柑橘衰退病毒基因p23 RNAi载体的构建及转化
李 芳,邓子牛,赵 亚,李大志,戴素明
(湖南农业大学园艺园林学院/国家柑橘改良中心长沙分中心,长沙 410128)
柑橘衰退病毒基因p23 RNAi载体的构建及转化
李 芳,邓子牛,赵 亚,李大志,戴素明
(湖南农业大学园艺园林学院/国家柑橘改良中心长沙分中心,长沙 410128)
【目的】构建柑橘衰退病毒(Citrus tristeza virus,CTV)含p23的RNAi载体,以获得具有抗性的柑橘转基因植株。【方法】基于转化病毒基因介导抗性,根据NCBI公布的CTV基因组序列,查找p23保守序列,设计并克隆两条不同长度的片段。对两条片段和植物表达载体pBI 121进行双酶切和连接来构建RNAi载体。初步预测所构建的载体发生RNAi抗病毒的可行性。利用农杆菌介导的瞬时表达技术将含RNAi载体的农杆菌注射入CTV指示植物墨西哥莱蒙的叶片,利用GUS组织化学染色法观察叶片中载体发生瞬时表达的情况。发生瞬时表达的叶片接种CTV T36基因型,利用酶联免疫反应(ELISA)检测病毒含量。同时,提取叶片的RNA并反转录为cDNA,利用实时荧光定量PCR(q-PCR)检测CTV p20,通过该基因的表达量反映叶片中的病毒含量。通过农杆菌介导的遗传转化将RNAi载体转入大红甜橙实生苗上胚轴节间茎段,抗生素筛选得到的芽嫁接至枳橙实生试管苗。提取大红甜橙叶片的DNA,通过PCR扩增确定其是否为转基因阳性;目的基因检测为阳性的植株二次嫁接至温室保存的酸橙实生苗;根据插入的p23基因序列设计q-PCR引物,检测转基因植株中p23的表达情况。取 CTV T36基因型寄主的带皮芽,用腹接法接种大红甜橙转基因植株。取接种后新萌发枝梢上的叶片,用检测瞬时表达叶片同样的方法分析植株的抗病性。对于第1次接种后未检测出病毒感染的植株,进行第2次接种并检测分析。【结果】克隆得到CTV p23 513 bp的长片段和291 bp的短片段,与载体pBI121连接后成功构建含发夹结构的来自病原且能靶向目的基因的RNAi载体,命名为p23-RNAi。注射p23-RNAi的墨西哥莱蒙叶片经GUS染色后能够产生蓝色斑点,表明农杆菌p23-RNAi可以在叶片中发生瞬时表达;接种CTV后第15和30天,瞬时表达p23-RNAi的墨西哥莱蒙叶片ELISA检测结果均为阴性,同时q-PCR检测结果显示其CTV p20的积累水平和增加速度明显低于对照植株,表明瞬时表达的p23-RNAi在一定时间内可以对CTV的侵染产生抑制。p23-RNAi经农杆菌介导遗传转化大红甜橙获得抗性芽,通过普通PCR的扩增结果证明得到7个转基因植株;q-PCR检测结果进一步表明7个转基因植株间p23的含量呈现一定差异,植株E的含量最高,其次是C、F、H、A、B和G。接种CTV后,p20的表达量在7个转基因植株间也表现出一定差异,表达量最高的是植株A,其次是G、F、E、B、H、C,且与对照植株相比,呈现不同程度的抗病性。转基因植株对病毒的抗性与外源基因的表达水平没有相关性,外源基因表达水平最高的植株E并没有表现强的CTV抗性。经过两次病毒接种,转基因植株C在接种后具有完全抗性。【结论】p23-RNAi载体能引起植物抗柑橘衰退病毒;瞬时表达技术可快速鉴定RNAi载体的抗病性,有利于筛选高效率的RNAi载体。
柑橘衰退病毒;p23;RNAi;大红甜橙;瞬时表达;遗传转化
0 引言
