AOX介导的线粒体与叶绿体之间的信号传导
林宏辉,张大伟,李鹏旭,翟晨蕾,韩雪莹
(四川大学 a.生物资源与生态环境教育部重点实验室;b.生命科学学院,成都 610064)
AOX介导的线粒体与叶绿体之间的信号传导
林宏辉,张大伟,李鹏旭,翟晨蕾,韩雪莹
(四川大学a.生物资源与生态环境教育部重点实验室;b.生命科学学院,成都 610064)
交替氧化酶是植物抗氰呼吸途径重要的末端氧化酶。在植物转绿过程中,AOX缺陷突变体表现出叶绿素积累延迟的现象。最近的研究表明。这种叶绿素积累的延迟在高光条件下更为显著,主要原因在于相关质体酶的转运受阻,而这些酶可以催化四吡咯或叶绿醇的生物合成。同时,抑制抗氰呼吸也伴随着质体NADPH/NADP+比率的增加,尤其是在高光处理条件下。因此表明,光照强度可以通过一个代谢信号在植物转绿过程中影响叶绿素的合成,而这个代谢信号很可能与AOX改变质体中 NADPH/NADP+的比率有关。总之,AOX介导的线粒体和叶绿体之间信号传导对维持光合作用能力至关重要。
交替氧化酶;代谢信号;NADPH/NADP+比率;光合效率
0 引 言
细胞核,质体和线粒体是植物细胞中3种储存基因组遗传信息的细胞器。核编码基因调控质体和线粒体功能以及发育过程的大多数方面。反之,质体和线粒体也可以将反向信号传递给细胞核[1]。其中一个反向信号与包括镁-原卟啉 IX(Mg-Proto IX)在内的叶绿素生物合成前体物质(四吡咯)相关,尽管这种观点还存在很大争议[2-6]。另外一个反向信号是由抑制质体基因的表达(PGE)所诱导的[7]。糖类、光照、活性氧(ROS)以及光合电子传递链的氧化还原状态也会参与到这些反向信号通路中[8,9]。近些年有研究发现类异戊二烯的前体物质2-C-甲基-D-赤藓糖醇-2,4-环二磷酸(MEcPP)在胁迫情况下会引起核编码质体相关基因的表达[10]。此外,3,5'二磷酸腺苷(PAP)从叶绿体进入到细胞核的过程中也会产生反向信号[11]。光敏色素相关蛋白磷酸酶5(PAPP5)和叶绿体膜包被的植物同源结构域转录因子PTM也可能参与到质体和细胞核之间的信号通路中[12,13]。最近的研究表明线粒体和叶绿体之间存在一些信号通路,其中AOX发挥着至关重要的作用[14-18]。
本文是我们对AOX介导的叶绿体和线粒体之间的细胞动力学代谢信号途径相关研究的一些见解。此外,对信号途径中的突出问题进行了初步探讨。
1 AOX在优化光合作用过程中的作用
高等植物中线粒体呼吸链包含ATP偶联的细胞色素(cyt)途径和AOX途径。AOX途径是呼吸电子传递链的分支途径,它在辅酶Q分支,省略掉了细胞色素途径的最后几步,其末端氧化酶是AOX[19-21]。AOX可以直接利用来自水的氧催化泛醌。同时 AOX不是质子泵,AOX途径作为电子储存槽会降低ATP的产生。因此,AOX可能会防止细胞的过度还原[22]。AOX催化一种能量耗散的呼吸途径,在佛焰花序的产热,细胞应对环境胁迫如减少活性氧(ROS)的形成和优化光合作用过程中发挥重要作用[15,17,23]。
