Nicotiana tabacum Lin.- N. plumbaginifolia Viv.杂种的鉴定及其育性和黑胫病抗性的初步分析
党江波,赵申清玉,邓红红,曹帅,梁国鲁,张艳
1 西南大学,园艺园林学院,重庆市北碚区天生路2号 400716;2 中国烟草总公司重庆市公司,烟草研究所,重庆市北碚区天生路2号,400716
Nicotiana tabacumLin.-N. plumbaginifoliaViv.杂种的鉴定及其育性和黑胫病抗性的初步分析
党江波1,赵申清玉1,邓红红1,曹帅1,梁国鲁1,张艳2
1 西南大学,园艺园林学院,重庆市北碚区天生路2号 400716;2 中国烟草总公司重庆市公司,烟草研究所,重庆市北碚区天生路2号,400716
为明确一株云烟87八倍体(2n=8x=96)与N. plumbaginifoliaViv.(2n=2x=20)的杂交后代的部分生物学特性。对该后代进行了形态观测,细胞学遗传学分析,育性分析,并进行黑胫病抗性的离体鉴定。结果显示,该杂交后代(无性系)大部分外观形态指数介于云烟87四倍体与N. plumbaginifolia之间;花冠颜色与云烟87相近,花药颜色与N. plumbaginifolia相近。其染色体数目为58条,其中含有来自N. plumbaginifolia的10条染色体。花粉离体培养未见萌发;与云烟87四倍体杂交,做母本时坐果率为100%,平均每个蒴果获得102.60±25.12粒可萌发的种子,以其为父本及自交时未获得成熟蒴果。该杂交后代(无性系)对黑胫病抗性强于云烟87,与N. plumbaginifolia相近。综上所述,该杂交后代为云烟87与N. plumbaginifolia的真杂种(2n=5x=58);其雄性败育,但雌性可育;且具较强的黑胫病抗性。
N. tabacum;N.plumbaginifolia;杂种;育性;黑胫病抗性
远缘杂交是作物育种的主要方法和有效途径之一,烟草育种中便较早利用远缘杂交进行品种改良[1-2]。1761-1765 年Koelreuter进行的烟草属种间杂交(Nicotiana rustica×N. paniculata)成为植物远缘杂交的经典案例[3]。自上世纪20年代开始,烟草远缘杂交得到较为系统的开展,且取得较大成就,为普通烟草导入大量优良抗病虫害基因:先后从N.glutinosa中导入抗TMV基因,从N. longi fl oraa中导入抗野火病基因,从N. debneyi中导入抗根结线虫病基因,从N. plumbaginifolia、N. longi fl ora、N.stocktonii、N. nesophila和N. repanda中导入抗黑胫病基因,从N. benthamiana中导入抗Spodoptera litura基因,从N. africana中导入抗PVY基因[2,4-11]。可见,远缘杂交对烟草抗病虫害育种有重要的意义。
Nicotiana plumbaginifolia属烟草属花烟草组(Alatae),与普通烟草(N. tabacum)杂交亲和,对黑胫病、野火病、根黑腐病、根结线虫病、角斑病等烟草生产中的常见病害具有较强的抗性[12-13]。其中黑胫病对我国烟草生产的危害最大,造成的损失仅次于病毒病[14]。研究证实,N. plumbaginifolia对烟草疫霉菌0号和1号生理小种均具较强抗性,是烟草抗黑胫病的优良抗源[15-16]。利用N. plumbaginifolia进行抗黑胫病育种可改变目前我国烟草育种中黑胫病抗源单一的局面[17-18]。虽然早在1951 年Chaplin便开始了将N. plumbaginifolia的抗黑胫病能力转移到N.tabacum的研究,且后续育种家以N. plumbaginifolia为抗源育成抗黑胫病品种NC2326等[18],但是这些品种在生产上的应用有限。目前,还未见以N.plumbaginifolia育成抗其它病害的烟草品种。在以往对N. plumbaginifolia与烟草的杂交报道中,并未对杂交后代的主要特征如外观性状、育性等进行报道,这对利用N. plumbaginifolia进行育种无疑是较大遗憾[7]。
