纳米银颗粒对游仆虫细胞结构的影响❋
任余廷, 倪 兵, 范鑫鹏, 顾福康
(华东师范大学生命科学学院,上海 200241)
纳米银颗粒对游仆虫细胞结构的影响❋
任余廷, 倪兵❋❋, 范鑫鹏, 顾福康
(华东师范大学生命科学学院,上海 200241)
本文利用微分干涉显微术、扫描及透射电镜术研究了不同浓度的50nm粒径三角平板状纳米银颗粒对游仆虫(Euplotessp.)细胞结构的影响。结果表明游仆虫细胞对于此类型纳米银的毒性较敏感。纳米银对细胞结构的影响主要体现在:(1)线粒体肿胀变形,内膜嵴状结构缺失;(2)细胞大核核仁聚集减少,染色质凝聚;(3)纤毛器发生顺序性脱落。除此之外,观察到纳米银颗粒存在于细胞表膜、细胞质、线粒体膜及其内部。初步推测,纳米银颗粒除可经过胞口之外,亦可直接通过细胞膜侵入细胞,主要通过损伤线粒体及细胞核,进而致使其他胞器结构紊乱,细胞形态及功能失常。
纳米银;游仆虫;线粒体;细胞核;自噬溶酶体
引用格式:任余廷, 倪兵, 范鑫鹏, 等. 纳米银颗粒对游仆虫细胞结构的影响[J]. 中国海洋大学学报(自然科学版), 2016, 46(10): 57-64.
REN Yu-Ting, NI Bing, FAN Xin-Peng, et al. The influence of silver nanoparticles on the cell structure ofEuplotessp. (Ciliophora, Hypotrichida)[J]. Periodical of Ocean University of China, 2016, 46(10): 57-64.
纳米银是目前应用最广泛的纳米材料,因具有独特的量子效应、小尺寸效应以及极大的比表面积,其在抗氧化能力、导电性能、抗菌能力方面具有极大的优势,因而广泛应用于医疗保健、电子电器、化妆品等日常生活用品领域[1-3]。然而,体外细胞实验证实纳米银是毒性最强的金属纳米材料之一[4-5],而释放到水环境中的纳米银会对水体产生何种影响,对水生生物、食物链以及接触水体的动物产生何种影响也逐渐引起了科学界的重视[6]。原生动物处于水体食物链的底端,且对环境条件有一定的要求,因此很早就被应用于环境检测及毒性评价中[7-8]。目前纳米银对原生动物毒性的研究报道甚少[9],游仆虫(Euplotessp.)取材容易、培养方便、对环境敏感度高且细胞体积大,便于观察处理。研究纳米银对游仆虫细胞结构的影响,为深入探索纳米银对细胞的毒性机制及侵入细胞的方式提供实验依据。
1 材料和方法
1.1 材料
游仆虫细胞采自上海市闵行区紫竹园内一封闭式淡水池塘,经分离纯化后,用麦粒发酵液培养的鞭毛虫草履唇滴虫(Chilomonasparamecium)喂食培养,培养温度24℃。
纳米银颗粒原液由华东师范大学生命科学学院生物材料实验室提供,使用前15000r/min离心10min后弃去上清液,自然烘干后加培养水,超声解聚,配置成3mg/L的颗粒悬浮母液,现配现用。
1.2 不同浓度纳米银处理后细胞半致死浓度(LC50)的测定
