PAN纤维炭化过程致密化机理研究
马全胜, 高爱君, 童元建, 张佐光
(1.北京航空航天大学 材料科学与工程学院,北京100191;2.北京化工大学 国家碳纤维工程技术研究中心,北京100029)
PAN纤维炭化过程致密化机理研究
马全胜1, 高爱君2, 童元建2, 张佐光1
(1.北京航空航天大学 材料科学与工程学院,北京100191;2.北京化工大学 国家碳纤维工程技术研究中心,北京100029)
研究了PAN纤维在不同炭化温度下(900~1 400 ℃)的致密性变化规律及机理。研究表明,随炭化温度升高,纤维密度出现增大-减小-增大-减小的变化规律,在炭化温谱[900,T]与[T,0]下密度具有相同的变化规律,但出现极值的温度不同,而两种温谱下密度随元素含量的变化则完全一致。在炭化温谱[900,T]下,1 050 ℃之前以缩聚反应为主,小分子气体快速逸出,密度快速增大;纤维在1 050 ℃左右出现最大失重速率,石墨微晶片层增长速度变缓,氮气释放量最大;1 050 ℃之后以裂解反应为主,元素大量裂解逸出使纤维密度迅速下降;1 250 ℃之后纤维中只有氮气逸出,石墨化转变与氮元素快速逸出的竞争反应使得密度先增后降,在1 350 ℃出现极大值。
炭纤维; 致密化; 炭化; 缩聚; 裂解
1 Introduction
Polyacrylonitrile (PAN)-based carbon fibers are the most attractive as reinforcement for advanced composites[1-7], in the aerospace and national defense industry. Carbonization is a necessary step to convert PAN fibers to high-strength carbon fibers[8, 9]and its importance is the same as stabilization[10, 11]. Carbonization is typically carried out in an inert atmosphere at a high temperature (900-1 600 ℃). During this process, non-carbon elements are released from the high-molecular-weight polymers to enrich carbon and the density of the fibers increases. In carbonization, stabilized fibers are subjected to heat treatment with an increasing temperature profile within a total residence time of only a few minutes[12-16]. To avoid thermal shock, low-temperature heat treatment usually between 300 and 800 ℃ is applied prior to carbonization.
Densification of carbon fibers is very important to obtain high performance product. In this work, we prepared PAN pre-carbonized fibers, which were then carbonized in a high temperature carbonization furnace in the temperature range of 900-1 400 ℃. Various techniques were used to analyze the densification mechanism in relation to the chemical compositions, structures, and the reaction types. The results of our studies can provide guidelines for choosing more reasonable temperature schemes of carbonization to get highly-densified carbon fibers.
2 Experimental
Each tow of PAN fibers used to prepare the fiber samples contained 12 000 strands of monofilament (QF0901, Jilin Qifeng Chemical Fiber Co., LTD, China). Before high temperature heat treatment, PAN fibers were pretreated by passing first through a stabilization furnace and then a pre-carbonization furnace s to get the “pre-carbonized” fibers. The stabilization furnace was divided into six zones (2 m per zone) set at different temperatures (200, 218, 223, 237, 254, and 257 ℃). The pre-carbonization furnace was divided into three zones (0.3 m per zone) set at 350, 450, and 680 ℃. The pre-carbonized fibers were then heat treated at a high temperature carbonization furnace with two zones (0.3 m per zone). Two temperature schemes denoted as [900, T] and [T, 0] for high-temperature carbonization were used. [900, T] means that the first zone was fixed at 900°C and the second zone with variable experimental temperatures. [T, 0] means that the temperature of the first zone was variable and the heating device of the second zone was switched off.
Elemental analysis was carried out with an Elementar Vario MICRO CUBE (Germany) elemental analyzer. The carbon, hydrogen, and nitrogen contents were analyzed in the carbonization.
TG-MS was carried out in an Ar atmosphere using a Netzsch STA449C system at a heating rate of 30 ℃/min from 30 to 1 400 ℃.