本文以烟草八倍体(2n=8x=96)与N.plumbaginifolia(2n=2x=20)的杂交后代为材料,采用形态观测、细胞学方法进行了杂种鉴定;通过花粉活力测定和正反杂交、自交结实开展了育性分析;并通过接种病原菌进行该杂交后代材料的黑胫病抗性鉴定。这对利用N. plumbaginifolia及其与烟草的杂交后代进行烟草抗病育种,特别是抗黑胫病育种有重要的参考价值。此外,烟草与N. plumbaginifolia杂种的获得对开展N. plumbaginifolia功能基因组研究及其抗病基因的定位大有益处,对利用N. plumbaginifolia进行抗根黑腐病、根结线虫病、角斑病等育种也具有一定的意义。
1 材料与方法
1.1 材料
普通烟草(Nicotiana tabacumLin)品种云烟87四倍体(2n=4x=48)、野生烟草N. plumbaginifoliaViv.(2n=2x=20)种子均由国家烟草中期库提供,烟草黑胫病病原菌寄生疫霉烟草致病型(Phytophthora parasiticavat. nicotianae)由西南大学资源环境学院李振轮教授赠送,分离自重庆市奉节县。
1.2 方法
1.2.1 杂交后代的获得及扩繁
云烟87四倍体经组织培养获得无菌苗,无菌苗茎尖经0.2%秋水仙素溶液浸泡48 h后,经分化、增殖、生根培养获得八倍体植株,移栽田间。盛花期,以其为母本与N. plumbaginifolia杂交获得种子。种子萌发成苗后移栽至田间,选取其中一株正常生长,经鉴定染色体数目为58的植株为研究对象。
该植株经组织培养获得无性系植株,诱导培养基为MS+1.0 mg·L-16-BA+0.2 mg·L-1NAA,增殖、分化培养基为MS+0.2 mg·L-16-BA+0.5 mg·L-1NAA,生根培养基为1/2 MS+2.0 mg/L IBA。
1.2.2 杂交后代(无性系)的形态学观测
为初步进行杂种鉴定,至初花期时,参考烟草农艺性状调查方法(YC/T 142-1998)并根据两个亲本基因型云烟87四倍体植株及N. plumbaginifolia植株形态差异对比结果,对该杂交后代(无性系)的在云烟87四倍体和N. plumbaginifolia中差异较大的性状如株高,茎围,着生片数,最大叶长、宽和节距进行测定,对其花器官形态进行观察,拍照记录。
以云烟87四倍体植株及N. plumbaginifolia植株为对照,每个材料测定株数为10株。
1.2.3 杂交后代的细胞学分析
有丝分裂中期染色体核型观察:
有丝分裂中期染色体制片参照陈瑞阳等[19]的方法并略有改进,取植株幼嫩叶片,于0.002 mol·L-1的8-羟基喹啉溶液中处理6 h,后用卡洛氏固定液(甲醇:冰醋酸=3:1)固定过夜。材料经蒸馏水清洗后分解为1 mm2大小,于混合酶液(3%纤维素酶+0.3%果胶酶)中37℃孵育40 min,后吸出酶液,蒸馏水浸泡10 min,吸出蒸馏水,加入卡洛氏固定液。约1 h后于清洁的载玻片上涂抹制片,干燥后用5%吉姆萨染液染色后镜检,拍照。
基因组原位杂交(Genomic in situ hybridization,GISH)分析:
GISH参照Brammer 等[20]的方法并加以改进,以N. plumbaginifolia基因组DNA为探针,采用DIGHigh Prime(罗氏)进行标记,云烟87基因组DNA 10倍浓度为封阻,探针终浓度为2.5 ng·μL-1。以Anti-Digoxigenin- fl uorescein (罗氏)对探针进行染色,DAPI衬染,Olympus荧光显微镜(日本)进行检测、拍照,随机软件cellSens Standard 1.9 (Olympus,Japan)对照片进行处理。信号颜色模拟为红色,背景颜色为蓝色。
1.2.4 杂交后代(无性系)的育性分析
花粉活力测定:
花粉活力检测参照谢朝添等[21]的方法配制烟草花粉离体萌发培养基: 0.01%KH2PO4,0.01%CaCl2,0.01% H3BO3和15%蔗糖。25℃培养4 h后显微镜下观察,以花粉管长度大于花粉粒直径作为萌发标准,统计花粉萌发率。
杂交试验:
以杂交后代(无性系)为父本、母本与云烟87四倍体进行杂交并进行自交,统计坐果率及种子活力。
1.2.5 杂交后代(无性系)对黑胫病抗性的初步观测
烟草疫霉菌孢子液准备:
