Preparation and characterization of ammoniated PAN nanofiber adsorbent
Zang Chuanfeng, Ren Yu, Zhang Guangyu
(School of Textile and Clothing, Nantong University, Nantong 226019)
Preparation and characterization of ammoniated PAN nanofiber adsorbent
Zang Chuanfeng, Ren Yu, Zhang Guangyu
(School of Textile and Clothing, Nantong University, Nantong 226019)
An ammoniated PAN nanofibers (PAN-NH2) absorbent was prepared by grafting amino hyperbranched polymer (HBP-NH2) onto polyacrylonitrile (PAN) nanofiber in presence of glutaraldehyde as a crosslinking agent. The structure and performance of PAN-NH2were characterized. The results indicated that HBP-NH2was successfully grafted onto PAN nanofibers surface by chemical crosslinking modification; PAN-NH2nanofiber provided the adsorption capacity up to 28.02 mg/g while used as an absorbent in the treatment of water containing Cu2+; and the amino mass fraction of PAN-NH2fiber was 5.4%.
polyacrylonitrile fiber; nanofiber; absorbent; chemical crosslinking modification; heavy metal ion adsorption
Nanofibers prepared by electrostatic spinning technique have become the focus of adsorption research in recent years[1-3]due to the advantage of large specific surface area and strong adsorption ability. Adsorption materials prepared by modified polyacrylonitrle (PAN) nanofibers have received much attention. Zhang Haitao et al.have prepared a kind of adsorption material by grafting chitosan onto PAN nanofiber membrane[2]. Khalid Saeed and Parvin Karimi Neghlani et al. have prepared ammoniated PAN nanofiber adsorption material by chemical modification[3-4]. The ammoniated PAN nanofibers had very good adsorption properties for Cu2 +and Pb2 +. However, the previous studies have been focused on grafting small molecular compounds to a nanofiber. It has not been reported that amino hyperbranched polymer (HBP-NH2) can be grafted to PAN nanofibers for preparing of adsorbents for heavy metals up to now. Here we report an ammoniated PAN(PAN-NH2) nanofiber adsorbent for heavy metals prepared by grafting HBP-NH2to PAN nanofibers.
1 Experiment
1.1Raw material
PAN with the relative molecular mass of 8×104was purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai,China. All of the used chemical reagents, such as N,N-dimethylformamide (DMF), sodium hydroxide, hydrochloric acid, methyl acrylate, tetraethylenepentamine, methanol, glutaraldehyde (25% solution by mass fraction), salicylaldehyde, pyridine, sodium methoxide, phenolphthalein and copper(II) nitrate hydrate, were all analytical grade and were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China.
1.2Electrospinning process
The spinning solution was prepared by dissolving 1 g PAN in 19 mL DMF aqueous and stirring for 12 h at room temperature in sealed state. The prepared spinning solution was pumped in a 20 mL plastic syringe of gauges with stainless steel needle. The electrolspinning voltage was maintained at 30 kV, the distance between the spinneret and collector was fixed as 16 cm, and the fitting speed was controlled at 0.015 mL/min.
1.3Fiber pretreatment
A certain amount of PAN nanofibers were putted into NaOH solution with the mass fraction of 15% at the bath ratio of 1:50, reacting for 60 min at 50 ℃. Then the reaction products were putted into HCl solution with the concentration of 1 mol/L, reacting at room temperature for 120 min[2]. The products were washed, dried and stoved for futher using.
1.4Preparation of HBP-NH2
HBP-NH2was synthesized by reacting tetraethylenepentamine (0.5 mol) with methyl acrylate (0.5 mol) according to the method described in literature [5].