Changes in crystal structure that occurred during heat treatment were determined by using a Philips X’Pert PRO MDP diffractometer (operated at 40 kV and 40 mA) with Ni-filtered CuKαradiation. Data were collected over the 2θrange from 10° to 90° at a scan rate of 1(°)/min. The apparent crystallite size was calculated from the fully corrected and resolved peak profiles. The crystallite size was calculated from the Scherrer equation (Eq.1) using the diffraction peak position and the full widths at half maximum (FWHM):
L(n/k)=Kλ/Bcosθ
(1)
whereθis the diffraction peak position of the (nlk) plane,λ=0.154 06 nm is the wavelength of the X-rays,Bis the FWHM the peak in radians of andKis the Scherer geometric or shape factor. The crystallite correlation length (La) along the fiber axis was determined by the (110) diffraction peak of the meridian scan. The shape factorKis 1.84 forLa.
Fiber tension was measured on-line during the high temperature carbonization by a tension meter (Schmidt DTMX-2000, German).
The density was measured at 25 ℃ in a density-gradient column (LLORD, UK), prepared with a mixture of 1,2-dibromoethane and carbon tetrachloride, which gave a density gradient from about 1.65 to 1.85 g/cm3from the top to the bottom.
3 Results and discussion
3.1 TG curves of the pre-carbonized fibers in He atmosphere
The TG curve of the pre-carbonized fibers at a heating rate of 30 ℃/min is shown in Fig. 1.
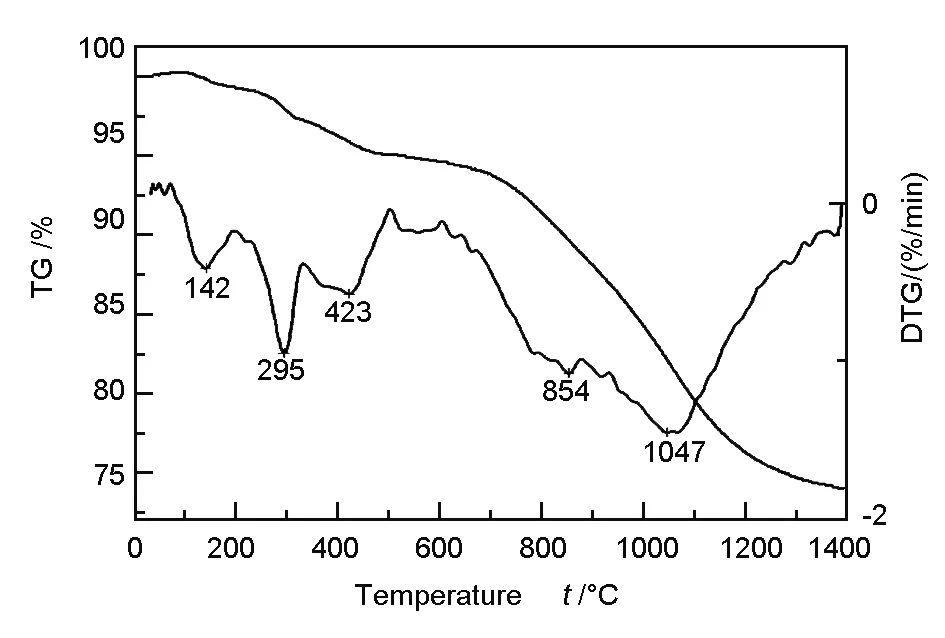
Fig. 1 TG and DTG curves of the pre-carbonized fibers.
The mass of the fibers decreases with temperature. Below 700 ℃, only a small amount of weight loss is observed because the fibers had been treated at 680 ℃ in the pre-carbonization. Between 700 ℃ and 1 050 ℃, the amount of weight loss increases appreciably, owing to the release of non-carbon elements from chemical reactions occurring at elevated temperature. Above 1 050 ℃, the weight loss in the TG curve begins to level off, which is indicative of a low content of non-carbon elements. The total weight loss of the fibers during heat treatment was 26.2%. In the derivative themogravimetric analysis (DTG) curve in Fig. 1, two peaks were detected, which represented two discrete weight-loss temperature regimes: 700-900 ℃ and 900-1 400 ℃, corresponding to weight losses of 6.2% and 14.9% respectively.
3.2 Elemental analysis
In the pre-carbonized fibers, there was a large amount of nitrogen, hydrogen, and oxygen, the content of which changed during the high-temperature carbonization, as shown in Fig. 2. Under the [900, T] and [T, 0] temperature schemes, the carbon and nitrogen contents varied in a similar manner, although their changing rate was faster under the [900, T] temperature scheme than the [T, 0] one mainly because the carbonization at low temperature was prolonged. As mentioned in the previous section, there was a mass loss at 700-900 ℃. For the [900, T] temperature scheme, there was a significant drop in nitrogen content above 900 ℃ with a maximum releasing rate occurring at around 1 050 ℃. Below 900 ℃, the increase in the relative carbon content was slow and the release of nitrogen was insignificant. However, above 900 ℃, the relative carbon content in the fibers increased markedly owing to the rapid release of nitrogen.