孢子诱导采用KNO3溶液诱导的方法[22-24],将培养2 周以上的菌丝挑入0.1%的KNO3溶液中(每个直径为9 cm的培养皿中的菌丝转入20 mL KNO3溶液中),培养3 d后,经4℃处理40 min后取出,组织破碎仪打断菌丝,25℃放置20 min后加入1%葡萄糖接种。
病原菌接种及结果观测:
取杂交后代无性系植株团棵期植株中部的新鲜叶片,置于铺有湿润吸水纸的方形搪瓷盘中,用无菌针头于叶片主脉一侧刺穿叶片,取上述菌液10 μL覆盖于针头穿刺处。取团棵期茎段,自根茎连接处截取地上部分,去除叶片,下端切口处于上述菌液中浸泡约5 s,待稍干燥后水平置于铺有湿润吸水纸的方形搪瓷盘中,切口处用湿润吸水纸覆盖。搪瓷盘用封口膜密封保湿,置于30℃黑暗处。分别于接种后4 d、10 d观察。
2 结果与分析
2.1 杂交后代(无性系)的形态学特征
通过对云烟87四倍体、N. plumbaginifolia及杂交后代(无性系)的株高,茎围,有效叶数,最大叶片长、宽及花器官等若干特征的观察测定显示:该杂交后代(无性系)的株高,茎围,最大叶长、叶宽和着生叶数均介于云烟87四倍体与N. plumbaginifolia之间,其中云烟87均为最大;而杂交后代(无性系)的节距较云烟87四倍体与N. plumbaginifolia短,N.plumbaginifolia的节间距最大。在叶形方面杂交后代(无性系)与云烟87四倍体更为相近,叶脉更明显,二级叶脉更清晰且排列规则,叶面密被小绒毛,绒毛腺体不明显。
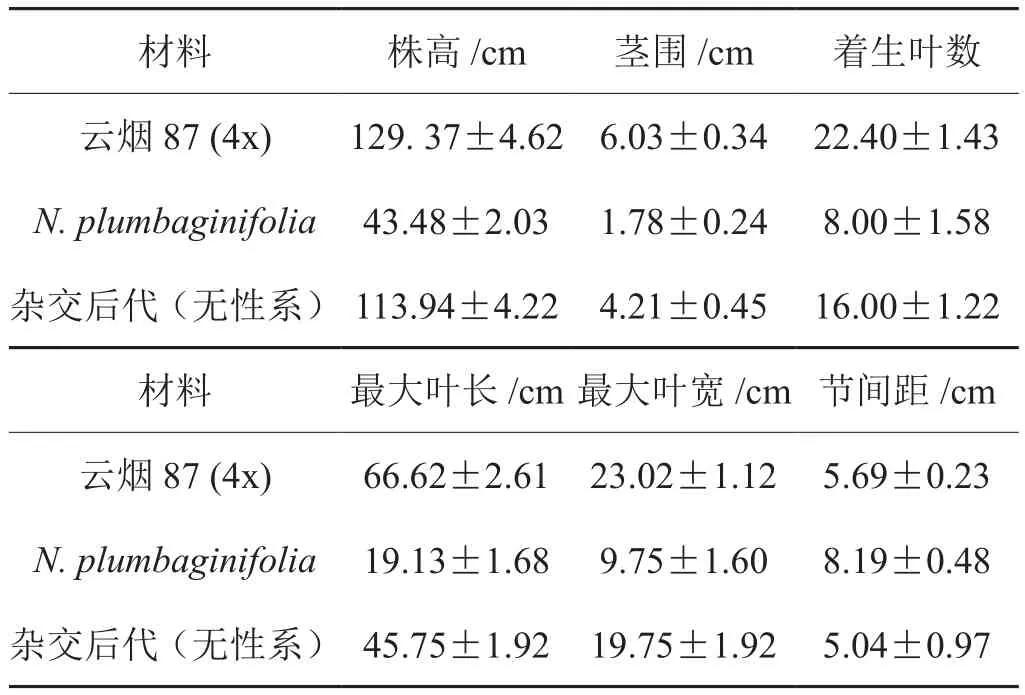
表1 杂交后代(无性系)与云烟87四倍体、N. plumbaginifolia部分形态比较Tab. 1 Part of morphological comparison between Yunyan87, N.plumbaginifolia and offspring plants

图1 杂交后代(B)与云烟87四倍体(A)和N. plumbaginifolia(C)的植株Fig.1 Plant of hybrid offspring plant (B), Yunyan87 tetraploid (A)and N. plumbaginifolia (C)
外观形态中,花器官也呈现一定的差异。杂交后代(无性系)花器官较云烟87四倍体与N. plumbaginifolia大,其次是云烟87,N.plumbaginifolia的花器官则最小;且花冠颜色存在差异,杂交后代(无性系)的花冠颜色为粉红色,而云烟87为浅红色,N. plumbaginifolia为白色(图2),可见杂交后代(无性系)花冠颜色与云烟87更为相似;但值得注意的是,杂交后代(无性系)植株花药颜色成熟未开裂时为褐色,开裂后则为深紫红色,这与N. plumbaginifolia花药颜色相同,这应为该杂交后代(无性系)遗传自N. plumbaginifolia的性状;杂交后代(无性系)植株花药大小和云烟87相近,N.plumbaginifolia的花药则较小。
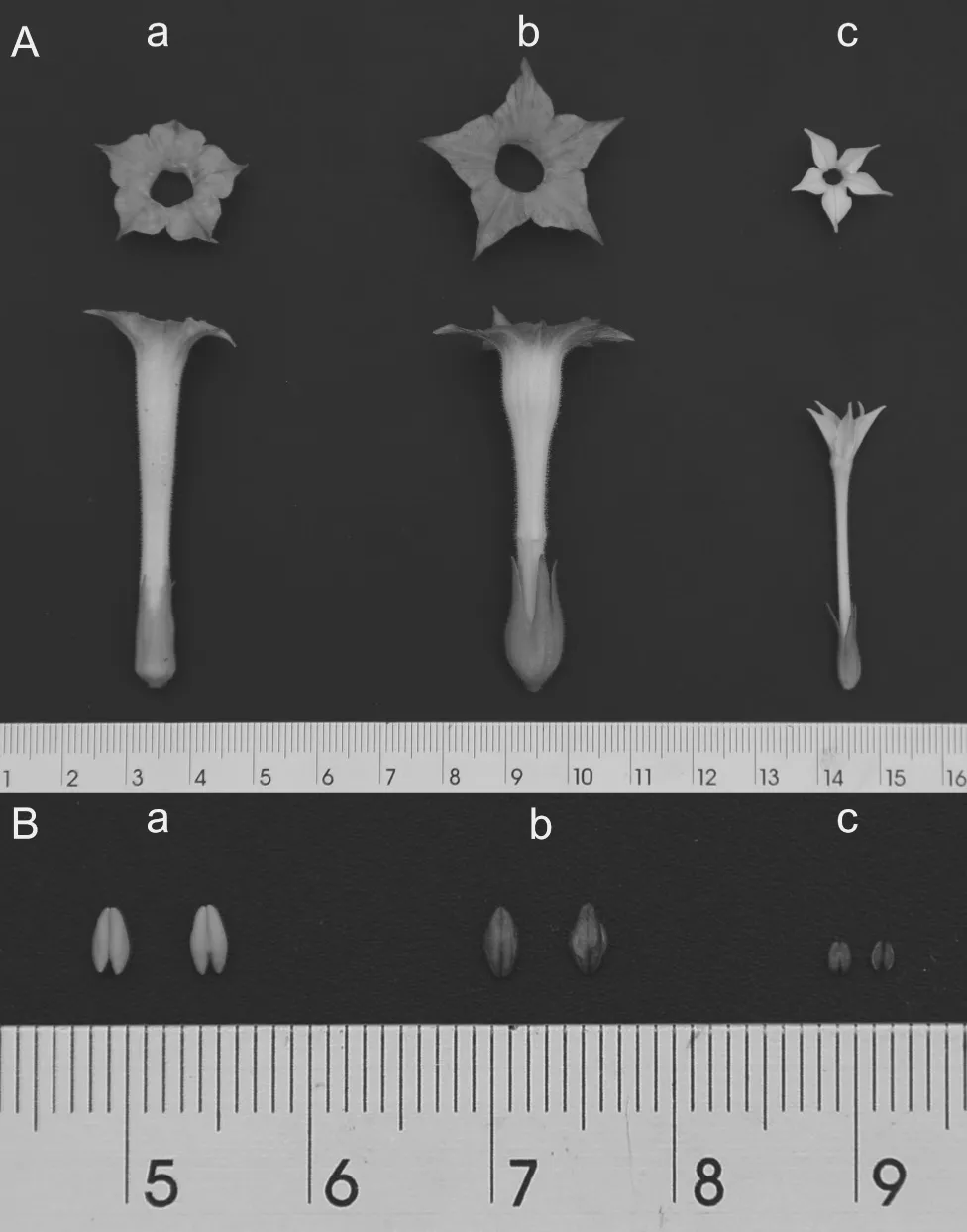
图2 杂交后代(b)、云烟87四倍体(a)和N. plumbaginifolia(C)的花(A)及花药(B)Fig.2 Flower (A) and anther (B) of hybrid offspring plant (b),Yunyan87 tetraploid (a) and N. plumbaginifolia (c)
2.2 杂交后代的细胞学特征
该杂交后代具有58条染色体(图3B)。由于云烟87四倍体染色体数目为48(图3A),而野生烟草N. plumbaginifolia具有染色体20条(图3C),因此可以初步鉴定该杂交后代植株为五倍体,即2n=5x=58,可能为2个完整云烟87染色体组外附加1个N. plumbaginifolia的染色体组。这一结论在GISH结果中得到证实(图3D)。