【研究意义】由柑橘衰退病毒(Citrus tristeza virus,CTV)引起的柑橘衰退病严重影响柑橘产业发展。目前为止,该病害难以防治。田间种植柑橘无病毒苗木受虫媒传播影响,很难做到持久性的无毒化。弱毒株交叉保护作用(MSCP)受到株系专化性、寄主、环境等因素的影响[1],而在柑橘生产应用中受到限制。因此,寻找高效的CTV防治方法对于柑橘产业健康发展具有重要意义。【前人研究进展】RNA interference(RNAi)系统是植物天然的病毒防御系统。利用RNAi赋予植物对病毒抗性的原理,可人为将与病毒同源的dsRNA导入植物体内,使其引发植物体内的RNAi机制,阻止病毒的复制扩散。这种抗性途径具有抗病性强、抗性持久、生物安全性高等特点,已成为植物抗病毒基因工程研究中的一种高效抗性手段[2]。SOLER等[3]将CTV的p23、p20和p25基因片段串联构建发夹结构RNAi载体,获得抗CTV的转基因柑橘植株;CHENG等[4]通过两个不同长度的p20基因片段构建发夹结构RNAi载体,也获得抗CTV的转基因柑橘植株。与SOLER等[3]构建的RNAi载体不同,CHENG等[4]选择病原序列作为内含子。VOINNET等[5]报道来自病原的内含子序列能提高RNAi效率。【本研究切入点】已获得的转基因柑橘植株均未达到完全抗性,利用RNAi获得柑橘对CTV的抗性有待挖掘更高效的RNAi载体。p23是柑橘衰退病毒的沉默抑制子之一[6-7],是重要的致病因子[8-9],目前,以p23两个不同长度的片段构建发夹结构RNAi载体尚为空白。RNAi载体产生的抗CTV作用依赖柑橘稳定遗传转化方法进行鉴定,而该方法存在效率低、周期长等困难[10],使得高效率RNAi载体的筛选很难进行。【拟解决的关键问题】通过选择合适的插入载体和酶切位点,构建以两个不同长度的p23基因片段形成发夹结构的RNAi载体。对所构建的载体通过瞬时表达技术和稳定遗传转化技术鉴定其抗病作用,为筛选高效率RNAi载体提供参考。
1 材料与方法
试验于2009—2014年在国家柑橘改良中心长沙分中心完成。
1.1 试验材料
供试材料包括大红甜橙 [Citrus sinensis (L.) Osb.]和枳橙 [C. sinensis (L.) Osb.×Poncirus trifoliate (L.)Raf] 种子,1年生酸橙(C. aurantium L.)实生苗,2年生墨西哥莱蒙[C. aurantifolia (Christm.) Swingle;CTV指示植物],CTV毒源为感病3年的冰糖橙(基因型为T36)植株。所有材料均由国家柑橘改良中心长沙分中心提供并保存于温室。
植物表达载体pBI 121,转化所用的农杆菌菌株EHA 105由国家柑橘改良中心长沙分中心提供,载体pGM-T购自天根生化科技(北京)有限公司,所用引物合成及测序均由上海生工生物工程有限公司完成。
1.2 试验方法
1.2.1 柑橘衰退病p23 RNAi载体的构建 根据GenBank公布的CTV基因组的序列,设计用于p23 RNAi载体构建的长片段和短片段的上、下游引物(表1)。以感染CTV的冰糖橙叶片提取的核酸为模板,进行RT-PCR扩增。将扩增产物纯化回收,连接克隆载体pGM-T,测序确认后分别命名为pGM-L和pGM-S。提取两个载体的质粒,用内切酶Bam HI 和Xba I双酶切pGM-L和植物表达载体pBI 121,温度为37℃,回收后用T-4 DNA连接酶连接得到中间载体命名为pBI-L。用内切酶Bam HI和Sma I双酶切pBI-L和pGM-S,温度为30℃,回收后连接得到RNAi载体,长片段L和短片段S置于CaMV 35 s启动子的下游,gus基因的上游(图1)。长片段L为反向插入载体,短片段S为正向插入载体,在转录后两者有部分序列可以发生碱基互补配对,形成发夹结构的臂,不能发生互补的长片段的部分序列片段则形成发夹结构的环,充当内含子结构。为检测p23的长片段和短片段是否正确插入目的载体,提取质粒并纯化,分别用限制性内切酶酶切鉴定,并且进行测序验证,得到RNAi载体p23-RNAi。用热激法将p23-RNAi导入农杆菌EHA105。