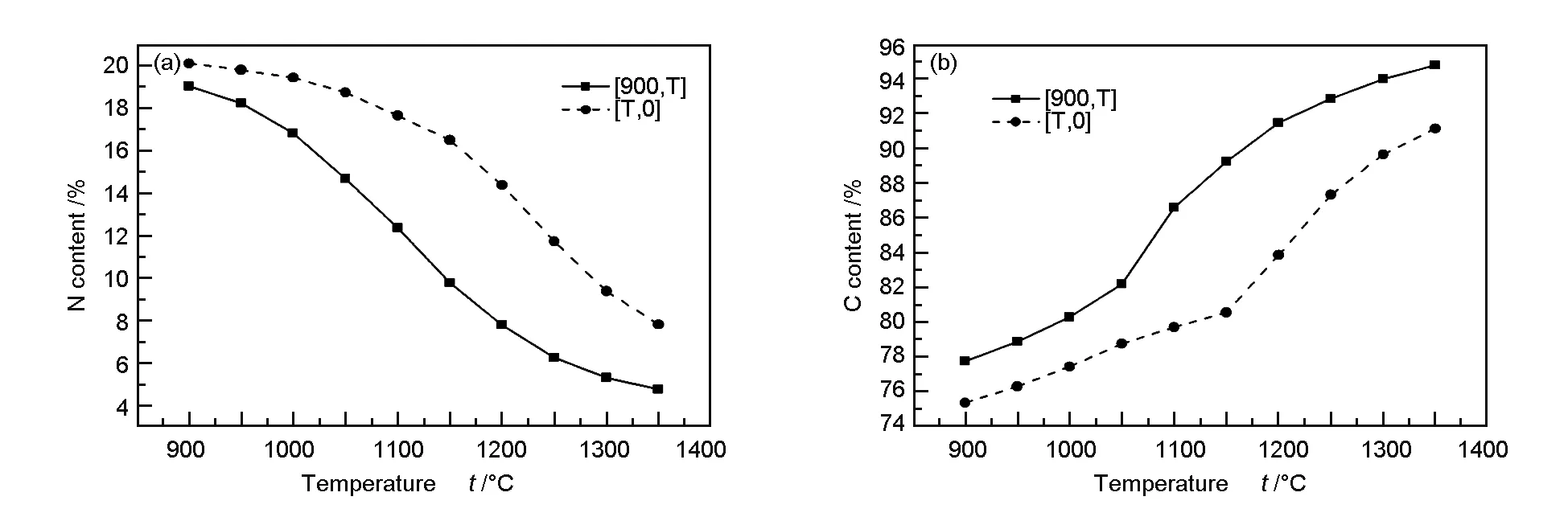
Fig. 2 Relative contents of elemental (a) nitrogen and (b) carbon as a function of temperature.
TG-MS tests were conducted from 30 to 1 400 ℃ with a rate of 30 ℃/min and the results are shown in Fig. 3. Mass number 2 corresponds to H2, 15 to CH4, 16 to CH4/NH3, 17 to NH3, 18 to H2O, 26 and 27 to HCN, 28 to N2/CO and 44 to CO2. With increasing temperature, nitrogen was released from the fibers in the form of NH3, HCN and N2. Below 900 ℃, nitrogen was released more slowly in the form of NH3and HCN. When the temperature reached 900 ℃, the maximum release corresponded to N2. The rate of N2release correlates with the decrease in the elemental nitrogen content in the fibers. At around 1 050 ℃, the release rate of N2(Fig.3) and rate of decrease in elemental nitrogen content (Fig.2) in the fibers both reached their peak, which corresponded to the maximum weight-loss rate of the fibers(Fig.1) .
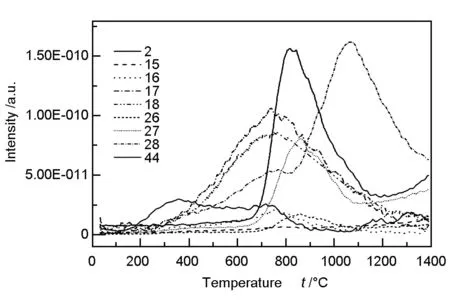
Fig. 3 Release of small gas molecules as a function of temperature by TG-MS test.