图3 杂交后代(2n=58,B)与云烟87四倍体(2n=4x=48,A)、N. plumbaginifolia(2n=2x=20,C)有丝分裂中期染色体及以N. plumbaginifolia基因组DNA为探针进行基因组原位杂交的杂交后代有丝分裂中期染色体形态(D,红色示源于N.plumbaginifolia的10条染色体)Fig.3 Mitosis metaphase chromosome of hybrid offspring plant(2n=58, B), Yunyan87 tetraploid (2n=4x=48, A), N. plumbaginifolia(2n=2x=20, C) and GISH result of offspring plant metaphase chromosomes when N. plumabginifolia DNA was used as probes(D, red showed 10 chromosomes from N. plumabginifolia)
2.3 杂交后代(无性系)的育性
光学显微镜下观察到,杂交后代(无性系)花粉空瘪且大小不均一,而云烟87四倍体和N.plumbaginifolia的花粉饱满且较为均一(图4)。经离体萌发试验检测,杂交后代(无性系)花粉未见萌发,而云烟87四倍体花粉萌发率为47.31%,N.plumbaginifolia花粉萌发率为55.98%。
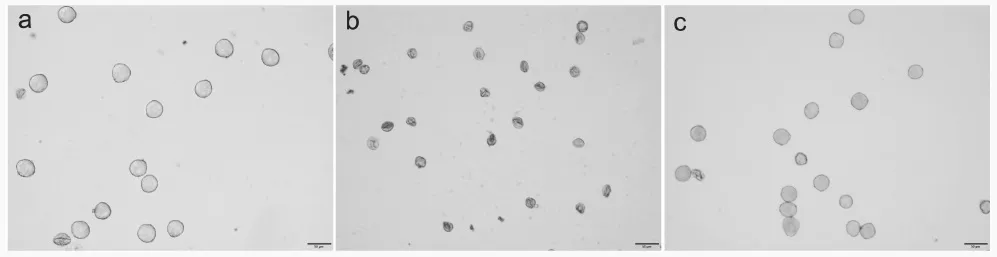
图4 光学显微镜下杂交后代(无性系)(b)及云烟87四倍体(a)和N. plumbaginifolia的花粉(c)Fig.4 Pollen of hybrid offspring plant (b), Yunyan87 tetraploid (a)and N. plumbaginifoli under optical microscope (c)
以杂交后代(无性系)为母本,云烟87四倍体为父本,所有杂交花序均能坐果。蒴果中获得了57-156粒有可以萌发的种子,平均每个蒴果获得102.60±25.12粒可萌发的种子。但以杂交后代(无性系)为父本与云烟87四倍体杂交以及杂交后代自交均未能获得成熟蒴果。可见,该杂交后代为雌性可育而雄性败育。
2.4 杂交后代(无性系)对烟草黑胫病的抗性
黑胫病菌接种后,对照云烟87四倍体叶片自第2d开始出现水渍样病斑,第3d病斑迅速扩大且发病部位软化程度加重,第4d病斑超过主脉延伸至叶片另一侧。而N. plumbaginifolia和杂交后代(无性系)叶片则未见任何病斑出现(图5A)。接种后第10d,云烟87四倍体整张叶片全部严重软化并呈略透明的水渍状,N. plumbaginifolia叶片则出现黄化现象,有不连续黄褐色坏死斑,但未出现水渍样病斑。杂交后代(无性系)叶片则既未出现黄化亦未出现水渍样病斑更未出现坏死斑。
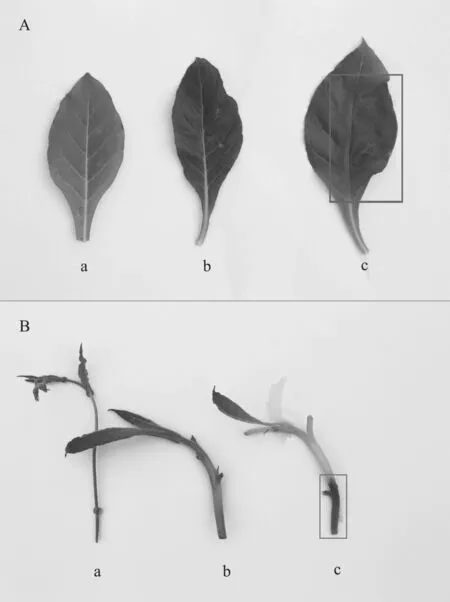
图5 杂交后代(b)及云烟87四倍体(c)和N.plumbaginifolia (a)的叶片(A)及茎段(B)接种烟草疫霉菌后4 d的表现Fig.5 Performance of leaf (A) and stem (B) of hybrid offspring plant (b), Yunyan87 tetraploid (c) and N. plumbaginifolia (a) 4 d after infected by Phytophthora parasitica
云烟87茎段自接种后第2 d自接种切口处开始出现黑褐色略透明的水渍样病斑,第3 d开始迅速沿茎段轴向延伸,第4 d延伸至茎段接近1/2处(图5B)。而N. plumbaginifolia和杂交后代(无性系)并未出现坏死现象。接种后第10 d,云烟87茎端全部呈黑褐色软化半透明水渍状,N. plumbaginifolia茎端亦全部坏死生长出杂色菌丝,但未呈现水渍状病斑,为杂菌浸染所致。杂交后代(无性系)则未出现任何病斑。可见,在对重庆分离的黑胫病病原菌的抗性方面,该杂交后代远远强于云烟87四倍体,可能是野生烟草对黑胫病的抗性随基因组的转移所致。
3 讨论
对杂种进行鉴定的方法较多,包括形态学、细胞学以及分子生物学等方法[25-26]。在形态学中,植株形态和花器官形态是主要的判定依据,一般来说杂种的性状介于亲本之间[27]。在本研究中,以云烟87四倍体和N. plumbaginifolia为对照,部分性状,如植株高度、叶长、叶宽、节间长度是介于二者之间的,而花器官则有所不同,大小及颜色均超过云烟87四倍体和N. plumbaginifolia,这可能是由于该杂交后代的倍性大于四倍体所致。