取预实验获得的9个浓度梯度(0、0.05、0.1、0.2、0.3、0.6、0.8、1.0、1.25mg/L)的纳米银溶液各0.5mL于表面皿中,加入200个细胞,实验4h,设4个平行,结果取其平均值,每0.5h观察1次。将纳米银质量浓度扩大100倍后换算成对数作为横坐标,根据死亡百分数-几率单位换算表将死亡百分数换算成死亡几率作为纵坐标[10],利用回归法求出LC50的值。
1.3 样品制备及观察
取6个干净的表面皿,根据LC50数据以及大量实验观察确定纳米银溶液浓度(0、0.2、0.5、1.0、1.5、3.0mg/L),同时在每个培养皿中放入100只生长状态良好,形态大小相似的游仆虫细胞,处理4h后,用吸管吸取0.2mL虫液于载玻片上,加盖玻片,在体视镜及Olympus BX 51微分干涉显微镜下观察细胞形态以及运动状态。
扫描电镜样品按顾福康等[11]报道的方法,1%的锇酸和饱和升汞溶液按1∶6的比例混合后,4℃固定样品10min,蒸馏水漂洗,梯度乙醇脱水之后,进行临界点干燥、喷金,于Hitachi S-4800扫描电镜下观察。
透射电镜样品制备按顾福康等[12]报道的方法并稍作修改,2.5%戊二醛和2%锇酸混合液4℃固定样品10min,蒸馏水漂洗,再用1%锇酸固定1h,漂洗后经常规脱水包埋、超薄切片,于Hitachi HT7700透射电镜下观察。
2 结果
2.1 LC50的值及显微水平下游仆虫细胞形态和行为的变化
由图1可知,游仆虫细胞的死亡几率和纳米银颗粒的浓度对数呈线性关系,4h的回归方程为y=0.4529x+4.0627,R2=0.7645,得出LC50=0.94mg/L。
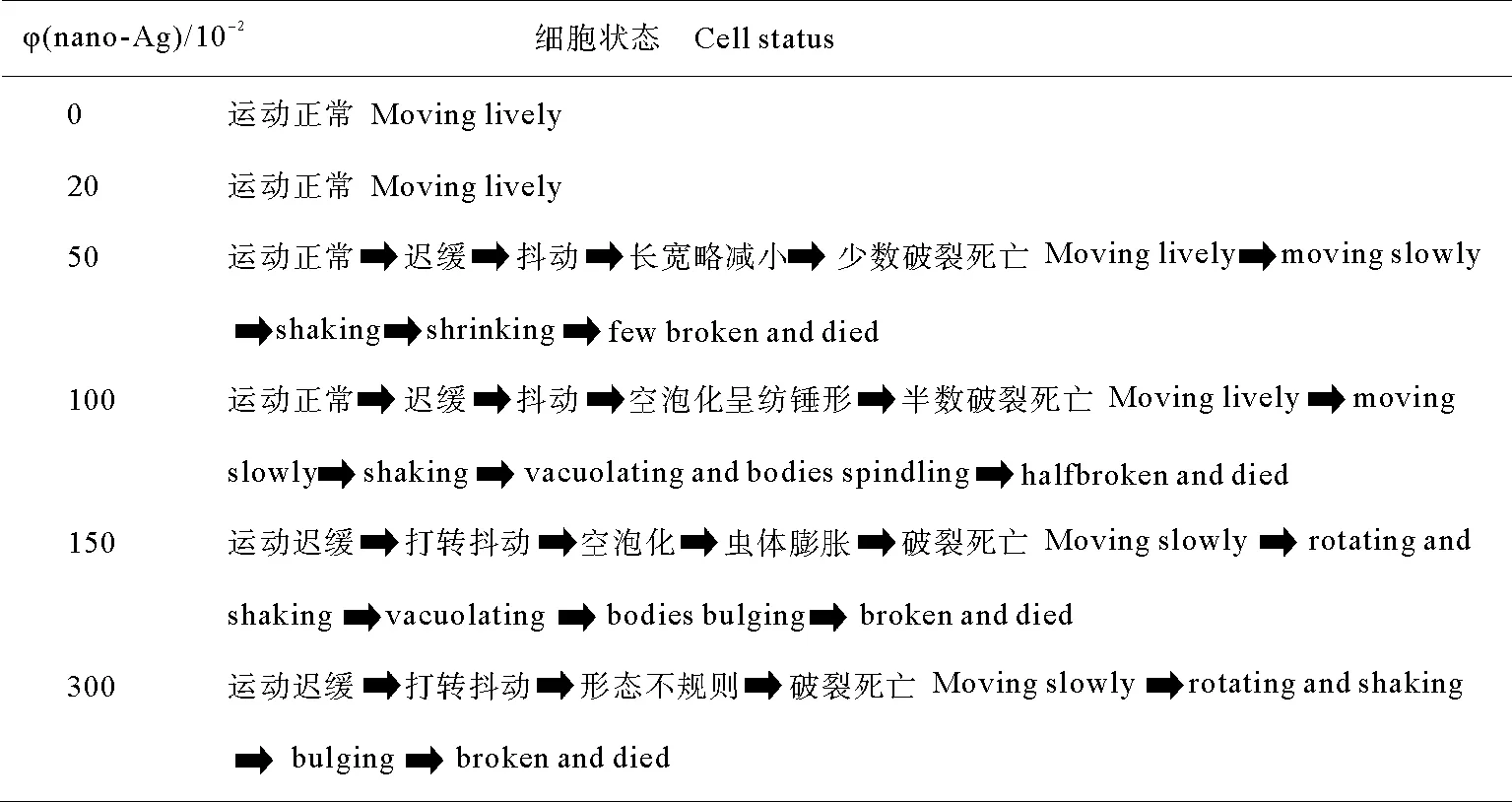
光学显微镜下观察,对照组游仆虫细胞体长130~140μm,宽80~90μm(见图1A、B),运动活泼,当经纳米银浓度低于0.3mg/L处理时,与对照组细胞比较,细胞形态与细胞运动未见异常,口纤毛器及体纤毛器均无明显变化。由0.5~0.7mg/L纳米银处理后,细胞体略减小,细胞内部出现直径不等的空泡;1.0~1.5mg/L纳米银处理后,与对照组相比,细胞体长缩短至原体长的3/4(见图1C~E);而随着纳米银颗粒处理浓度的增大细胞的形态及行为变化如表1。
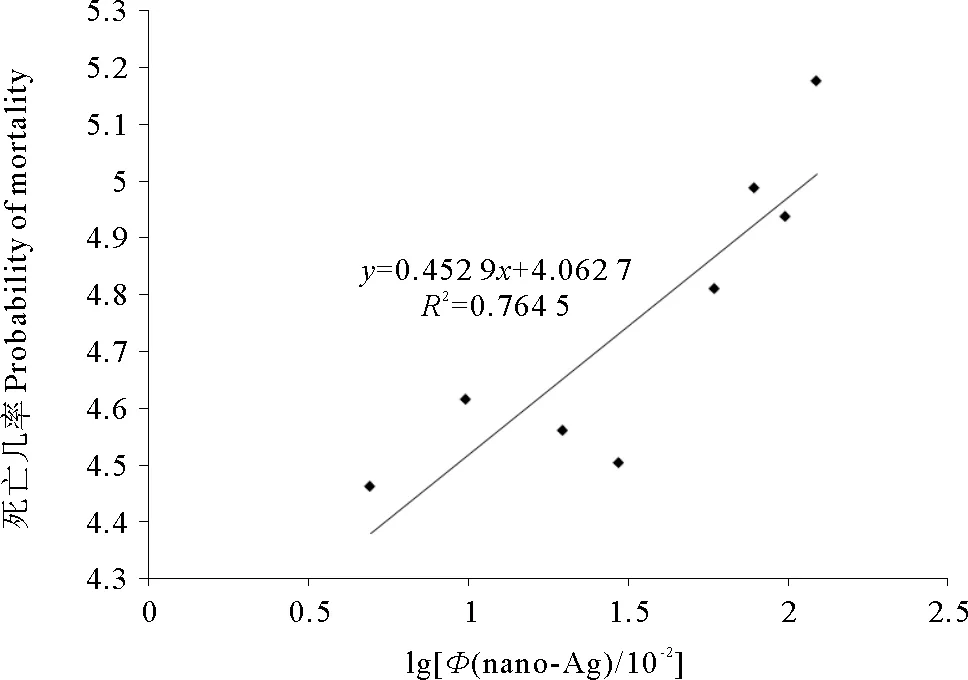
图1 游仆虫细胞对纳米银颗粒悬浮液4h急性 毒性反应浓度对数-死亡几率曲线Fig.1 4 hours log of dosage-mortality curve of Euplotes sp. under different concentrations of silver nanoparticles medium表1 50 nm三角平板状纳米银颗粒对游仆虫细胞4h毒性作用Table 1 4 hours toxic effects of 50 nm sized triangle plate shaped SNPs on Euplotes sp.