3.3 X-ray diffraction
The values ofLa increased as a function of heat-treatment temperature, as shown in Fig. 4.
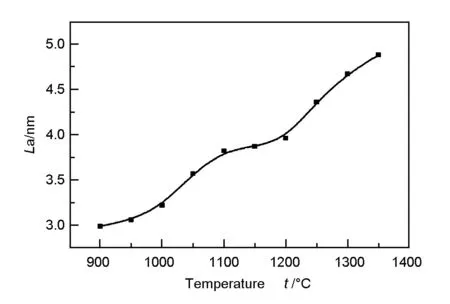
Fig. 4 La as a function of temperature.
At low temperatures,La increased more rapidly because the molecules were cross-linked by condensation reaction and small graphite crystallites were transformed into largenes. At around 1 050 ℃, pyrolysis reactions begin to dominate while the influence of the condensation reaction was reduced, leading to a slow increasing rate ofLa with temperature between 1 050 and about 1 200 ℃. However, above 1 200 ℃, the high temperature facilitated graphitization andLa increased sharply.
3.4 Fiber tension
The fiber tension during high-temperature treatment is shown in Fig. 5. The fiber tension initially increased with increasing temperature, and then levelled off. For the temperature scheme [900, T], the fiber tension increased to a maximum value at 1 200 ℃, whereas for [T, 0], the maximum was detected at 1 300 ℃. The tension and fiber deformation capacity varied according to the type of reaction that was dominant. At low temperatures, the condensation reaction rate was progressively intensified with increasing temperature and significant fiber contraction resulted in the increase of tension rapidly. At a critical temperature, the tension stopped increasing because the condensation reaction was slowed and the fiber contraction in the pyrolysis reaction was small.
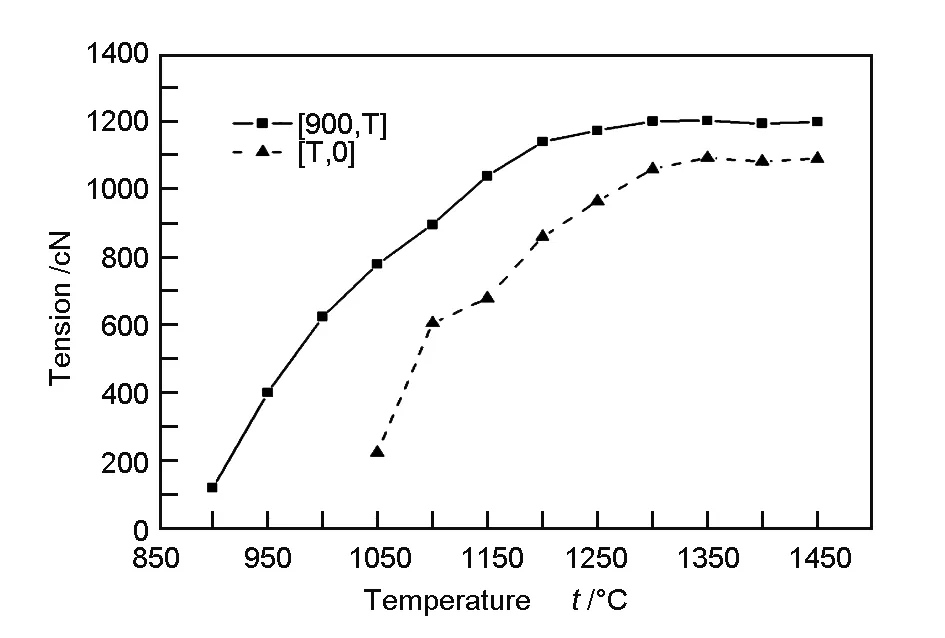
Fig. 5 Fiber tension as a function of temperature.