1.5Preparation of PAN-NH2nanofiber
A solution was prepared by adding 0.05 g PAN nanofi bers into a 100 mL conical flask containing 5 mL distilled water, stirring and heating up to 70 ℃. A certain volume of glutaraldehyde was mixed with 2 mL distilled water, drippd into the solution containing PAN nanofibers, and then kept at 70 ℃ for 0.5 h. And a certain volume of 100 g/L HBP-NH2aqueous solution was added into the above solution, reacting for 2 h at 70 ℃. Hence, a tan PAN nanofiber adsorbent modified by HBP-NH2was obtained[2], which was filtered, washed and dried for adsorption. The mass fraction of amino was tested as 5.4% in the adsorbent by the salicylaldehyde method[6]. The reaction route is shown in Fig.1.

Fig.1 Preparation of PAN-NH2 nanofiber adsorbent
1.6Analysis and test
The surface morphology of samples were observed by a S- 4800 scanning electron microscope (SEM) (Hitachi, Japan). The FTIR spectra were recorded on a Nicolet 5700 FTIR spectrometer (Thermo Nicolet, USA) with a detector at 4 cm-1resolution from 500 cm-1to 4 000 cm-1and 32 scans per sample. The heavy metal concentration of the initial and adsorbed solutions were measured by a Vista MPX inductively coupled plasma atomic emission spectrometer (ICP-OES) (Spectro Analytical Instruments GmbH, Germany).
2 Results and discussion
2.1SEM analysis
It can be seen from Fig.2 that PAN fibers had the even fineness in a clearly separated state.

Fig.2 SEM photographs of PAN and PAN-NH2 nanofibers
After modification by HBP-NH2, PAN nanofibers became thicker, and the fiber surface was covered with a layer of crosslinking matter, which was the crosslinking product ofHBP-NH2and glutaraldehyde. It was shown that HBP-NH2was linked onto PAN nanofibers surface via chemical grafting.
2.2FTIR spectrometry
As shown in Fig.3, new characteristic peaks appeared in the infrared spectrum of PAN nanofiber modified by HBP-NH2. The bending vibration peak of amino groups appeared at 1 635.41 cm-1and 1 558.27 cm-1, the stretching vibration peak of aldehyde group appeared at 2 931.39 cm-1and 2 829.18 cm-1[2,7-8]. The emergence of these new characteristic peaks showed that HBP-NH2had been grafted to the surface of PAN nanofibers via glutaraldehyde crosslinking,which furtherly confirmed the result of the SEM images.

Fig.3 FTIR spectra of PAN and PAN-NH2 nanofibers1—PAN nanofiber;2—PAN-NH2 nanofiber
2.3Heavy metal ions adsorption
0.1 g PAN nanofiber and 0.1 g PAN-NH2nanofiber were put into two 100 mL conical flasks separately and were mixed with 50 mL 100 μg/g Cu2+solution, shaking for 4 h at 30 ℃ in water bath. The adsorption results were shown in Tab.1.
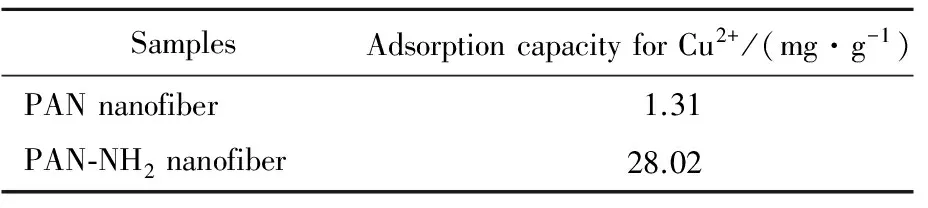
Tab.1 Contrast of Cu2+ adsorption between PAN and PAN-NH2 nanofibers
As seen in Tab.1, the adsorption capacity of PAN nanofibers for Cu2+was 1.31 mg/g while the adsorption of PAN-NH2nanofibers for Cu2+was up to 28.02 mg/g. PAN-NH2nanofiber had the adsorption capacity for Cu2+greatly improved and showed excellent heavy metal adsorption ability due to the chelation between amino groups and Cu2+metal ions[9]. An abundant of amino groups which could chelate with Cu2+were introduced to PAN nanofibers after HBP-NH2grafting onto the fiber surface, so the adsorption of PAN-NH2nanofibers for Cu2+had been greatly improved after modification by HBP-NH2.3Conclusions
a.A PAN-NH2nanofiber absorbent was prepared by the modification of PAN nanofibers using HBP-NH2.