2.2 对照组游仆虫细胞的亚显微结构
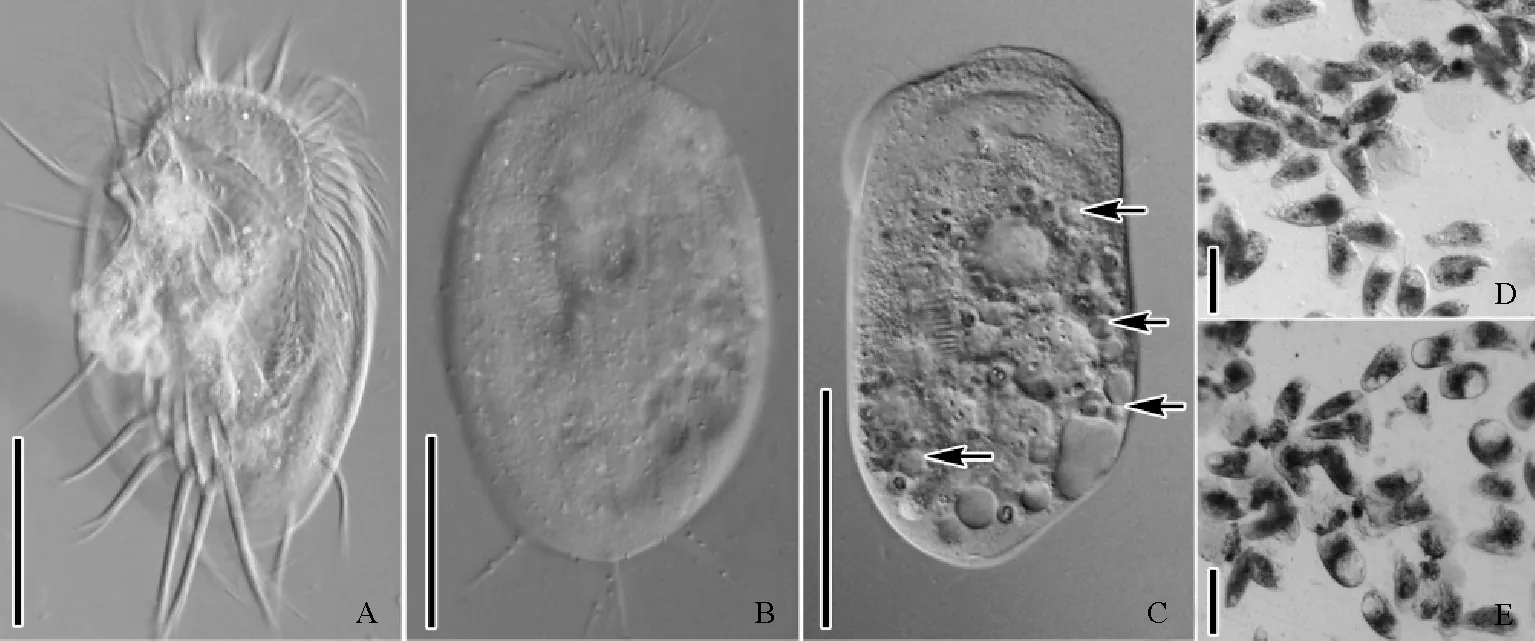
扫描电镜显示,游仆虫细胞呈椭圆形(见图2A、B)。腹面纤毛器中,口围带自基部在口腔左后侧起始向前口缘弯曲,终止于口缘右前端,呈倒“C”型,约占体长的2/3,含40~50片整齐排列的小膜;波动膜位于口围带基部右侧,它的毛基体向口围带方向伸出聚集成形似刷子的纤毛;额腹棘毛9根,横棘毛5根,尾棘毛2根和左缘棘毛2根,按一定方式定位于细胞表面的特定区域。细胞背面隆起,其中8列背触毛成纵向排列,每列含13~16根背触毛。
透射电镜观察到,游仆虫细胞质致密,在口围带波动膜区皮层内含有大量的长条形咽肋片结构,其肋片成紧密、整齐排列(见图3G);在口纤毛区皮层附近,线粒体呈椭球形,由双层膜构成,其内、外膜清晰,内膜向内折叠成长短不等、形如管状或片状的嵴;细胞核大核中,核膜界限明显,大核染色质电子密度较高,在核内成团块状稀疏分布,其间并含有大量的电子密度较低的核仁颗粒(见图4A)。
(A、B:正常游仆虫腹面观(A)与背面观(B);C:1.5mg/L处理组,箭头示纳米银处理后细胞出现空泡化;D、E:1.0mg/L(D),1.5mg/L(E)处理组低倍镜下细胞整体观。A~C:标尺=50μm;D、E:标尺=100μm。A, B: Ventral view (A) and dorsal view (B) of normal cells; C: 1.5mg/L treatment group, arrows show cytoplasmic vacuolation after treatment with silver nanoparticles; D, E: The general view of cells at low magnification in 1.0mg/L (D) and 1.5mg/L (E) treatment groups respectively. A-C: Bar=50μm; D, E: Bar=100μm.)