3.5 Density of fibers
The bulk density of the pre-carbonized fibers was 1.63 g/cm3. The bulk density of the fibers after high-temperature carbonization is shown in Fig. 6. After the heat treatment between 900 and 1 400 ℃, the bulk density became much higher than that of the pre-carbonized fibers owing to mainly the condensation reactions. For [900, T], the bulk density rapidly increased with increasing temperature and reached a maximum value of 1.83 g/cm3at 1 000 ℃. It decreased to 1.78 g/cm3at 1 050 ℃, but then slowly increased again as the temperature increased, reaching another peak of 1.79 g/cm3at 1 250 ℃. The density for [T, 0] and [900, T] varied in the same manner, but the characteristic temperature corresponding to the density maximum was 100 ℃ higher in the former than that in the latter.
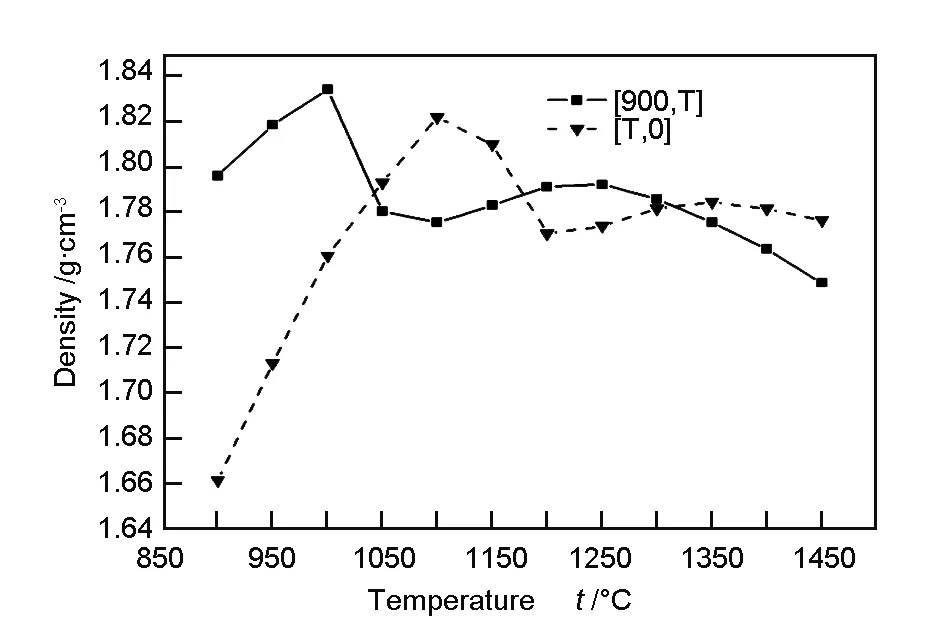
Fig. 6 Fiber density as a function of temperature.
3.6 Densification mechanism
The variation of the fiber density with increasing the elemental contents of nitrogen and carbon is shown in Fig. 7.
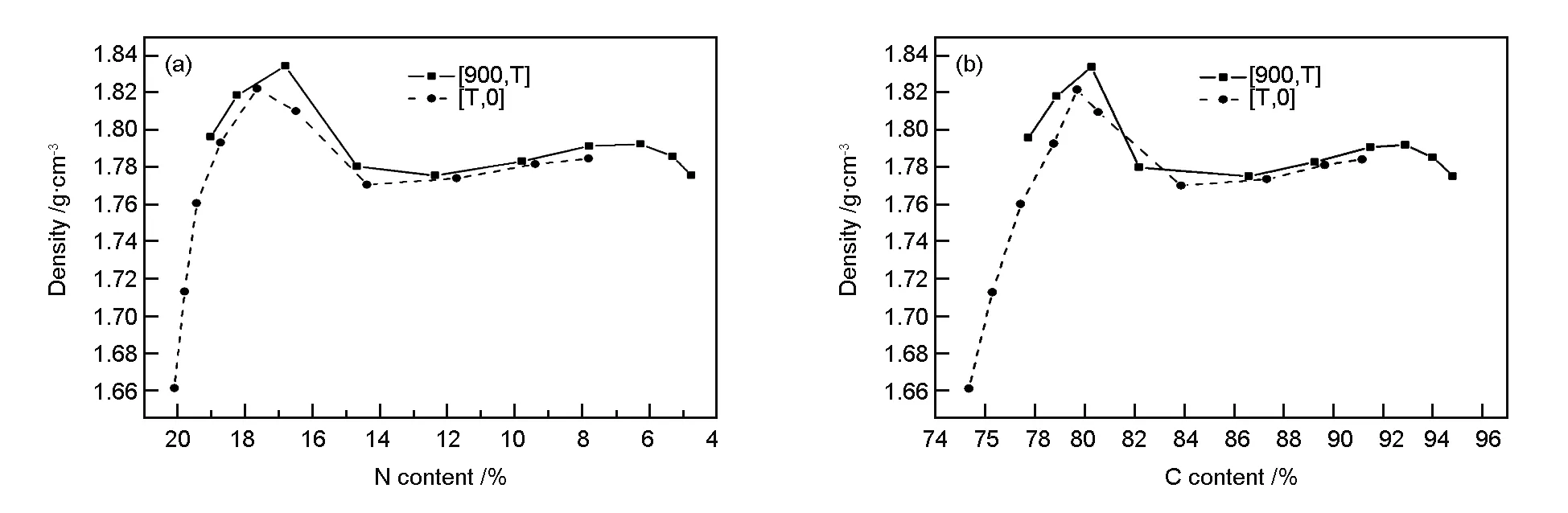
Fig. 7 Density as a function of elemental contents: (a) nitrogen and (b) carbon.