b.The experimental results showed that the amino mass fraction of PAN-NH2was up to 5.4% after modification and HBP-NH2was linked onto the surface of PAN nanofibers via chemical grafting.
c.The adsorption of PAN-NH2nanofibers for Cu2+had been greatly improved after modification by HBP-NH2, the adsorption capacity for Cu2+reached 28.02 mg/g, and the PAN-NH2nanofiber was a good absorbent for Cu2+heavy metal treatment in water.
[1]Zhao Rui,Li Xiang,Sun Bolun,et al.Preparation of phosphorylated polyacrylonitrile-based nanofiber mat and its application for heavy metal ion removal[J].Chem Eng J, 2015, 268: 290-299.
[2]Zhang Haitao,Nie Huali,Yu Dengguang,et al.Surface modification of electrospun polyacrylonitrile nanofiber towards developing an affinity membrane for bromelain adsorption[J].Desalination, 2010,256(1/2/3): 141-147.
[3]Neghlani P K,Rafizadeh M,Taromi F A.Preparation of aminated-polyacrylonitrile nanofiber membranes for the adsorption of metal ions: Comparison with microfibers[J].J Hazard Mater, 2011, 186(1): 182-189.
[4]Saeed K,Haider S,Oh T J,et al.Preparation of amidoxime-modified polyacrylonitrile (PAN-oxime) nanofibers and their applications to metal ions adsorption[J].J Membrane Sci, 2008, 322(2):400-405.
[5]Zhang Feng,Chen Yuyue,Zhang Desuo,et al.Preparation and properties of amino-terminated hyperbranched polymers and its quaternary ammonium Salt[J].Polym Mater Sci Eng,2009,25(8):141-144.
[6]Zhang Zhixian,Zhang Ruihao.Quantitative analysis of organic functional groups[M].Beijing:Chemical Industry Press,1990:227-234.
[7]Zhang Feng,Chen Yuyue,Lin Hong,et al.HBP-NH2grafted cotton fiber: Preparation and salt-free dyeing properties[J].Carbohyd Polym,2008,74:250-256.
[8]Tao Tingxian,Wu Zhichuan,Zhao Zeqing.Preparation of chelating fibers-modification of polyacrylonttrile fiber[J].Syn Fiber Chin,2001,30(4):32-38.
[9]Ma Nianfang.Preparation of chelating fibers containing amine froup and their adsorption behavior for heavy metal ions[D].Guangzhou:Sun Yat-sen University,2010.
多氨基改性PAN纳米纤维吸附剂的制备与表征
臧传锋,任煜,张广宇
(南通大学纺织服装学院,江苏 南通 226019)
以聚丙烯腈(PAN)纳米纤维为基体,利用多氨基超支化聚合物(HBP-NH2)末端所具有的大量氨基官能团,采用戊二醛作为交联剂,将HBP-NH2接枝到PAN纳米纤维上制备了多氨基改性PAN纳米纤维(PAN-NH2)吸附剂,对PAN-NH2的结构与性能进行了表征。结果表明:HBP-NH2通过化学交联成功接枝到PAN纳米纤维的表面; PAN-NH2用于含重金属离子Cu2+的水体吸附,对Cu2+的吸附量可达28.02 mg/g;PAN-NH2纤维的氨基质量分数高达5.4%。
聚丙烯腈纤维纳米纤维吸附剂化学交联改性重金属离子吸附性能
date:17- 05- 2016; revised date: 20- 08- 2016.
Natural Science Foundation for Young Scholars of Jiangsu Province(BK20140431).
TQ342+.34Document code:AArticle ID: 1001- 0041(2016)05- 0050- 03
Biography: Zang Chuanfeng(1979-), male, lecturer, Ph. D, is engaged in the research of fiber material modification and application. E-mail:zang.cf@ntu.edu.cn.