图2游仆虫细胞活体观察
Fig.2Morphology ofEuplotessp. from life
2.3处理组游仆虫细胞扫描电镜观察
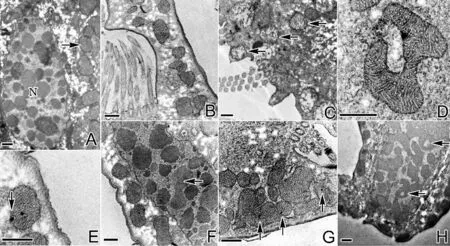
进一步采用扫描电镜观察,经0.5~0.7mg/L纳米银处理后,口围带小膜的纤毛自前端开始脱落,小膜纤毛脱落后,其中领部小膜基体完全裸露(见图2C、D);额-腹棘毛中其纤毛聚集在一起形成的纤毛复合结构出现纤毛散乱,纤毛杆逐渐断裂,纤毛基体显露。1.0~1.5mg/L纳米银处理后,口围带小膜的纤毛脱落逐渐从领部向翻领部扩展(见图2E),同时前端小膜裸露的基体发生脱落(见图2F),翻领部小膜纤毛部分脱落,基体裸露(见图2G),底端小膜的纤毛仍然保留;额-腹棘毛中其聚集的纤毛完全断裂脱落,可见其基部毛基体列(见图2H);横棘毛的纤毛复合结构中纤毛杆也出现脱落,基体显露(见图2J)。3mg/L纳米银处理后,细胞体呈椭球形,仅口围带底端小膜的纤毛保留,构成额-腹-横棘毛纤毛复合结构的纤毛全部断裂脱落(见图2I、K)。
(A:正常游仆虫细胞腹面观(AZM-C,口围带领部;AZM-L,口围带翻领部;UM,波动膜;FVC,额-腹棘毛;TC,横棘毛;LMC,左缘棘毛);B:正常游仆虫细胞背面观(DK,背触毛);C:0.5mg/L处理组,口围带及额-腹棘毛;D:0.5mg/L处理组,口围带领部(箭头示裸露的小膜基体);E:1.5mg/L处理组,游仆虫腹面观;F:1.5mg/L处理组,口围带领部小膜毛基体脱落(箭头示毛基体脱落);G:1.5mg/L处理组,口围带翻领部(箭头示裸露的小膜基体);H:1.5mg/L处理组,额棘毛纤毛杆脱落;I:3.0mg/L处理组,游仆虫腹面观;J:1.5mg/L处理组,横棘毛(箭头示横棘毛脱落后裸露的毛基体);K:3.0mg/L处理组,额棘毛基体断裂。A~C、E、I、J:标尺=10μm;D、F、G、H、K:标尺=1μm。A, B: Ventral view (A) and dorsal view (B) of normal cells (AZM-C, collar region of adoral zone of membranelles; AZM-L, lapel region of adoral zone of membranelles; UM, undulating membranes; FVC, frontal-ventral cirri; TC, transverse cirri; LMC, left marginal cirri; DK, dorsal kineties); C: Adoral zone of membranelles and frontal-ventral cirri in 0.5 mg/L treatment group; D: Collar region of adoral zone of membranelles in 0.5 mg/L treatment group (arrows refer to the bare kinetosomes of membranelles); E: Ventral view in 1.5 mg/L treatment group; F: Kinetosomes of membranelles in collar region desquamated (arrows) in 1.5 mg/L treatment group; G: Lapel region of adoral zone of membranelles in 1.5 mg/L treatment group (arrows refer to the bare kinetosomes of membranelles); H: Cilia of frontal cirri ruptured in 1.5 mg/L treatment group; I: Ventral view in 3.0 mg/L treatment group; J: Cilia of transverse cirri ruptured in 1.5 mg/L treatment group, (arrow shows the bare kinetosomes); K: Cilia of frontal cirri ruptured in 3.0 mg/L treatment group. A~C, E, I, J: Bar=10 μm; D, F, G, H, K: Bar=1 μm.)
图3游仆虫细胞扫描电镜观察
Fig.3Scanning electron microscopy observation ofEuplotessp.
2.4 处理组游仆虫细胞透射电镜观察
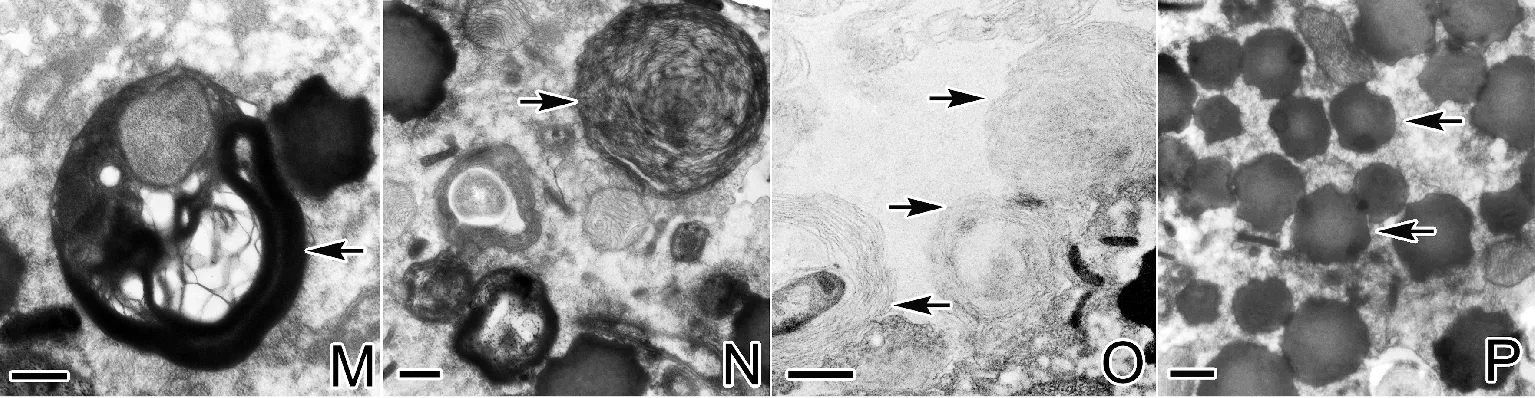