表1 用于RNAi载体构建的长片段L和短片段S引物Table1 The primers of segments L and S used for RNAi vector construction
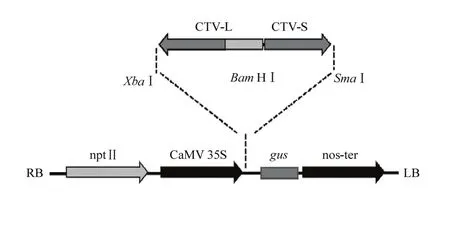
图1 CTV p23片段连接入p23-RNAiFig. 1 p23 fragments of CTV ligated into plasmid p23-RNAi
1.2.2 p23-RNAi的瞬时表达 以农杆菌EHA105为阴性对照,按照瞬时表达体系(暂未公布)将p23-RNAi用注射缓冲液重悬后在28℃培养基静置培养2 h,用1 mL无菌注射器注射入墨西哥莱蒙的叶片。叶片用GUS组织化学染色法[11]染色观察。
1.2.3 转基因植株的获得 p23-RNAi转化大红甜橙参照敖小平等[12]的方法获得抗性芽,抗性芽嫁接至试管中25 d苗龄的枳橙实生苗。按照CTAB法提取叶片的DNA为模板,用载体上长片段L的引物进行PCR扩增,筛选的转基因植株嫁接在酸橙实生苗。根据载体的发夹结构的环状部分设计引物q-s23-h(F:CACACTCCTATTATTCTCG;R:ATGAATCCCTCGTTATCG),以CsEF1α(F:TTGGACAAGCTCAAGGC TGAACG;R:ATGGCCAGGAGCATCAATGACAGT)为内参基因,用q-PCR鉴定转基因植株。
1.2.4 病毒接种 为检测瞬时表达的p23-RNAi对病毒的抗性反应,摘取感染CTV的冰糖橙叶片用打孔器打出带有主脉的圆片,瞬时表达p23-RNAi的墨西哥莱蒙叶片打出的圆片丢弃,带毒圆片与墨西哥莱蒙叶片的主脉对合后在叶片两面用胶带粘好[13]。瞬时表达农杆菌EHA105的墨西哥莱蒙叶片为对照。
待转基因大红甜橙长至30 cm左右时接种CTV。取感染CTV冰糖橙的带皮芽用腹接法接种至大红甜橙枝条,每株接种3个芽且全部成活以保证接种成功。以非转基因大红甜橙同时接种为阳性对照,未接种的为阴性对照。第1次接种后,对于未检测出病毒感染的转基因植株,同样的方法进行第2次接种CTV。
1.2.5 抗病性鉴定 取接种CTV后的转基因大红甜橙新萌发枝条上的成熟叶片,用酶联免疫法和q-PCR两种方法进行抗病性鉴定。酶联免疫法按照试剂盒说明书(ACD VS216-K1)操作;提取叶片的RNA,反转录后的cDNA为模板,用q-PCR检测CTV p20[14]的表达量,内参基因为nad5[15]。
2 结果
2.1 RNAi载体的获得
按照图1,将p23基因扩增得到的两个片段插入植物表达载体pBI121,获得RNAi载体(p23-RNAi)。该载体分别用3组双酶切反应(Bam HI-Xba I、Bam HI-Sma I、Xba I-Sma I)进行分析,发现酶切片段分别为500、300和800 bp左右,与插入片段大小一致(图2)。通过对载体插入片段进行测序(结果未显示),进一步说明载体构建成功。
2.2 RNAi载体瞬时表达的有效性分析
利用农杆菌介导的瞬时表达技术,将p23-RNAi载体快速导入叶片。该载体的gus位于插入的发夹结构下游,共同受CaMV 35S启动子控制。通过GUS染色,结果显示注射p23-RNAi的叶片出现蓝色斑点(图3),说明瞬时表达技术能引起RNAi载体在墨西哥莱蒙叶片中的表达。