The trends are similar for both temperature schemes. This indicates that the temperature influences the density by inducing chemical reactions that alter the elemental compositions. With the elevation of carbonization temperature, the concentrations of nitrogen, hydrogen, and oxygen, as well as the rate of condensation reactions between molecules are gradually reduced. The pyrolysis reactions become dominant in the fibers and then produce a large number of holes left by releasing of small molecules, causing the density to decrease with increasing carbonization temperature. Meanwhile, the graphitization in which amorphous carbon is transformed into graphitic carbon is activated, which increases the extent of ordering of graphene layers and thereby the density pf carbon fibers. With a further increase in carbonization temperature, a small amount of residual nitrogen undergoes a rapid pyrolysis[17], leading to a gradual reduction in density.
4 Conclusions
The density evolution of carbon fibers during high-temperature carbonization between 900 ℃ and 1 400 ℃ was investigated by compositional and structural analysis. Two maximum values of fiber density were observed at different temperatures, depending on the temperature schemes applied. For a carbonization temperature scheme of [900, T], a maximum rate of fiber weight loss occurred at around 1 050 ℃. Above 1 250 ℃, only N2escaped from the fibers. The influences of three major reactions were identified in the carbonization: condensation, pyrolysis and graphitization. At low temperatures, the condensation reactions were dominant because of the large amounts of heteroatoms of nitrogen, hydrogen, and oxygen in the fibers, leading to an increase of the fiber density. A further increase of the temperature caused the heteroatom content and rate of condensation to reduce, while pyrolysis reactions occurred, thereby reducing the fiber density. Graphitization and pyrolysis to release nitrogen were accelerated at high temperatures. Competition between these two reactions resulted in the maximum fiber density with increasing temperature.
如我在《物体在水中的沉浮》一课的教学中,物体的沉浮和它们的大小、轻重有关吗?这一问题的提出,我先引用猜一猜的方法,然后让学生在推理的基础上进行实验,最后学生通过亲手实验,将实验的结果与猜测进行比较,激发了学生的学习欲望。
[1] REN Gui-zhi, CHEN Cong-jie, DENG Li-hui, et al. Microstructural heterogeneity on the cylindrical surface of carbon fibers analyzed by Raman spectroscopy[J]. New Carbon Materials, 2015, 30(5): 476-480.
[2] Li M, Gu Y Z, Liu Y N, et al. Interfacial improvement of carbon fiber/epoxy composites using a simple process for depositing commercially functionalized carbon nanotuves on the fibers[J]. Carbon, 2013, 52: 109-121.
[3] Li W, Long D H, Miyawaki J, et al. Structural feature of polyacrylonitrile-based carbon fibers[J]. Journal of Materials and Science, 2012, 47(2): 919-928.
[4] ZHAO Yu-hua, LI Qi-feng, WANG Jun-wei, et al. Preparation and properties of carbon fiber/polyether polyurethane composites[J]. New Carbon Materials, 2014, 29(6): 454-453.
[5] Tong Y J, Wang X Q, Su H, et al. Oxidation kinetics of polyacrylonitrile-based carbon fibers in air and the effect on their tensile properties[J]. Corrosion Science, 2011, 50(8): 2484-2488.
[6] Wu G P, Li D H, Yang Y, et al. Microvoid evolution in carbon fibers during graphitization for the preparation of carbon/carbon composites[J]. New Carbon Materials, 2014, 29(1): 41-46.