在处理组游仆虫细胞表面及内部观察到纳米银颗粒(见图3A~D),此外,在线粒体的膜及线粒体内部也发现纳米银颗粒的存在(见图4E、G)。(1)0.5mg/L纳米银溶液处理后,细胞口皮层区部分线粒体出现轻微肿胀现象(见图4B),并相伴出现少量含有自身结构物质的自噬体(见图3E),但细胞核及其他胞内结构未见损伤。(2)1.0mg/L纳米银处理后,细胞口围带基部皮层下聚集大量多层膜状结构(见图3F),同时口围带右侧皮层下咽肋片结构排列紊乱,散乱分布于口皮层细胞质(见图3G、H);位于口纤毛器基部皮层的线粒体其内膜形成的嵴状结构由外向内瓦解、缺失,附近的表膜下线粒体肿胀变形严重,且在线粒体内观察到纳米银颗粒(见图4C~E);大核核染色质凝聚成边缘凹凸不平的块状结构,核仁稀少(见图4F);此外,细胞内出现大量的膜性髓状结构以及含有自身物质且处于自噬中期的自噬溶酶体(见图3I~N)。(3)1.5mg/L纳米银处理后,相应于口围带基部的皮层下,膜状结构散乱且集聚在一起(见图3O),大量线粒体也出现团聚融合且极度肿胀的现象(见图4G);细胞核核膜界限模糊,核仁消失,染色质凝聚成不规则的大块状结构,部分染色质团内出现孔洞(见图4H);胞内密集分布着大量电子密度极高的处于自噬后期的自噬溶酶体(见图3P)。
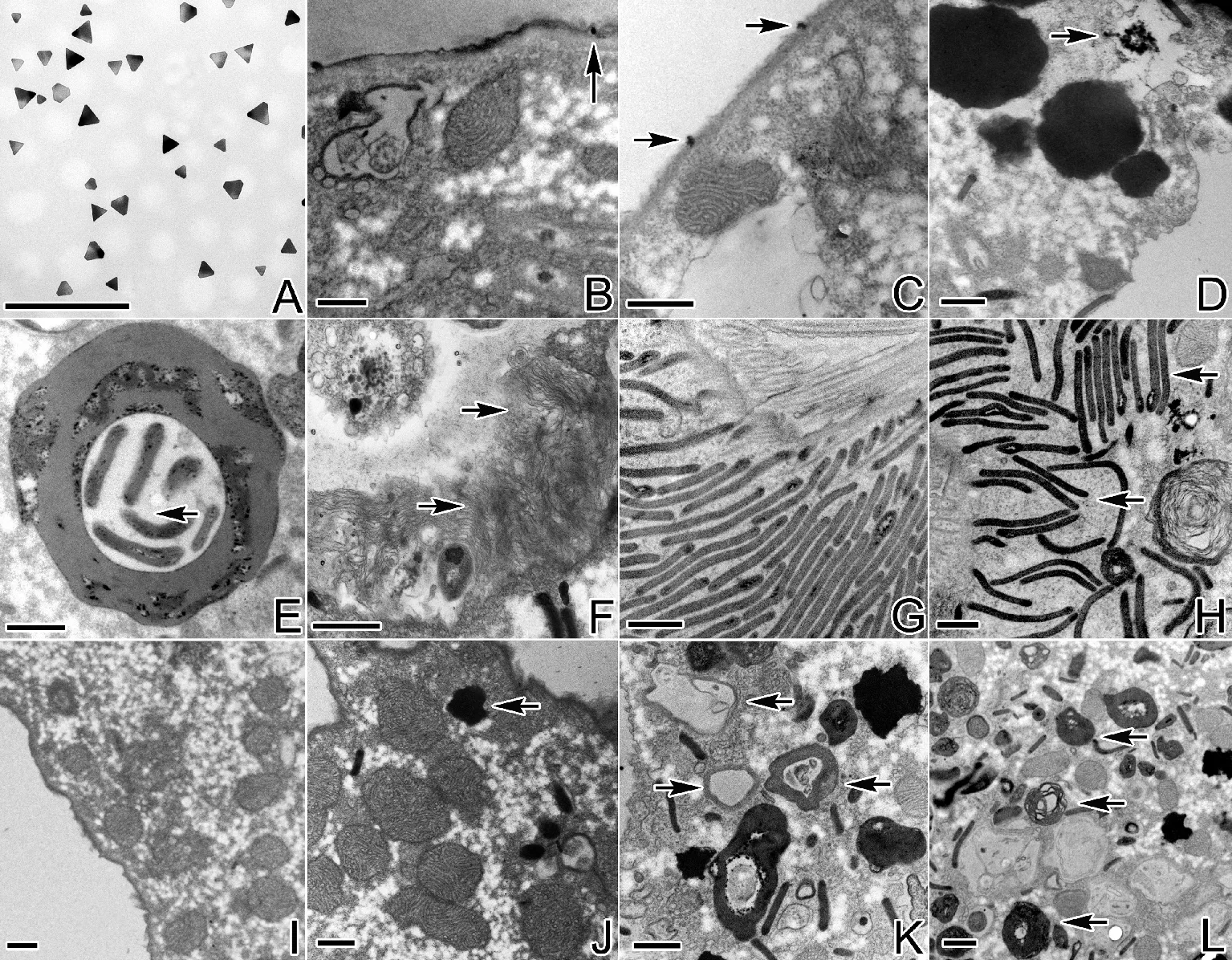
(A:粒径40~60nm纳米银颗粒;B、C:1.0mg/L处理组,细胞膜上的纳米银颗粒(箭头示纳米银颗粒);D:1.5mg/L处理组,箭头示细胞内聚集的纳米银颗粒;E:0.5mg/L处理组,内含肋片结构的自噬体(箭头示肋片结构);F:1.0mg/L处理组,箭头示多层膜性结构;G:对照组细胞中正常排列的口区皮层下肋片结构;H:1.0mg/L处理组,箭头示紊乱的肋片结构;I、J:对照组细胞质(箭头示自噬溶酶体);K、L:1.0mg/L处理组,箭头示膜性髓状结构及处于自噬中期的自噬溶酶体;M、N:1.0mg/L处理组,箭头示处于自噬中期的自噬溶酶体;O、P:1.5mg/L处理组,箭头示多层膜性结构及处于自噬后期的自噬溶酶体。A~P:标尺=0.5μm。A: 40~60 nm sized silver nanoparticles; B, C: Silver nanoparticles located in the cell membrane in 1.0 mg/L treatment group (arrows show silver nanoparticles); D: Arrow shows aggregation of silver nanoparticles in the cell in 1.5 mg/L treatment group; E: Autophagic lysosome containing pharyngeal disks in 0.5 mg/L treatment group (arrow shows the pharyngeal disks); F: Arrows show multilayer membrane structure in 1.0 mg/L treatment group; G: The normal arrangement of pharyngeal disks under the oral cortex; H: Arrows show the disorder of pharyngeal disks in 1.0 mg/L treatment group; I, J: The cytoplasm in the control group (arrow shows autophagic lysosome); K, L: Arrows show membranous medullary structures and autophagic lysosome at the middle stage of autophagy in 1.0 mg/L treatment group; M, N: Arrows show autophagic lysosome at the middle stage of autophagy in 1.0 mg/L treatment group; O, P: Arrows show multilayer structure and autophagic lysosome at the late stage of autophagy in 1.5 mg/L treatment group. A~P: Bar=0.5 μm.)
图4游仆虫细胞透射电镜观察
Fig.4Transmission electron microscopy observation ofEuplotessp.