进一步对p23-RNAi瞬时表达所产生的抗病性进行分析,q-PCR结果显示所有接种CTV的植株均检测有病毒感染,并且p20的表达量随着接种后时间的延长而增加(表2)。但是,在p23-RNAi瞬时表达的叶片中,p20基因积累水平和增加速度明显低于对照。同时,ELISA检测显示,p23-RNAi瞬时表达的叶片均显示阴性。由此说明,p23-RNAi能在柑橘中干扰CTV侵染。

图2 Xba I、Bam HI、Sma I组合双酶切p23-RNAiFig. 2 Double digestion of p23-RNAi with Bam HI, Xba I or Sac I
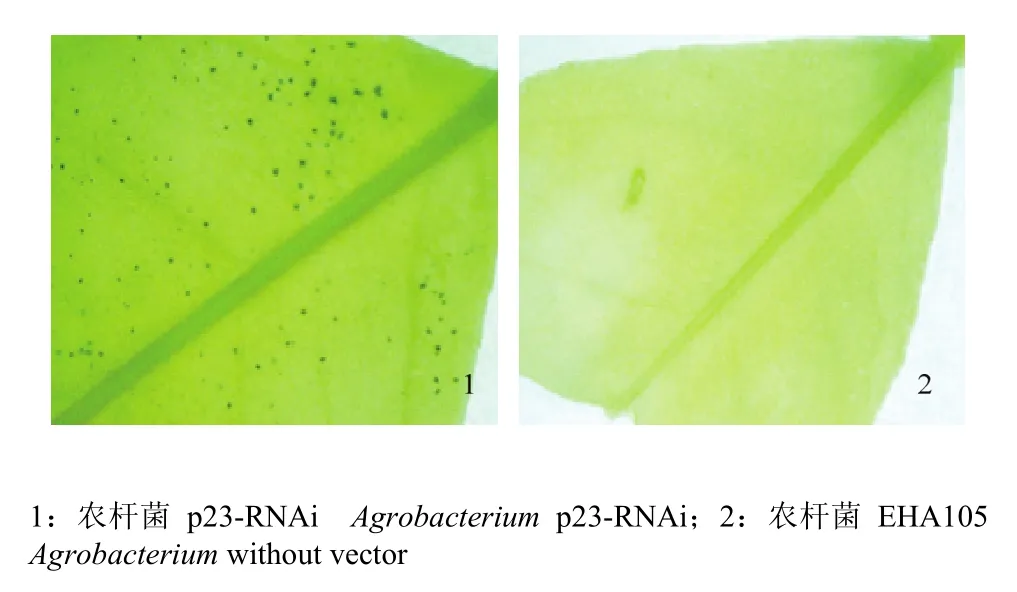
图3 GUS染色检测p23-RNAi载体的瞬时表达Fig. 3 Transient gus expression were observed with Mexican lime leaves by histochemical GUS staining
2.3 转基因大红甜橙的获得
通过农杆菌遗传转化,将p23-RNAi稳定转入大红甜橙实生苗。将获得转基因植株进行PCR鉴定,结果显示,7个转基因植株A、B、C、E、F、G、H(D二次嫁接时未成活)均扩增得到特异条带,大小为513 bp(图4)。进一步用q-PCR对转基因植株进行内含子结构表达分析,发现7个转基因植株均产生内含子结构的表达,且各个植株之间表达量存在显著差异。其中,转基因植株E表达的内含子结构含量最高,其次是C、F、H、A、B和G(图5)。
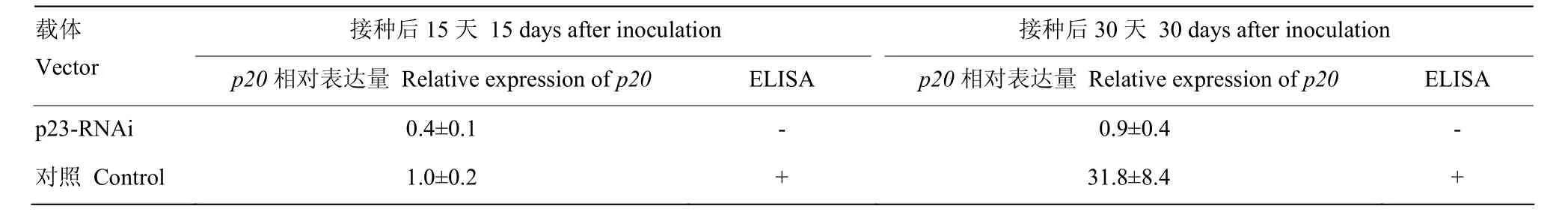
表2 p23-RNAi瞬时表达对CTV的抗性反应Table2 Resistance to CTV with p23-RNAi transient expression

图4 转基因大红甜橙的PCR检测Fig 4 PCR analysis of transgenic ‘Da Hong’ sweet orange

图5 q-PCR检测转基因植株的△Ct值Fig. 5 △Ct value of transgenic plants with q-PCR
2.4 转基因植株对柑橘衰退病的抗性分析
对转基因大红甜橙植株两次接种病毒所产生的抗性进行分析,q-PCR结果显示,除C外,所有接种CTV的植株均检测到病毒感染,p20的表达量在不同的植株间表现出差异,植株B和E的表达量较低,但是C几乎检测不到基因表达(图6)。ELISA检测显示,C的叶片和未接种的叶片均为阴性。由此说明,转基因植株C对柑橘衰退病毒强毒株系具有完全抗性。

图6 转基因植株接种后p20相对表达量Fig. 6 p20 relative expression of transgenic plants inoculated with CTV
3 讨论
病毒基因介导的抗性是将病毒的一段序列构建成RNAi载体,产生的dsRNA在植物内与病毒基因发生沉默产生抗性,在多种植物的抗病毒中已有报道[16-18]。CHENG等[4]曾经试图用柑橘衰退病毒p23的长片段和短片段相连的方式构建RNAi载体以用于获得具有抗性的转基因酸橙,但是p23发夹结构存在限制性内切酶酶切位点Sac I,与植物表达量载体pCAMBIA 2301的酶切位点冲突,使得载体构建中止。本研究选用的p23保守区段,与CHENG等[4]的序列部分片段相同,所设计RNAi载体的长片段和同源的短片段与植物表达载体pBI 121没有冲突的酶切位点,使得载体构建成功,可以用于p23-RNAi的抗病性检测。