许多研究已经证明线粒体的代谢反应过程在优化光合作用过中具有重要作用[24,25]。近期研究表明,非生物胁迫情况下拟南芥的AOX对光合作用响应环境变化具有重要作用[16,17,26-29]。一般情况下,正常生长的AOX突变植株aox1a中光合作用只受到轻微影响,包括稍微降低的CO2吸收率和O2释放率[30,31],稍微升高的循环电子传递(CET)速率[16],以及高光处理后轻微增强的光抑制敏感性[28]。总之,AOX缺陷植株表现出光合作用能力的抑制,其潜在机制可能是线粒体AOX可以作为一个缓冲池通过苹果酸/草酰乙酸穿梭途径代谢由叶绿体光合作用产生的过多还原力[32,33]。与拟南芥相似,最近的研究发现生长在高光下缺失AOX的烟草植株光合作用速率降低10%—15%。同时,这项研究还表明较低的光合作用速率是由气孔导致的而不是因为光合作用的生物化学过程受到了限制,进一步研究证实AOX缺失植株保卫细胞中的NO稳态失衡导致了气孔运动的限制[34]。
2 AOX在叶绿体和线粒体信号传递过程中的作用
AOX可以通过更普遍的作用影响新陈代谢,比如ROS,活性氮,碳和氮的平衡和ATP/ADP比率等都是与AOX相关的代谢信号元件。但有一些报告是关于AOX衍生的叶绿体和线粒体之间的代谢信号。植物生长在不同的环境中要保持高效的光合作用,这需要线粒体AOX消耗来自叶绿体过量的还原当量[14-17]。在aox1a突变体中,叶绿素的累积受到延迟,同时植株对强光胁迫的耐受性较低[16,17]。AOX2可以利用自身的转运多肽进入到叶绿体,这可能与叶绿体发育早期AOX2参与补充质体末端氧化酶行使功能有关[35]。最近的研究证实线粒体AOX调控质体NADPH/NADP+比率的变化可以作为一种新陈代谢信号调控质体蛋白的转运和叶绿素的生物合成[18]。同样的,NADH脱氢酶是一种线粒体蛋白质复合物的组成元件,NADH脱氢酶基因缺失突变体表现出光合作用强度降低[36]。另一方面,在叶绿体发育突变体albostrians中线粒体的发育也被抑制[36]。
十余年的研究发现线粒体呼吸途径在维持光合作用效率的过程中存在着精细的调控机制[28,35,37]。在高光下aox1a突变体的光系统II运作效率受到影响,并且光系统I周围的循环电子传递(CET-PSI)的活性是增加的[16]。相应的在低光照条件下,CEI-PSI缺失突变体在叶绿体基质中累积过量的还原当量,并且AOX和参与苹果酸/草酰乙酸穿梭途径的酶具有较高的活性[15]。这其中可能存在一种调控机制,NADPH可能作为一种连接线粒体和叶绿体的代谢信号分子(图1)。同时,许多与光合作用和叶绿素合成相关的叶绿体蛋白质的转运受到这种代谢信号的调控(图1)。
光是一种对植物生长至关重要的环境因子,它调控植物的光合作用和呼吸作用[17]。当AOX途径受阻时,线粒体中将累积过多的NADH,然后通过苹果酸/草酰乙酸穿梭途径将还原当量传递到叶绿体中以NADPH存在。最终质体中NADPH/NADP+比率升高导致质体处于过还原状态。然后,包括卡尔文-本森循环相关酶和光合电子传递链相关元件在内的质体蛋白转运受到阻碍,因此会扰乱光合作用的光反应和暗反应。光照可以诱导ROS的产生,AOX基因表达和抗氰呼吸[23]。另外,光受体,光敏色素,向光素和隐花色素直接促进AOX基因的表达[17]。这个反馈回路对植物的高光胁迫抗性是很重要的。通过这种代谢信号,植物细胞监测线粒体的活动,然后及时调整质体的功能来保持更好的氧化还原稳态。
先前的大多数关于叶绿体和线粒体之间相互关系的研究是在成熟植物中进行的[14-17]。最近的研究表明在植物幼苗中这两种细胞器之间也存在着密切的联系。这种代谢信号的生理学意义在于幼苗期调控植物的初期发育,成苗期调控植物对环境胁迫的适应性。