细胞学鉴定的方法包括染色体数目、形态,基因组原位杂交等技术[28-30]。一般进行精确鉴定时,会对染色体的形态以及显带技术较为重视,而大多在对杂交后代进行初步鉴定时,染色体数目鉴定应用较多[31]。本研究所获得杂交后代染色体数目为58条,且通过GISH结果可知该杂交后代中确有来源于N.plumbaginifolia的完整染色体组10条染色体。因此,可确定该杂交后代为真杂种,并可初步判定为五倍体。但是由于云烟87八倍体在减数分裂过程中可能会形成假整倍性配子,不能排除该杂交后代为类似单-三体类型的假五倍体的可能。
该杂交后代雌性可育,并拥有完整的N.plumbaginifolia基因组,N. plumbaginifolia对诸多烟草病害具有较强抗性,尤其是对烟草主要病害黑胫病、野火病、根黑腐病、根结线虫病的抗性,这在烟草品种的改良方面具有较大的潜力[2,7,13,16]。本研究结果证实该杂交后代对重庆地区黑胫病分离菌株的抗性与N. plumbaginifolia相当,明显强于该地区的现有主栽品种云烟87。Li等[24]报道N. plumbaginifolia对1号生理小种的抗性略高于Beinhart,重庆地区的黑胫病优势小种即为1号小种。Beinhart是目前所知烟草种内对黑胫病抗性最高的品种,由于其抗性与其它基因连锁,而未能在育种中得到广泛应用[17-18,32]。可见N.plumbaginifolia与烟草的杂交后代对改良黑胫病高发区域,特别是以黑胫病1号小种为优势小种的区域的黑胫病抗性育种具有重要的现实意义。
该杂种与云烟87四倍体回交获得了大量后代,这些后代中可能会筛选到含有少量N. plumbaginifolia染色体的附加的甚至是单体附加的植株,是较为难得的烟草育种和亚基因组研究材料[33]。该杂交后代经物理如γ射线辐射等手段处理,可使外源染色体断裂后重接,获得含有外源染色体片段的异源易位系材料,这也是远缘杂交育种中的理想材料,是获得优良新株系的重要基础[34-37]。
4 结论
该杂交后代为烟草与N. plumbaginifolia的真杂种,可能为五倍体(2n=5x=58);其雄性败育,但雌性可育;对重庆地区分离的黑胫病病原的抗性强于云烟87四倍体,与其父本N. plumbaginifolia相近。
[1] Liu Dengcai, Zhang Huaigang, Zhang Lianquan, et al.Distant hybridization: a tool for interspeci fi c manipulation of chromosomes[M] // Pratap A, Kumar J. Alien gene transfer in crop plants. New York: Springer, 2014:25-42.
[2] Burk L G, Heggestad H E. The genusNicotiana: a source of resistance to diseases of cultivated tobacco[J]. Economic Botany, 1966, 20(1): 76-88.
[3] 王伯毅, 李莲芬. 烟草的起源与演化[J]. 烟草科技,1981, 2: 1-4.Wang Boyi, Li Lianfen. Origin and evolution of tobacco[J].Tobacco science and technology, 1981, 2: 1-4. (in Chinese)
[4] 蒋予恩. 中美两国烤烟育种比较与分析[J]. 中国烟草学报, 1996, 3(1): 26-35.Jiang Yuen. Comparative analysis of flue-cured tobacco breeding in USA and China[J]. Acta Tabacaria Sinica, 1996,3(1): 26-35. (in Chinese)