柑橘的遗传转化受到多种因素限制,如转化效率低,成本费用高等[19-20],并且长时间的遗传转化后不一定能获得具有优异性状的转基因植株[21]。农杆菌介导的瞬时表达具有操作简单、省时、转化效率高等优点,可以用于高效、快速的分析基因功能[22-25]。将载体p23-RNAi注射入墨西哥莱蒙叶片,目的基因在进入细胞核后的短时间内可以表达,并且持续一定的时间[26-27]。笔者课题组前期的研究证明,柑橘叶片在注射农杆菌后15 d仍然能够检测到目的基因。本研究采用先注射p23-RNAi载体然后接种CTV的方式,使得在病毒入侵前就能够产生特异的siRNA,能够产生对CTV的抗性[3]。在此笔者发现瞬时表达技术能够检测p23-RNAi对CTV的抗性,结果与转基因植株一致,成功预测了载体的可行性,目前已有多个RNAi载体用于转化病毒基因介导抗性,此方法更有利于筛选优质载体,避免盲目的遗传转化,获得有效的RNAi载体和转基因植株。
转基因植株的抗病性与寄主和病毒沉默抑制子之间的相互作用程度,以及植株的遗传背景、外界的环境和植物自身的生长状态[28-29]相关,与目的基因的拷贝数没有关联[3,22]。本研究通过遗传转化p23-RNAi载体获得7个大红甜橙转基因植株,不同转基因植株间RNAi载体发夹结构部分的表达量存在差异,这种差异可能与外源基因在转基因植株中的表达量不同有关。接种病毒后,转基因植株表现出不同的抗病性,这种抗病性的趋势与外源基因表达量的差异趋势没有相关性。p23表达量最高的转基因植株E并没有表现出最强的CTV抗性,而表达量稍低的植株C两次接种后仍然具有完全抗性;同时,转基因植株F的p23表达量与植株C相同,对病毒的抗性结果却有较大的差异。这些研究结果进一步表明,转基因抗性与外源基因的拷贝数没有关联,与前人研究结果相符。
4 结论
成功构建了柑橘衰退病的RNAi载体,瞬时表达技术检测载体p23-RNAi对柑橘衰退病毒(CTV)具有抗病性。遗传转化大红甜橙获得的7株转基因植株中,植株C对CTV具有完全抗性,为柑橘衰退病的防治提供了资源。
[1] 周彦, 周常勇, 李中安, 王雪峰, 刘科宏. 利用弱毒株交叉保护技术防治甜橙茎陷点型衰退病. 中国农业科学, 2008, 41(12): 4085-4091.
ZHOU Y, ZHOU C Y, LI Z A, WANG X F, LIU K H. Mild strains cross protection against stem-pitting tristeza of sweet orange. Scientia Agricultura Sinica, 2008, 41(12): 4085-4091. (in Chinese)
[2] KREUZE J F, KLEIN I S, LÁZARO M U, CHUQUIYURI W C,MORGAN G L, MEJÍA P G C, GHISLAIN M, VALKONEN J P. RNA silencing-mediated resistance to a crinivirus (Closteroviridae) in cultivated sweetpotato (Ipomoea batatas L.) and development of sweet potato virus disease following co-infection with a potyvirus. Molecular Plant Pathology, 2008, 9(5): 589-598.