(A:对照组细胞核及线粒体(N,细胞核,箭头示线粒体);B:0.5mg/L处理组变形线粒体;C,D:1.0mg/L处理组,箭头示不同损伤程度的线粒体;E:1.0mg/L处理组,箭头示线粒体膜上的纳米银颗粒;F:1.0mg/L处理组,箭头示聚集的核仁;G:1.5mg/L处理组肿胀的线粒体,箭头示纳米银颗粒;H:1.5mg/L处理组,箭头示染色质上的空洞。A~H:标尺=0.5μm。A: The nucleus and mitochondria in the control group (N: nucleus, arrow refers to mitochondria); B: The deformation of mitochondria in 0.5 mg/L treatment group; C, D: Arrows show different damage degree of mitochondria in 1.0 mg/L treatment group; E: Arrow shows silver nanoparticles in the membrane of mitochondria in 1.0 mg/L treatment group; F: Arrow shows the deformation of the nucleolus in 1.0 mg/L treatment group; G: Mitochondria swelling dramatically in 1.5 mg/L treatment group, arrows refer to silver nanoparticles; H: Arrow shows the hole on the chromatin in 1.5 mg/L treatment group. A~H: Bar=0.5 μm.)
图5细胞核及线粒体的透射电镜观察
Fig.5Transmission electron microscopy observation of nucleus and mitochondria
3 讨论
3.1 纳米银颗粒对游仆虫细胞的毒理作用
目前研究表明原生动物四膜虫(Tetrahymena)LC50=38mg/L,草履虫(Paramecium)LC50=39mg/L,与其他水生无脊椎动物相比,其对于纳米银有更强的耐受性[13-14]。而本实验测得纳米银颗粒对游仆虫细胞的LC50=0.94mg/L,远远低于其他2种原生动物,出现这种差异的原因可能有2种:首先,四膜虫、草履虫为嗜污种类,主要大量出现于受污染以及有机物丰富的水体中[15-17],对于环境有极强的适应性,也常用于污水处理[18-19],而游仆虫多生活于水质较清的环境,因此对于毒性物质的敏感度高;其次,本实验选用的是50nm左右三角平板状的纳米银,在水中的分散能力极强,同时活性也强于前人报道中采用的棒状纳米银和球形纳米银[13-14,20],据此推测可能对实验对象的致死作用更明显。
3.2 纳米银颗粒对游仆虫细胞亚显微结构的影响
纳米银对细胞亚显微结构的影响主要体现在线粒体、细胞核、纤毛器微管胞器上。(1)与对照组相比,随着浓度的增加,处理组细胞的线粒体结构肿胀变形严重,而口皮层线粒体内膜嵴状结构的缺失由内向外也在不断加重。产生这种现象的原因可能是由于Ag+对于硫具有很高的亲和性,因此易与细胞含有巯基的蛋白质及酶结合,使酶蛋白失活,而这些蛋白质和酶都是细胞抵抗氧化损伤级联反应的重要组成[21],可见纳米银颗粒使得抗氧化机制耗竭,活性氧累积,而过量累积的活性氧又能引起线粒体功能的紊乱和丧失,致使线粒体损伤严重[22]。(2)随着纳米银处理浓度的增加,大核核膜界限逐渐模糊,核仁聚集且明显减少,染色质发生凝聚,部分染色质团内部出现孔洞。这种现象在其他纳米材料对细胞超微结构的研究中也有类似报道[23],可能是因为纳米银诱导了活性氧的产生,阻断ATP的合成,从而影响了细胞核功能的正常进行,致使其结构损伤严重[24]。(3)随着处理浓度的增加,口围带小膜的纤毛从领部至翻领部发生脱落,与此同时额-腹-横棘毛的纤毛也发生了顺序性脱落,毛基体裸露甚至脱落,造成这种现象的原因可能是纳米银颗粒可直接与纤毛器相接触,释放大量Ag+使其膜电位改变,从而损伤纤毛器[25],另外,细胞核及线粒体的损伤可能影响到细胞内微管基因的表达及能量的供应,从而阻碍了微管蛋白的合成组装,致使细胞骨架异常,纤毛器受损,因而,细胞无法保持正常形态,细胞运动受影响[26-27]。
3.3 纳米银进入小腔游仆虫细胞的方式
目前报道的关于纳米材料进入细胞的方式主要有3种机制:被吞噬进入细胞内部、存在于液体中被细胞胞饮以及受体介导的内吞作用[28]。而纳米材料进入原生动物的方式是通过吞噬形成食物泡[16,23],但有报道称SiO2和Fe3O4纳米颗粒可直接穿透细胞膜侵入细胞[29]。本研究在游仆虫细胞表膜及其内部,线粒体膜及其内部观察到纳米银颗粒,推测纳米银颗粒除由口结构摄食途径进入细胞外,还可能是通过表膜直接进入细胞,而进入细胞的纳米银颗粒会通过线粒体膜从而进入到线粒体内对细胞产生影响。
[1]白世贞, 沈欣.纳米银抗菌化妆品的研制[J]. 哈尔滨商业大学学报(自然科学版), 2010, 26(5): 619-621.
