S. Carbon-based materials as supercapacitor electrodes[J]. Chemical Society Reviews, 2009, 38(9): 2520-2531.
[4] Li Y F, Liu Y Z, Zhang W K, et al. Green synthesis of reduced graphene oxide paper using Zn powder forsupercapacitors[J]. Materials Letters, 2015, 157: 273-276.
[5] Miller J R, Simon P. Electrochemical capacitors for energy management[J]. Science Magazine, 2008, 321(5889): 651-652.
[6] Winter M, Brodd R J. What are batteries, fuel cells, and supercapacitors?[J]. Chemical Reviews, 2004, 104(10): 4245-4270.
[7] Pandolfo A G, Hollenkamp A F. Carbon properties and their role in supercapacitors[J]. Journal of Power Sources, 2006, 157(1): 11-27.
[8] Stoller M D, Park S, Zhu Y, et al. Graphene-based ultracapacitors[J]. Nano Letters, 2008, 8(10): 3498-3502.
[9] Wang Y, Shi Z, Huang Y, et al. Supercapacitor devices based on graphene materials[J]. The Journal of Physical Chemistry C, 2009, 113(30): 13103-13107.
[10] Kötz R, Carlen M. Principles and applications of electrochemical capacitors[J]. Electrochimica Acta, 2000, 45(15): 2483-2498.
[11] Wang G, Zhang L, Zhang J. A review of electrode materials for electrochemical supercapacitors[J]. Chemical Society Reviews, 2012, 41(2): 797-828.
[12] Aricò A S, Bruce P, Scrosati B, et al. Nanostructured materials for advanced energy conversion and storage devices[J]. Nature Materials, 2005, 4(5): 366-377.
[13] Choi D, Kumta P N. Nanocrystalline TiN derived by a two-step halide approach for electrochemical capacitors[J]. Journal of the Electrochemical Society, 2006, 153(12): A2298-A2303.
[14] Lee H, Cho M S, Kim I H, et al. RuOx/polypyrrole nanocomposite electrode for electrochemical capacitors[J]. Synthetic Metals, 2010, 160(9): 1055-1059.
[15] Shi H. Activated carbons and double layer capacitance[J]. Electrochimica Acta, 1996, 41(10): 1633-1639.
[16] Elmouwahidi A, Zapata-Benabithe Z, Carrasco-Marín F, et al. Activated carbons from KOH-activation of argan (Argania spinosa) seed shells as supercapacitor electrodes[J]. Bioresource Technology, 2012, 111: 185-190.
[17] Li X, Xing W, Zhuo S, et al. Preparation of capacitor’s electrode from sunflower seed shell[J]. Bioresource Technology, 2011, 102(2): 1118-1123.
[18] Bao L, Li X. Towards textile energy storage from cotton T-shirts[J]. Advanced Materials, 2012, 24(24): 3246-3252.
[19] Balathanigaimani M S, Shim W G, Lee M J, et al. Highly porous electrodes from novel corn grains-based activated carbons for electrical double layer capacitors[J]. Electrochemistry Communications, 2008, 10(6): 868-871.
[21] Rufford T E, Hulicova-Jurcakova D, Fiset E, et al. Double-layer capacitance of waste coffee ground activated carbons in an organic electrolyte[J]. Electrochemistry Communications, 2009, 11(5): 974-977.
[22] Rufford T E, Hulicova-Jurcakova D, Khosla K, et al. Microstructure and electrochemical double-layer capacitance of carbon electrodes prepared by zinc chloride activation of sugar cane bagasse[J]. Journal of Power Sources, 2010, 195(3): 912-918.
[23] Rufford T E, Hulicova-Jurcakova D, Zhu Z, et al. Nanoporous carbon electrode from waste coffee beans for high performance supercapacitors[J]. Electrochemistry Comm-unications, 2008, 10(10): 1594-1597.
[24] Subramanian V, Luo C, Stephan A M, et al. Supercapacitors from activated carbon derived from banana fibers[J]. The Journal of Physical Chemistry C, 2007, 111(20): 7527-7531.
[25] Wu F C, Tseng R L, Hu C C, et al. Effects of pore structure and electrolyte on the capacitive characteristics of steam-and KOH-activated carbons for supercapacitors[J]. Journal of Power Sources, 2005, 144(1): 302-309.
[26] Wang Q, Yan J, Wang Y, et al. Template synthesis of hollow carbon spheres anchored on carbon nanotubes for high rate performance supercapacitors[J]. Carbon, 2013, 52: 209-218.
[28] Biswal M, Banerjee A, Deo M, et al. From dead leaves to high energy density supercapacitors[J]. Energy & Environmental Science, 2013, 6(4): 1249-1259.
[29] Fan Z, Qi D, Xiao Y, et al. One-step synthesis of biomass-derived porous carbon foam for high performance supercapacitors[J]. Materials Letters, 2013, 101: 29-32.
[30] Thomberg T, Kurig H, Jänes A, et al. Mesoporous carbide-derived carbons prepared from different chromium carbides[J]. Microporous and Mesoporous Materials, 2011, 141(1): 88-93.
[31] Wang Y, Xia Y. Electrochemical capacitance characterization of NiO with ordered mesoporous structure synthesized by template SBA-15[J]. Electrochimica Acta, 2006, 51(16): 3223-3227.
[32] Raymundo-Pinero E, Kierzek K, Machnikowski J, et al. Relationship between the nanoporous texture of activated carbons and their capacitance properties in different electrolytes[J]. Carbon, 2006, 44(12): 2498-2507.