[3] SOLER N, PLOMER M, FAGOAGA C, MORENO P, NAVARRO L,FLORES R, PEÑA L. Transformation of Mexican lime with an intron-hairpin construct expressing untranslatable versions of the genes coding for the three silencing suppressors of Citrus tristeza virus confers complete resistance to the virus. Plant Biotechnology Journal, 2012, 10: 597-608.
[4] CHENG C Z, YANG J W, YAN H B, BEI X J, ZHANG Y Y, LU Z M,ZHONG G Y. Expressing p20 hairpin RNA of Citrus tristeza virus confers Citrus aurantium with tolerance/resistance against stem pitting and seedling yellow CTV strains. Journal of Integrative Agriculture, 2015, 14(9): 1767-1777.
[5] VOINNET O, LEDERER C, BAULCOMBE D C. A viral movement protein prevents spread of the gene silencing signal in Nicotiana benthamiana. Cell, 2000, 103: 157-167.
[6] LU R, FOLIMONOV A, SHINTAKU M, LI W X, FALK B W,DAWSON W O, DING S W. Three distinct suppressors of RNA silencing encoded by a 20-kb viral RNA genome. Proceedings of the National Academy of Sciences of the United States of America, 2004,101(44): 15742-15747.
[7] COSTA Â, MARQUES N, NOLASCO G. Citrus tristeza virus p23 may suppress systemic silencing but is not related to the kind of viral syndrome. Physiological & Molecular Plant Pathology, 2014, 87: 69-75.
[8] GHORBEL R, LÓPEZ C, FAGOAGA C, MORENO P, NAVARRO L,FLORES R, PEÑA L. Transgenic citrus plants expressing the Citrus tristeza virus p23 protein exhibit viral like symptoms. Molecular Plant Pathology, 2001, 2(1): 27-36.
[9] FAGOAGA C, LÓPEZ C, MORENO P, NAVARRO L, FLORES R,PEÑA L. Viral-like symptoms induced by the ectopic expression of the p23 gene of Citrus tristeza virus are citrus-specific and do not correlate with the pathogenicity of the virus strain. Molecular Plant-Microbe Interactions, 2005, 18(5): 435-445.
[10] JONES H D, DOHERTY A, SPARKS C A. Transient transformation of plants. Methods in Molecular Biology, Plant Genomics, 2009, 513: 131-152.
[11] 王关林, 方宏筠. 植物基因工程. 2版. 北京: 科学出版社, 2002: 831-834. WANG G L, FANG H Y. Plant Genetic Engineering. 2nd ed. Beijing: Science Press, 2002: 831-834. (in Chinese)
[12] 敖小平, 胡新喜, 郭琛, 焦徕, 邓子牛, 熊兴耀. 用rol B基因转化大红甜橙的初步研究. 湖南农业大学学报 (自然科学版), 2005,31(6): 623-626.
AO X P, HU X X, GUO C, JIAO L, DENG Z N, XIONG X Y. Genetic transformation of ‘Dahong’ sweet orange with rol B gene. Journal of Hunan Agricultural University (Natural Sciences), 2005,31(6): 623-626. (in Chinese)
[13] BLUE R L, ROISTACHER C N, CARTIA G, CALAVAN E C. Leaf-disc grafting-a rapid indexing method for detection of some citrus viruses//Proceedings of the Seventh IOCV Conference. University of California, Riverside, 1976: 207-212.
[14] 刘红光, 王中康, 曹月青, 夏玉先, 殷幼平. 应用常规RT-PCR和荧光定量RT-PCR 检测柑桔衰退病毒. 植物病理学报, 2008, 38(1): 24-30.
LIU H G, WANG Z K, CAO Y E, XIA Y X, YIN Y P. Detection of Citrus tristeza virus using conventional and fluorescence quantitative RT-PCR assays. Acta Phytopathologica Sinica, 2008, 38(1): 24-30. (in Chinese)
[15] MENZEL W, JELKMANN W, MAISS E. Detection of four apple viruses by multiplex RT-PCR analysis assays with coamplification of plant mRNA as internal control. Journal of Virological Methods, 2002,99: 81-92.