[5] Clausen R E, Goodspeed T H. Interspecific hybridization inNicotiana. II. A tetraploid glutinosa-tabacum hybrid,an experimental verification of Winge’s hypothesis [J].Genetics, 1925, 10(3): 278-284.
[6] Holmes F O. Inheritance of resistance to tobacco-mosaic disease in tobacco[J]. Phytopathology, 1938, 28: 553-561.
[7] Chaplin J F. Transfer of black shank resistance from
Nicotiana plumbaginifoliato fl ue-curedN. tabacum[J]. Tob Sci, 1962, 6: 184-189.
[8] Reed S, Collins G B. Interspecific hybrids inNicotianathrough in vitro culture of fertilized ovulesN. stocktonii×N. tabacum;N. nesophila×N. tabacum;N. repanda×N.tabacum[J]. J Hered, 1978, 69 (5): 311-315.
[9] Charudattan R, Zettler F W, Cordo H A, et al. Partial characterization of a potyvirus infecting the milkweed vine,Morrenia odorata[J]. Phytopathology, 1980, 70(9): 909-913.
[10] Lewis R S. Transfer of resistance to potato virus Y (PVY)fromNicotianaafricanatoNicotianatabacum: possible influence of tissue culture on the rate of introgression[J].Theoretical and applied genetics, 2005, 110(4): 678-687.
[11] Doroszewska T. Transfer of tolerance to different Potato virus Y (PVY) isolates fromNicotianaafricana Merxm. toNicotianatabacumL[J]. Plant breeding, 2010, 129(1): 76-81.
[12] Lewis R S.Nicotiana[M]// Kole C. Wild Crop Relatives:Genomic and Breeding. New York: Springer, 2011: 185-208.
[13] 魏治中, 魏克强. 烟草远缘杂交育种[M]. 北京: 中国农业科学技术出版社, 2008.Wei Zhizhong, Wei Keqiang. Tobacco distant hybridization breeding[M]. Beijing: Chinese agricultural science and technology press, 2008. (in Chinese)
[14] 王志愿, 姜清治, 霍沁建. 烟草黑胫病的研究进展[J]. 中国农学通报, 2010, 26(21): 250-255.Wang Zhiyuan, Jiang Qingzhi, Huo Qinjian. Process of research on tobacco black shank[J]. Chinese Agricultural Science Bulletin. 2010, 26(21): 250-255. (in Chinese)
[15] Li B C, Bass W T, Cornelius P L. Resistance to tobacco black shank inNicotianaspecies[J]. Crop science, 2006,46(2): 554-560.