Bai S Z, Shen X. Development of antiseptic nano-silver cosmetics [J]. Journal of Harbin University of Commerce(Natural Sciences Edition), 2010, 26(5): 619-621.
[2]程定超, 杨洁, 赵艳丽. 纳米银抗菌材料在医疗器具与生活用品中的应用[J]. 医疗卫生装备, 2005, 25(11): 27-30.
Cheng D C, Yang J, Zhao Y L. Antibacterial materials of silver nanoparticles application in medical appliances and appliances for daily use [J]. Chinese Medical Equipment Journal, 2005, 25(11): 27-30.
[3]Chen X, Schluesener H J. Nanosilver: A nanoproduct in medical application [J]. Toxicology Letters, 2008, 176(1): 1-12.
[4]Hussain S M, Hess K L, Gearhart J M, et al. In vitro toxicity of nanoparticles in BRL 3A rat liver cells [J]. Toxicology in Vitro, 2005, 19(7): 975-983.
[5]Bar-Ilan O, Albrecht R M, Fako V E, et al. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos [J]. Small, 2009, 5(16): 1897-1910.
[6]Wijnhoven S W P, Peijnenburg W J G M, Herberts C A, et al. Nano-silver-a review of available data and knowledge gaps in human and environmental risk assessment [J]. Nanotoxicology, 2009, 3(2): 109-138.
[7]周可新, 许木启, 曹宏, 等. 土壤原生动物在环境监测中的应用[J]. 动物学杂志, 2003, 38(1): 80-84.
Zhou K X, Xu M Q, Cao H, et al. Soil protozoa as monitors of the environment [J]. Chinese Journal of Zoology, 2003, 38(1): 80-84.
[8]王正方, 齐小花, 邹明强, 等. 三聚氰胺对纤毛类原生动物梨形四膜虫的细胞毒性[J]. 生态毒理学报, 2009, 4(1): 35-39.
Wang Z F, Qi X H, Zhou M Q, et al. Cytotoxicity assessment of melamine using the ciliated protozoanTetrahymenapyriformis[J]. Asian Journal of Ecotoxicology, 2009, 4(1): 35-39.
[9]Ivask A, Juganson K, Bondarenko O, et al. Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: A comparative review [J]. Nanotoxicology, 2014, 8(8): 57-71.
[10]Broderius S J, Smith L L. Lethal and sublethal effects of binary mixtures of cyanide and hexavalent chromium, zinc, or ammonia to fathead minnow (Pimephalespromelas) and rainbow trout (Salmogairdneri) [J]. Journal of the Fisheries Research Board of Canada, 1979, 36(2): 164-172.
[11]顾福康, 倪兵. 包囊游仆虫休眠包囊的超微结构研究[J]. 实验生物学报, 1995, 28(2): 163-171.
GU F K, Ni B. An ultrastructural study on resting cyst ofEuplotesencysticus[J]. Acta Biologiae Experimentalis Sinica, 1995, 28(2): 163-171.
[12]顾福康, 倪兵. 原生动物的扫描电镜样品制备方法探讨[J]. 电子显微学报, 1993(6): 526-529.
GU F K, Ni B. The exploration of preparing protozoan specimens for scanning electron microscopy [J]. Journal of Chinese Electron Microscopy Society, 1993(6): 526-529.
[13]Bondarenko O, Juganson K, Ivask A, et al. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review [J]. Archives of Toxicology, 2013, 87(7): 1181-1200.
[14]Kvitek L, Vanickova M, Panacek A, et al. Initial study on the toxicity of silver nanoparticles (NPs) againstParameciumcaudatum[J]. The Journal of Physical Chemistry C, 2009, 113(11): 4296-4300.
[15]Gerhardt A, Ud-Daula A, Schramm K W.Tetrahymenaspp. (Protista, Ciliophora) as test species in rapid multilevel ecotoxicity tests [J]. Acta Protozoologica, 2010, 49(4): 271-280.
[16]Juganson K, Mortimer M, Ivask A, et al. Extracellular conversion of silver ions into silver nanoparticles by protozoanTetrahymenathermophile[J]. Environmental Science, 2013, 15(1): 244-250.
[17]Curds C R, Cockburn A. Protozoa in biological sewage-treatment processes—II. Protozoa as indicators in the activated-sludge process [J]. Water Research, 1970, 4(3): 237-249.
[18]Amanchi N R. Acute toxicity and cytogenetic effects of monocrotophos inParameciumcaudatumandOxytrichafallax[J]. Indian Journal of Fundamental and Applied Life Sciences, 2011, 1(3): 65-70.
[19]Sauvant M P, Pepin D, Piccinni E.Tetrahymenapyriformis: A tool for toxicological studies, A review [J]. Chemosphere, 1999, 38(7): 1631-1669.