[16] YADAV J S, OGWOK E, WAGABA H, L. PATIL B, BAGEWADI B,ALICAI T, GAITAN-SOLIS E, TAYLOR N J, FAUQUET C M. RNAi-mediated resistance to Cassava brown streak Uganda virus in transgenic cassava. Molecular Plant Pathology, 2011, 12(7): 677-687.
[17] PATIL B L, OGWOL E, WAGABA H, MOHAMMED I U, YADAV, J S, BAGEWADI B, TAYLOR N J, KREUZE J F, MARUTHI M N,ALICAI T, FAUQUET C M. RNAi-mediated resistance to diverse isolates belonging to two virus species involved in cassava brown streak disease. Molecular Plant Pathology, 2011, 12(1): 31-41.
[18] FAGOAGA C, LÓPEZ C, MENDOZA A H D, MORENO P,NAVARRO L, FLORES R, PEÑA L. Post-transcriptional gene silencing of the p23 silencing suppressor of Citrus tristeza virus confers resistance to the virus in transgenic Mexican lime. Plant Molecular Biology, 2006, 60: 153-165.
[19] KAPILA J, RYCKE R D, MONTAGU M V, ANGENON G. An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Science, 1997, 122(1): 101-108.
[20] ALMEIDA W A B, MOURÃO FILHO F A A, PINO L E,BOSCARIOL R L, RODRIGUEZ A P M, MENDES B M J. Genetic transformation and plant recovery from mature tissues of Citrus sinensis L. Osbeck. Plant Science, 2003, 164: 203-211.
[21] LÓPEZ C, CERVERA M, FAGOAGA C, MORENO P, NAVARRO L,FLORES R, PEÑA L. Accumulation of transgene-derived siRNAs is not sufficient for RNAi-mediated protection against Citrus tristeza virus in transgenic Mexican lime. Molecular Plant Pathology, 2010,11(1): 33-41.
[22] BHASKAR P B, VENKATESHWARAN M, WU L, ANÉ J M,JIANG J M. Agrobacterium-mediated transient gene expression and silencing: a rapid tool for functional gene assay in potato. PLoS ONE,2009, 4(6): e5812.
[23] LLAVE C, KASSCHAU K D, CARRINGTON J C. Virus-encoded suppressor of posttranscriptional gene silencing targets a maintenance step in the silencing pathway. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(24): 13401-13406.[24] XU K D, HUANG X H, WU M M, WANG Y, CHANG Y X, LIU K,ZHANG JU, ZHANG Y, ZHANG F L, YI L M, LI T T, WANG R Y,TAN G X, LI C W. A rapid, highly efficient and economical method of Agrobacterium-mediated in planta transient transformation in living onion epidermis. PLoS ONE, 2014, 9(1): e83556.
[25] SENDIN L N, FILIPPONE M P, ORCE I G, RIGANO L, ENRIQUE R, PEÑA L, VOJNOV A A, MARANO M R, CASTAGNARO A P. Transient expression of pepper Bs2 gene in Citrus limon as an approach to evaluate its utility for management of citrus canker disease. Plant Pathology, 2012, 61: 648-657.
[26] WYDRO M, KOZUBEK E, LEHMANN P. Optimization of transient Agrobacterium-mediated gene expression system in leaves of Nicotiana benthamiana. Acta Biochimica Polonica, 2006, 53(2): 289-298.
[27] KIM M J, BAEK K, PARK C M. Optimization of conditions for transient Agrobacterium-mediated gene expression assays in Arabidopsis. Plant Cell Reports, 2009, 28: 1159-1167.
[28] PANG S Z, JAN F J, CARNEY K, STOUT J, TRICOLI D M,QUEMADA H D, GONSALVES D. Post-transcriptional transgene silencing and consequent tospovirus resistance in transgenic lettuce are affected by transgene dosage and plant development. The Plant Journal, 1996, 9(6): 899-909.
[29] KALANTIDIS K, PSARADAKIS S, TABLER M, TSAGRIS M. The occurrence of CMV-specific short RNAs in transgenic tobacco expressing virus-derived double-stranded RNA is indicative of resistance to the virus. Molecular Plant-Microbe Interactions, 2002,15(8): 826-833.