[16] 杨铁钊. 烟草育种学[M]. 北京: 中国农业出版社, 2003.Yang Tiezhao. Tobacco breeding[M]. Beijing: China Agriculture Press, 2008. (in Chinese)
[17] 陈学平, 王彦亭. 烟草育种学[M]. 合肥: 中国科学技术大学出版社, 2002.Chen Xueping, Wang Yanting. Tobacco breeding[M]. Hefei:Press of University of Science and Technology of China,2002. (in Chinese)
[18] 佟道儒. 烟草育种学[M]. 北京:中国农业出版社,1997.Tong Daoru. Tobacco breeding[M]. Beijing: China Agriculture Press, 1997. (in Chinese)
[19] 陈瑞阳, 宋文芹, 李秀兰. 植物染色体标本制备的去壁-低渗法及其在细胞遗传学中的意义[J]. 遗传学报, 1982,9(2): 151-159.Chen Ruiyang, Song Wenqin, Li Xiulan. Wall degradation hypotonic method of preparing chromosome samples in plant and its signi fi cance in the cytogenetics[J]. Journal of Genetics and Genomics, 1982, 9(2): 151-159. (in Chinese)
[20] Brammer S P, Vasconcelos S, Poersch L B, et al. Genomic in situ hybridization in Triticeae: a methodological approach[M]//Andersen S B. Plant Breeding from Laboratories to Fields. Intech, 2013: 2-44.
[21] 谢朝添, 邱义兰, 葛丽丽, 等. 烟草花粉萌发和花粉管生长期间柱头和花柱中的钙分布[J]. 植物生理与分子生物学学报, 2005, 31(1): 53-61.Xie Chaotian, Qiu Yilan, Ge lili, et al. The distribution of calcium in the stigma and style of tobacco during pollen germination and tube growth[J]. Journal of Plant Physiology and Molecular Biology, 2005, 31(1): 53-61. (in Chinese)
[22] 王万能, 全学军, 肖崇刚. 烟草疫霉菌的产孢和接种方法研究[J]. 植物保护学报, 2005, 32(1): 18-22.Wang Wanneng, Quan Xuejun, Xiao Chonggang. Research on the methods of producing zoosporangia and inoculation of Phytophthora parasitica var.nicotianae[J]. Acta Phytophylacica Sinica, 2005, 32(1): 18-22. (in Chinese)
[23] 孙常伟. 重庆地区烟草黑胫病菌交配型及生理小种研究[D]. 重庆: 西南大学. 2009.Sun Changwei. Mating type and physiological race ofPhytophthoranicotianaein Chongqing[D]. Chongqing:Southwest University, 2009. (in Chinese)
[24] 刘廷利. 重庆地区烟草黑胫病菌致病性分化及小种鉴定[D]. 重庆: 西南大学. 2009.Liu Tingli. Pathogenicity differentiation and identification of race ofPhytophthora Parasiticain Chongqing[D].Chongqing: Southwest University, 2009. (in Chinese)
[25] 苗明军. SSR 标记在甘蓝遗传多样性及杂种鉴定中的应用研究[D]. 重庆: 西南大学, 2010.Miao Mingjun. Application of SSR marker on cabbage germplasm genetic diversity and purity identification[D].Chongqing: Southwest University, 2010. (in Chinese)
[26] 毕晓颖, 李卉, 娄琦, 等. 野鸢尾和射干属间杂交亲和性及杂种鉴定[J]. 园艺学报, 2012, 39(5): 931-938.Bi Xiaoying,Li Hui, Lou Qi, et al. Studies on inter-generic compatibility ofIris dichotomaandBelamcanda chinensisand their hybrids identification[D]. Acta Horticulturae Sinica, 2012, 39(5): 931-938. (in Chinese)
[27] 王晓玉. 尾叶紫薇与紫薇种间杂交育种研究[D]. 北京:北京林业大学, 2012.Wang Xiaoyu. Interspecific hybridizing betweenLagerstroemia indicaandL. caudate[D]. Beijing: Beijing Forestry University, 2012. (in Chinese)
[28] 刘文荣, 邓祖湖, 张木清, 等. 甘蔗斑茅的杂交利用及其杂种后代鉴定系列研究Ⅲ. 甘蔗斑茅远缘杂交后代细胞遗传分析[J]. 作物学报, 2005, 30(11): 1093-1096.Liu Wenrong, Deng Zuhu, Zhang Muqing, et al. Use and characterization of the genuine intergeneric hybrids from the cross ofSaccharumspp. andE. arundinaceumRetz. Ⅲ.Cytogenetic analysis for the hybrid and backcross progeny ofS. officinarumL. andErianthussect. Ripidium [J]. Acta Agronomica Sinica, 2005, 30(11): 1093-1096. (in Chinese)
[29] 于卓, 云锦凤, 马有志, 等. 加拿大披碱草×野大麦三倍体杂种染色体的分子原位杂交鉴定[J]. 遗传学报,2004, 31(7): 735-739.Yu Zhuo, Yun Jingfeng, Ma Youzhi, et al. Identification of the triploid hybrid chromosomes ofElymus canadensisL.×Hordeum brivisubulatumLink. by genomic in situ hybridization[J]. Journal of Genetics and Genomics, 2004,31(7): 735-739. (in Chinese)
[30] 徐霞. 小麦及其亲缘属植物染色体显带技术的研究和利用[J]. 国外农学: 麦类作物, 1993, 6: 48-50.Xu Xia. Research and application of shromosome banding technology in wheat and its relatives[J]. Agronomy abroad:Triticeae crops, 1993, 6: 48-50. (in Chinese)
[31] 钟少斌, 姚景侠. 簇毛麦及其与普通小麦杂种双二倍体的C-分带分析[J]. 遗传, 1992, 14(3): 7-9.Zhong Shaobin, Yao Jingxia. C band analysis ofHaynaldia villosa– wheat amphidiploid[J]. Hereditas, 1992, 14(3):7-9. (in Chinese)
[32] 王元英, 周健. 中美主要烟草品种亲源分析与烟草育种[J]. 中国烟草学报, 1995, 2(3): 11-22.Wang Yuanying, Zhou Jian. Parentage analysis of major tobacco varieties and tobacco breeding in America and China[J]. Acta Tabacaria Sinica, 1995, 2(3): 11-22. (in Chinese)
[33] Kaneko Y, Bang S W. Interspecific and intergeneric hybridization and chromosomal engineering of Brassicaceae crops[J]. Breed Sci, 2014, 64(1): 14–22.