[20]Edwards-Jones V. The benefits of silver in hygiene, personal care and healthcare [J]. Letters in Applied Microbiology, 2009, 49(2): 147-152.
[21]Blokhina O, Virolainen E, Fagerstedt K V. Antioxidants, oxidative damage and oxygen deprivation stress: A review [J]. Annals of Botany, 2003, 91(2): 179-194.
[22]Xia T, Kovochich M, Brant J, et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an axidative stress paradigm [J]. Nano Letters, 2006, 6(8): 1794-1807.
[23]杨林颖, 马瑞, 翟楠, 等. 纳米二氧化钛对纤毛虫阔口游仆虫超微结构的影响研究[J]. 生物学杂志, 2014, 31(5): 37-40.
Yang L Y, Ma R, Zhai N, et al. The influence of nano-TiO2on the ultrastructure ofEuploteseurystomus( Ciliophora, Hypotrichida) [J]. Journal of Biology, 2014, 31(5): 37-40.
[24]Asharani P V, Mun G L K, Hande M P, et al. Cytotoxicity and genotoxicity of silver nanoparticles in human cells [J]. American Chemical Society Nano, 2009, 3(2): 279-290.
[25]Kim K J, Sung W S, Suh B K, et al. Antifungal activity and mode of action of silver nano-particles onCandidaalbicans[J]. Biometals, 2009, 22(2): 235-242.
[26]尹飞, 赵柳, 周瑶, 等. 华美游仆虫皮层细胞骨架的超微结构及免疫电镜观察[J]. 中山大学学报, 2009, 48(5): 97-102.
Yin F, Zhao L, Zhou Y, et al. Ultrastructure and immunoelectron microscopic observations on cortical microtubular cytoskeleton ofEuploteselegans(Ciliophora: Hypotrichida) [J]. Acta Scientiarum Naturalium Universitatis Sunyatseni, 2009, 48(5): 97-102.
[27]顾福康, 邹士法, 李艺松, 等. 镰游仆虫腹面皮层细胞骨架的扫描电镜观察[J]. 动物学报, 2003, 49(4): 514-521.
Gu F K, Zhou S F, Li Y S, et al. Scanning electron microscopic observations on the ventral cortical cytoskeleton ofEuplotesharpa(Protozoa, Ciliophora) [J]. Acta Zoologica Sinica, 2003, 49(4): 514-521.
[28]Lorenz M R, Holzapfel V, Musyanovych A, et al. Uptake of functionalized, fluorescent-labeled polymeric particles in different cell lines and stem cells [J]. Biomaterials, 2006, 27(14): 2820-2828.
[29]杨勇骥, 汤莹, 于洋, 等. 纳米材料侵入细胞的电镜研究[J]. 电子显微学报, 2008, 27(5): 400-403.
Yang Y J, Tang Y, Yu Y, et al. Electron microscopic study on the ultrastructure of cell invaded by nanomaterials [J]. Journal of Chinese Electron Microscopy Society, 2008, 27(5): 400-403.
责任编辑高蓓
The Influence of Silver Nanoparticles on the Cell Structure ofEuplotessp. (Ciliophora, Hypotrichida)
REN Yu-Ting, NI Bing, FAN Xin-Peng, GU Fu-Kang
(School of Life Sciences, East China Normal University, Shanghai 200241, China)
Silver nanoparticles (SNPs) are being used increasingly in health care, electronic products, cosmetics, and various household products. However, it is more likely that these SNPs are released from consumers and household products into the environment, which will be a threat to aquatic organisms or animals exposed to them. Meanwhile, the ciliated protists are sensitive to environmental changes, and therefore are often used as bio-indicators. The influence of theEuplotessp. structure in response to different concentration of 50 nm sized triangle plate shaped SNPs was visualized by differential interference contrast microscopy, scanning electron microscopy and transmission electron microscopy. The results show thatEuplotessp. is more sensitive to the toxicity of SNPs. The median lethal concentrations (LC50 values) was obtained by means of probit method, and the LC50, 4 hof silver nanoparticles medium onEuplotessp. were 0.94 mg/L. And the toxic effects of SNPs on cell structure reflected mainly in the following aspects: (1) in different treatment groups, the morphology ofEuplotessp. cells changed obviously, such as, the length and width decreasing significantly, cilia motion slowing down, body shape changing from normal oval to spindle or even flat-spherical, and cytoplasmic vacuolation present; (2) most of the ciliary shafts of membranelles in the collar region and lapel region and frontal-ventral-transverse cirri ruptured sequentially, and kinetosomes of certain frontal-ventral-transverse cirri were also desquamated; (3) under the oral cortex, the lack of mitochondrial crista gradually aggravated from inside to outside, and under the pellicle, mitochondria swelled dramatically; (4) a large number of multilayer membrane structure gathered under the cortex of adoral, and pharyngeal disks dispersedly distributed in the cytoplasm; (5) with the nucleolus and chromatin gathering, the macronucleus membrane gradually became blurred, and holes appeared on the part of the chromatin mass; and (6) a large number of autophagic lysosomes and the medullary structures formed in the cytoplasm. In addition, SNPs were observed to be located in cell membrane, cytoplasm, mitochondria membrane and its interior. According to available data, it is speculated that SNPs can be invaded through the cell membrane besides of cytostome, and mainly by damaging the mitochondrial and macronucleus the toxicity can causes damages of other organelles which leads to cell morphology and functional disorders.
silver nanoparticles;Euplotessp.; mitochondria; nucleus; autophagic lysosome
国家自然科学基金项目(31572223)资助
2016-01-28;
2016-03-21
任余廷(1992-),硕士生。E-mail: Renyt1992@163.com
❋❋通讯作者:E-mail:bni@bio.ecnu.edu.cn
Q952;Q954
A
1672-5174(2016)10-057-08
10.16441/j.cnki.hdxb.20160003
Supported by the National Natural Science Foundation of China(31572223)