(责任编辑 岳梅)
Construction and Transformation of RNAi Vector for Citrus tristeza virus Gene p23
LI Fang, DENG Zi-niu, ZHAO Ya, LI Da-zhi, DAI Su-ming
(Horticulture and Landscape College, Hunan Agricultural University/National Center for Citrus Improvement (Changsha),Changsha 410128)
【Objective】 The objective of this study is to construct RNAi vector containing p23 gene of Citrus tristeza virus(CTV), and obtain transgenic orange plants with virus resistance. 【Method】 Based on the pathogen-derived resistance and the CTV genome sequences published by NCBI, two specific conserved fragments of p23 with different lengths were cloned. Two segments and vector pBI 121 were double-digested and connected for RNAi construction. Subsequently, to initially estimate the antiviral feasibility, the Mexican lime (CTV indicator plant) leaves were injected with Agrobacterium contained the RNAi vector for transient expression, and observed using GUS histochemical staining method. The leaves were inoculated with CTV T36 isolate, and detected by enzyme-linked immunosorbent assay (ELISA). The RNA of leaves was also extracted and reverse transcript to cDNA. Quantitative real-time PCR (q-PCR) was performed to observe the CTV p20 gene expression which could reflect the virus in hosts. The RNAi vector was also transferred into the epicotyl stem of ‘DA HONG’ sweet orange seedlings via Agrobacterium-mediated transformation. Resistant buds were engrafted onto Carrizo Citrange in vitro seedlings. DNA extracted from the ‘DA HONG’ sweet orange leaves was used as the PCR template to identify the transgenic plants. Plants containing the target gene were re-engrafted onto sour orange seedlings and stored in the greenhouse. The expression of the p23 within the transgenic plants was evaluated by q-PCR. The skin buds of CTV T36 isolate hosts were collected and inoculated onto the transgenic sweet orange. The leaves from the sprouted branch tips were collected and analyzed the pathogen resistance capability with the same method that the transient expression leaves detected. Plants without detectable virus infection after the first inoculation were also inspected and analyzed by the same manner in the second round. 【Result】 Long (513 bp) and short (291 bp) fragments of the p23 were cloned. These p23 fragments are then cloned into the pBI121 vector, named p23-RNAi. This p23-RNAi vector was then delivered in the Mexican lime leaves using the Agrobacterium-mediated transient expression assay. The leaves were identified based on the presence of blue stains after GUS staining, indicating that the Agrobacterium contained vector p23-RNAi may induce transient expression in Mexican lime leaves. On the 15th and 30th day after the CTV inoculation, the ELISA detection results for the transgenic Mexican lime leaves in p23-RNAi plants were all negative, whereas the q-PCR detection results showed that the accumulation level of p20 expression was significantly lower than that of the control plants. It indicated that the transient expression of p23-RNAi may, in a defined period,inhibit the CTV infection. Introduction of p23-RNAi via Agrobacterium-mediated genetic transformation led to the production of resistant buds for the ‘DA HONG’ sweet orange, and PCR amplification confirmed a total of seven transgenic plants. The expression of the p23 in all seven transgenic plants was further confirmed by q-PCR amplification, and the gene expression level exhibited a certain degree of difference, with expression level in plant E being the highest, followed by C, F, H, A, B, and G. After inoculation with CTV, the level of expression of p20 varied among these seven transgenic plants, with plant A having the highest level of p20 expression, followed by G, F, E, B, H and C. The transgenic plants showed higher pathogen resistance, albeit to different degrees,when compared to the control plants. However, the virus resistance degrees in the transgenic plants were not closely related to the expression levels of the exogenous gene. For example, plant E, which had the highest expression level of the exogenous gene, did not exhibit powerful CTV resistance. By contrast, the transgenic plant C displayed complete resistance after the two rounds of virus inoculation. 【Conclusion】The p23-RNAi construct that generated confers plant disease resistance to CTV. Transient expression assay can be applied for the high efficiency identification of resistance and screening for high efficiency RNAi vectors.
Citrus tristeza virus (CTV); p23; RNAi; ‘DA HONG’ sweet orange; transient expression; genetic transformation
2016-04-15;接受日期:2016-08-19
国家自然科学基金(30900972,3157211)、国家公益性行业(农业)科研专项(201203076-06)、湖南省研究生科研创新项目(CX2013B290)
联系方式:李芳,E-mail:lifang200709@126.com。通信作者戴素明,Tel:0713-84635302;E-mail:dsm531@126.com