[33] Ania C O, Khomenko V, Raymundo-Piero E, et al. The large electrochemical capacitance of microporous doped carbon obtained by using a zeolite template[J]. Advanced Functional Materials, 2007, 17(11): 1828-1836.
[34] Qu T, Guo W, Shen L, et al. Experimental study of biomass pyrolysis based on three major components: hemicellulose, cellulose, and lignin[J]. Industrial & Engineering Chemistry Research, 2011, 50(18): 10424-10433.
[35] Nakagawa K, Mukai S R, Suzuki T, et al. Gas adsorption on activated carbons from PET mixtures with a metal salt[J]. Carbon, 2003, 41(4): 823-831.
[36] Kim C, Lee J W, Kim J H, et al. Feasibility of bamboo-based activated carbons for an electrochemical supercapacitor electrode[J]. Korean Journal of Chemical Engineering, 2006, 23(4): 592-594.
[37] Hao G P, Mi J, Li D, et al. A comparative study of nitrogen-doped hierarchical porous carbon monoliths as electrodes for supercapacitors[J]. New Carbon Materials, 2011, 26: 197-203.
[38] Chen M D, Kang X Y, Wumaier T, et al. Preparation of activated carbon from cotton stalk and its application in supercapacitor[J]. Solid State Electrochem, 2013, 17: 1005-1012.
[39] Jang Y, Jo J, Choi Y M, et al. Activated carbon nanocomposite electrodes for high performance supercapacitors[J]. Electrochimica Acta, 2013, 102: 240-245.
[40] Basri N H, Dolah B N M. Physical and electrochemical properties of supercapacitor electrodes derived from carbon nanotube and biomass carbon[J]. Int J Electrochem Sci, 2013, 8: 257-273.
[41] Guo Y, Qi J, Jiang Y, et al. Performance of electrical double layer capacitors with porous carbons derived from rice husk[J]. Materials Chemistry and Physics, 2003, 80(3): 704-709.
[42] Kim Y J, Lee B J, Suezaki H, et al. Preparation and characterization of bamboo-based activated carbons as electrode materials for electric double layer capacitors[J]. Carbon, 2006, 44(8): 1592-1595.
[43] Ruan C, Ai K, Lu L. Biomass-derived carbon materials for high-performance supercapacitor electrodes[J]. RSC Advances, 2014, 4(58): 30887-30895.
[44] McDonough J R, Choi J W, Yang Y, et al. Carbon nanofiber supercapacitors with large areal capacitances[J]. Applied Physics Letters, 2009, 95(24): 243109.
[45] Zheng G, Hu L, Wu H, et al. Paper supercapacitors by a solvent-free drawing method[J]. Energy Environ Sci, 2011, 4(9): 3368-3373.
Porous carbons produced by the pyrolysis of green onion leaves and their capacitive behavior
YU Jing1, GAO Li-zhen1, LI Xue-lian1, WU Chao2, GAO Li-li1,3, LI Chang-ming2
(1.SchoolofEnvironmentalScienceandEngineering,TaiyuanUniversityofTechnology,Taiyuan030024,China;2.InstituteforCleanEnergy&AdvancedMaterials,SouthwestUniversity,Chongqing400715,China;3.Labofgreenenergymaterialsandstoragesystems,TaiyuanUniversityofTechnology,Taiyuan030024,China)
Porous carbons were prepared by the simple carbonization of green onion leaves at temperatures from 600 to 800 ℃ and used as the electrode materials of supercapacitors. SEM, FESEM, EDX, AAS, XRD, TGA and nitrogen adsorption were used to characterize their morphology, pore structure and surface elemental composition. Cyclic voltammetry, electrochemical impedance spectroscopy and galvanostatic charge/discharge were carried out to evaluate their specific capacitance, resistance and cycling life. Results showed that the initial mineral elements present in the leaves such as calcium (Ca) and potassium (K) play an activating role during the carbonization. All samples have a bimodal pore distribution of micropores (mainly 0.6-1.2 nm) and mesopores (mainly 3-5 nm). The carbon prepared at 800 ℃ had the highest surface area of 551.7 m2/g, a specific capacitance of 158.6 F/g at 0.2 A/g and an effective areal capacitance of 28.8 μF/cm2. The effective areal capacitance of the carbon prepared at 800 ℃ is higher than of most porous carbons reported in the literature, which is ascribed to its pore size distribution that favors ion access to its pores.
Porous carbon; Green onion leaves; One-step carbonization and activation; Effective areal capacitance
GAO Li-li, Post-doctor, Lecturer. E-mail: gaolili@tyut.edu.cn
山西省青年科技研究基金资助项目(2013021011-3);山西省留学人员科研基金资助项目(2013-041);太原理工大学人才引进资助项目(tyut-rc201110a).
高丽丽,博士后,讲师. E-mail:gaolili@tyut.edu.cn
1007-8827(2016)05-0475-10
X712
A
10.1016/S1872-5805(16)60026-4
Receiveddate: 2016-06-10;Reviseddate: 2016-07-28
Foundation: Shanxi Province Science Foundation for Youths (2013021011-3); Shanxi Scholarship Council of China (2013-041); Project for Importing Talent of Taiyuan University of Technology(tyut-rc201110a).
English edition available online ScienceDirect ( http:www.sciencedirect.comsciencejournal18725805 ).