在受到AOX调控的体质蛋白中,铁氧化还原蛋白NADP+-氧化还原酶(FNR)是整合碳同化、碳异化、氮同化,硫同化和其他重要代谢过程至关重要的酶。一方面,它是一种光合作用电子传递链组成元件。另一方面,它作为质体氧化还原传感器。在光合作用电子传递反应过程中叶型FNR-L1催化最后的反应(电子从铁氧化还原蛋白(Fd)转移到 NADP+产生NADPH),并形成一种异源二聚体FNR-L2[38-40]。此外,Tic62是一种质体膜易位子元件(膜嵌入蛋白复合体),并且可以与FNR相互作用。Tic62调控的质体蛋白在叶绿体膜和基质间的穿梭过程受氧化还原状态(NADPH/NADP+比率)的影响[27,38,40].它可能是NADPH/NADP+比率调控质体蛋白质转运的关键步骤(图1)。除了对光合作用和氧化还原稳态的影响,还原型铁氧化还原蛋白也提供电子使亚硝酸盐还原酶催化亚硝酸盐还原,或者供给参与同化反应的许多其他铁氧化还原蛋白相关的酶[41]。
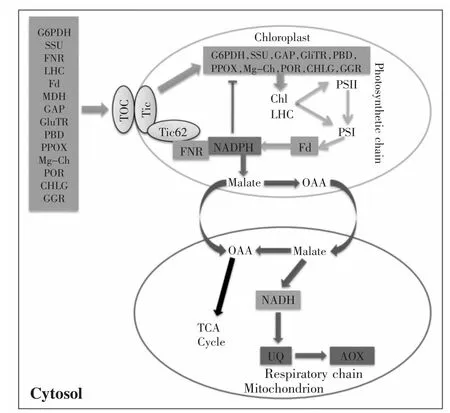
图1 叶绿体和线粒体之间信号通路模型图
3 总结和展望
叶绿体和线粒体是植物细胞中重要的细胞器。叶绿体和线粒体到细胞核的反向信号已经得到充分的研究。然而,很少有报道是关于叶绿体和线粒体之间的信号传递和相关的信号元件。AOX衍生的质体 NADPH/NADP+比率的变化和苹果酸/草酰乙酸穿梭系统可能作为一种代谢信号调控质体蛋白的转运和叶绿素的合成,但这不是连接叶绿体和线粒体间的唯一途径。叶绿体和线粒体之间的联系也可能由抗坏血酸合成途径所介导[42]。瓜氨酸-鸟氨酸穿梭系统和谷氨酸-谷氨酰胺穿梭系统也可能与叶绿体和线粒体之间的代谢信号相关[37]。遗传学,基因组学和蛋白组学相结合能使我们在系统生物学水平上理解叶绿体和线粒体之间的信号传递。显然,在下一个十年将有更令人兴奋的发现。
[1]NOTT A,JUNG H S,KOUSSEVITZKY S,et al.Plastid-to-nucleus retrograde signaling[J].Annu Rev Plant Biol,2006,57:739-759.
[2]STRAND A,ASAM IT,ALONSO J,et al.Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrin IX[J].Nature,2003,421(6918):79-83.
[3]MOCHIZUKIN,TANAKA R,TANAKA A,et al.Tetrapyrrole profiling in Arabidopsis seedlings reveals that retrograde plastid nuclear signaling is not due to Mg-protoporphyrin IX accumulation[J].Proc Natl Acad Sci USA,2008,105(39):15178-15183.
[4]MOULIN M,MCCORMAC A C,TERRY M J,et al.The level of Mg-protoporphyrin IX is not a determinant of plastid-to-nucleus signaling in Arabidopsis[J].Proc Natl Acad Sci USA,2008,105:15184-15189.
[5]WOODSON JD,PEREZ-RUIZ JM,CHORY J.Heme Synthesis by plastid ferrochelatase I regulates nuclear gene expression in plants[J].Curr Biol,2011,21(10):897-903.