[7] Gao A J, Gu Y Z, Wu Q, et al. Influence of processing temperature on interfacial behavior of HKT800 carbon fiber with BMI and epoxy matrices[J]. Chinese Journal of Aeronautics, 2015, 28(4): 1255-1262.
[8] Mittal J, Konno H, Inagaki M, et al. Denitrogenation behavior and tensile strength increase during carbonization of stabilized PAN fibers[J]. Carbon, 1998, 36: 1327-1330.
[9] Ko T H, Li C H. The influence of pre-carbonization on the properties of pan-based carbon fibers developed by two-stage continuous carbonization and air oxidation[J]. Polymer Composites, 1995, 16: 224-232.
[10] Kalashinik A T. The role of different factors in creation of the structure of stalilized acrylic fibers[J]. Fibre Chemistry, 2002, 34: 11-17.
[11] Gupta A, Harrison I R. New aspects in the oxidative stabilization of PAN-base carbon fibers[J]. Carbon, 1996, 34: 1427-1445.
[12] Liu J, Wang P H, Li R Y. Continuous carbonization of polyacrylonitrile based oxidized fibers: aspects on mechanical properties and morphological structure[J]. Journal of Applied Polymer Science, 1994, 52(7): 945-950.
[13] Li L Y, Huang Q Z, Zhang H B. Study on the carbonization of polyacrylonitrile-based preoxidized fibres[J]. Materials Science and Engineering or Powder Metallargy, 2000, 5(1): 69-74.
[14] Watt W. Nitrogen evolution during the pyrolysis of polyacrylonitrile[J]. Nature: Physical Science, 1972, 235: 10-11.
[15] Ko T, Day T, Perng J. The characterization of PAN-based carbon fibers developed by two stage continuous carbonization[J]. Carbon, 1993, 31(5): 765-771.
[16] Ko T. The influence of pyrolysison physical properties and microstructure of modified PAN fiber during carbonization[J]. Journal of Applied Polymer Science, 1991, 43(3): 589-600.
[17] Gao A J, Su C J, Luo S, et al. Densification mechanism of polyacrylonitrile-based carbon fiber during heat treatment[J]. Journal of Physics and Chemistry of Solids, 2011, 72: 1159-1164.
The densification mechanism of polyacrylonitrile carbon fibers during carbonization
MA Quan-sheng1, GAO Ai-jun2, TONG Yuan-jian2, ZHANG Zuo-guang1
(1.SchoolofMaterialsScienceandEngineering,BeihangUniversity,Beijing100191,China;2.NationalCarbonFiberEngineeringResearchCenter,BeijingUniversityofChemicalTechnology,Beijing100029,China)
The densification mechanism of polyacrylonitrile carbon fibers during carbonization from 900 to 1 400 ℃ was investigated. The density, elemental composition, microstructure and weight loss of the fibers, as well as the gases released during the process were analyzed to reveal the mechanism. Results indicated that the density of the fibers was strongly dependent on the carbonization temperature and reactions involved indifferent temperature regimes. Condensation, pyrolysis and graphitization reactions were dominant at low (<1 050 ℃), medium (1 050-1 250 ℃) and high (>1 250 ℃) temperatures, respectively. The amount of small molecule gas released and the fiber density both increased rapidly with temperature when condensation reactions dominated. The fiber density decreased as a result of nitrogen release when pyrolysis reactions dominated above 1050 °C while the fiber density increased due to the growth and increase in order of the graphene layers during graphitization. The two fiber density maxima found with increasing carbonization temperature were attributed to the different reactions.
Carbon fiber; Densification; Carbonization; Condensation; Pyrolysis
National High Technology Research and Development Program of China(2015AA03A202).
GAO Ai-jun, Ph.D, Lecturer. E-mail: bhgaoaijun@163.com
国家863计划(2015AA03A202).
高爱君,讲师.E-mail: bhgaoaijun@163.com
马全胜,博士研究生.E-mail: terrymark@163.com
1007-8827(2016)05-0550-05
TQ342+.74
A
Authorintroduction: MA Quan-sheng, Ph.D, Candidate. E-mail: terrymark@163.com
10.1016/S1872-5805(16)60031-8
Receiveddate: 2016-08-15;Reviseddate: 2016-10-12
English edition available online ScienceDirect (http:www.sciencedirect.comsciencejournal18725805).