[34] 康雷. 全套甘蓝型油菜-菘蓝单体异附加系及新型CMS的创建[D]. 武汉: 华中农业大学, 2014.Kang Lei. Establish of complete set ofBrassica napusisatis tinctoriamonosomic alien addition line and new type CMS[D]. Wuhan: Huazhong Agricultural University, 2014.(in Chinese)
[35] 李洪杰, 郭北海, 张艳敏, 等. 利用组织培养和辐射诱变创造高频率小麦与簇毛麦染色体易位[J]. 遗传学报,2000, 27(6): 511-519.Li Hongjie, Guo Beihai, Zhang Yanmin, et al. High efficient intergeneric chromosomal translocations between wheat(TriticumaestivumL.) andDasypyrum viliosumarising from tissue culture and irradiation[J]. Journal of Genetics and Genomics, 2000, 27(6): 511-519. (in Chinese)
[36] 钟冠昌, 穆素梅, 张正斌. 麦类远缘杂交[M]. 北京: 科学出版社, 2003.Zhong Guanchang, Mu Sumei, Zhang Zhengbin. Distant hybridization of wheat[M]. Beijing: Science press, 2003. (in Chinese)
[37] Zhan Haixian, Zhang Xiaojun, Li Guangrong, et al. Molecular characterization of a new Wheat-Thinopyrum intermediumtranslocation line with resistance to powdery mildew and stripe rust[J]. Int J Mol Sci, 2015, 16(1): 2162-2173.
Identi fi cation ofN. tabacumLin. andN. plumbaginifoliaViv. hybrid and primary analysis of its fertility and resistance to black shank disease
DANG Jiangbo1, ZHAO Shenqingyu1, DENG Honghong1, CAO Shuai1, LIANG Guolu1, ZHANG Yan2
1 College of horticulture and landscape, Southwest University, Chongqing 400716, China;2 Chongqing Tobacco Research Institute, Chongqing Municipal Tobacco Company, Chongqing 400716, China
Plant morphology of an o ff spring between octoploid plant ofN. tabacumLin. cv. Yunyan87 (2n=8x=96) andN. plumbaginifoliaViv. (2n=2x=20) was observed. Its cytogenetic characteristics, fertility and its resistance to black shank were investigated. Results showed that most main morphology indexes were between Yunyan87 tetraploid plant andN. plumbaginifolia. Corolla color was similar to Yunyan87 while anther color was similar toN. plumbaginifolia. It had 58 chromosomes, among which 10 were fromN. plumbaginifoliadetected by GISH. Pollen did not germinate in vitro and no capsules were harvested when the hybrid (clones) was crossed to Yunyan87 tetraploid plants as male and self. 102.60±25.12 germinating seeds were obtained in every capsule when the hybrid crossed to Yunyan87 tetraploid plants as female. No black shank specks on hybrid leaf and stem were found afterP. parasiticainfection. This was the same withN. plumbaginifolia. The o ff spring was the sure hybrid ofN. tabacumandN. plumbaginifolia(2n=5x=58). It was male sterile but female fertile and has strong resistance to black shank etiology.
N. tabacum;N. plumbaginifolia; interspeci fi c hybrid; fertility; black shank resistance
党江波,赵申清玉,邓红红,等.Nicotiana tabacumLin.-N. plumbaginifoliaViv.杂种的鉴定及其育性和黑胫病抗性的初步分析[J]. 中国烟草学报,2016,22(2)
中国烟草公司重庆市公司资助项目(No: 2012044)
党江波(1984—),博士研究生,主要从事烟草细胞遗传学研究,Tel:023-68250383,Email:dangjiangbo@126.com
梁国鲁(1960—),主要从事植物细胞遗传学研究,Tel:023-68250383,Email:lianggl@swu.edu.cn
2015-06-08
:DANG Jiangbo, ZHAO Shenqingyu, DENG Honghong, et al. Identi fi cation ofN. tabacumLin. andN. plumbaginifoliaViv.hybrid and primary analysis of its fertility and resistance to black shank disease[J]. Acta Tabacaria Sinica, 2016, 22(2)