[6]ZHANG ZW,FENG L Y,CHENG J,et al.The roles of two transcription factors,ABI4 and CBFA,in ABA and p lastid signalling and stress responses[J].Plant Mol Biol,2013,83(4/5):445-458.
[7]ZHANG ZW,YUAN S,XU F,et al.The plastid hexokinase pHXK:A node of convergence for sugar and plastid signals in Arabidopsis[J].FEBS Lett,2010b,584(16):3573-3579.
[8]WAGNER D,PRZYBYLA D,OP DEN CAMP R,et al.The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana[J].Science,2004,306(5699):1183-1185.
[9]KOUSSEVITZKY S,NOTT A,MOCKLER TC,et al.Signals from chloroplasts converge to regulate nuclear gene expression[J].Science,2007,316(5825):715-719.
[10]XIAO Y,SAVCHENKO T,BAIDOO E E,et al.Retrograde signaling by the plastidialmetabolite MEcPP regulates expression of nuclear stress-response genes[J].Cell,2012,149(7):1525-1535.
[11]ESTAVILLO G M,CRISP P A,PORNSIRIWONG W,et al.Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis[J].Plant Cell,2011,23(11):3992-4012.
[12]SUN X,FENG P,XU X,et al.A chloroplast envelope-bound PHD transcription factormediates chloroplast signals to the nucleus[J].Nat Commun,2011,2:477.
[13]BARAJAS-LÓPEZ JD D,KREMNEV D,SHAIKHALIJ,et al.PAPP5 is involved in the tetrapyrrolemediated plastid signalling during chloroplast development[J].PLoSOne,2013,8(3):e60305.
[14]YOSHIDA K,TERASHIMA I,NOGUCHIK.Up-regulation ofmitochondrial alternative oxidase concomitant with chloroplast over-reduction by excess light[J].Plant Cell Physiol,2007,48(4):606-614.
[15]YOSHIDA K,WATANABE C K,TERASHIMA I,et al.Physiological impact ofmitochondrial alternative oxidase on photosynthesis and growth in Arabidopsis thaliana[J].Plant Cell Environ,2011,34(11):1890-1899.
[16]YOSHIDA K,WATANABE C K,KATO Y,et al.Influence of chloroplastic photo-oxidative stress on mitochondrial alternative oxidase capacity and respiratory properties:a case study with Arabidopsis yellow variegated 2[J].Plant Cell Physiol,2008,49(4):592-603.
[17]ZHANG D W,XU F,ZHANG ZW,et al.Effects of light on cyanide-resistant respiration and alternative oxidase function in Arabidopsis seedlings[J].Plant Cell Environ,2010,33(12):2121-2131.
[18]ZHANG DW,YUAN S,XU F,et al.Light intensity affects chlorophyll synthesis during greening process by metabolite signal from mitochondrial alternative oxidase in Arabidopsis[J].Plant Cell Environ,2014,doi:10.1111/pce.12438.
[19]VANLERBERGHE G C,MCINTOSH L.Alternative oxidase:from gene to function[J].Annu Rev Plant Physiol PlantMol Biol,1997,48:703-734.
[20]MILLAR A H,WHELAN J,SOOLE K L,et al.Organization and regulation ofmitochondrial respiration in plants[J].Annu Rev Plant Biol,2011,62:79-104.
[21]VANLERBERGHE G C.Alternative oxidase:amitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in p lants[J].Int JMol Sci,2013,14(4):6805-6847.
[22]DAHAL K,WANG J,MARTYN G D,et al.Mitochondrial alternative oxidase maintains respiration and preserves photosynthetic capacity during moderate drought in Nicotiana tabacum[J].Plant Physiol,2014,166(3):1560-1574.
[23]VANLERBERGHE G C,CVETKOVSKA M,WANG J.Is themaintenance of homeostatic mitochondrial signaling during stress a physiological role for alternative oxidase?[J].Physiol Plant,2009,137(4):392-406.
[24]HOEFNAGEL M H N,ATKIN O K,W ISKICH J T.Interdependence between chloroplasts and mitochondria in the light and the dark[J].Biochim Biophys Acta,1998,1366(3):235-255.
[25]RAGHAVENDRA A S,PADMASREE K.Beneficial interactions ofmitochondrialmetabolism with photosynthetic carbon assimilation[J].Trends Plant Sci,2003,8(11):546-553.
[26]GIRAUD E,HO L H M,CLIFTON R,et al.The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress[J].Plant Physiol,2008,147(2):595-610.
[27]STRODTKÖTTER I,PADMASREE K,DINAKAR C,et al.Induction of the AOX1D isoform of alternative oxidase in A.thaliana T-DNA insertion lines lacking isoform AOX1A is insufficient to optim ize photosynthesis when treated with antimycin A[J].Mol Plant,2009,(2):284-297.
[28]FLOREZ-SARASA I,FLEXAS J,RASMUSSON A G,et al.In vivo cytochrome and alternative pathway respiration in leavesof Arabidopsis thaliana p lants with altered alternative oxidase under different light conditions[J].Plant Cell Environ,2011,34(8):1373-1383.
[29]YOSHIDA K,WATANABE C K,HACHIYA T,et al.Distinct responses of the mitochondrial respiratory chain to long-and short-term high-light environments in Arabidopsis thaliana[J].Plant Cell Environ,2011,34(4):618-628.
[30]GANDIN A,DUFFESC,DAY D A,et al.The absence of alternative oxidase AOX1A results in altered response of photosynthetic carbon assimilation to increasing CO(2)in Arabidopsis thaliana[J].Plant Cell Physiol,2012,53(9):1627-1637.
[31]VISHWAKARMA A,BASHYAM L,SENTHILKUMARAN B,et al.Physiological role of AOX1a in photosynthesis and maintenance of cellular redox homeostasis under high light in Arabidopsis thaliana[J].Plant Physiol Biochem,2014,81:44-53.
[32]NOGUCHI K,YOSHIDA K.Interaction between photosynthesis and respiration in illuminated leaves[J].M itochondrion,2008,8(1):87-99.
[33]TANIGUCHIM,M IYAKE H.Redox-shuttling between chloroplast and cytosol:integration of intra-chloroplast and extra-chloroplastmetabolism[J].Curr Opin Plant Biol,2012,15(3):252-260.
[34]CVETKOVSKA M,DAHAL K,ALBER N A,et al.Knockdown ofmitochondrial alternative oxidase induces the‘stress state’of signaling molecule pools in Nicotiana tabacum,with implications for stomatal function[J].New Phytol,2014,203:449-461.
[35]FU A,LIU H,YU F,et al.Alternative oxidases(AOX1a and AOX2)can functionally substitute for plastid terminal oxidase in Arabidopsis chloroplasts[J].Plant Cell,2012,24(4):1579-1595.
[36]LEISTER D.Genomics-based dissection of the cross-talk of chloroplastswith the nucleus and mitochondria in Arabidopsis[J]. Gene,2005,354:110-116.
[37]NUNES-NESIA,SULPICE R,GIBON Y,et al.The enigmatic contribution ofm itochondrial function in photosynthesis[J].J Exp Bot,2008,59(7):1675-1684.
[38]STENGEL A,BENZ P,BALSERA M,et al.TIC62 redox-regulated translocon composition and dynamics[J].J Biol Chem,2008,283(11):6656-6667.
[39]STENGEL A,BENZ JP,BUCHANAN B B,et al.Preprotein import into chloroplasts via the Toc and Tic complexes is regulated by redox signals in Pisum sativum[J].Mol Plant,2009,2(6):1181-1197.
[40]BALSERA M,SOLL J,BUCHANAN B B.Redox extends its regulatory reach to chlorop last protein import[J].Trends Plant Sci,2010,15(9):515-521.
[41]NEUHAUSH E,EMESM J.Nonphotosynthetic metabolism in plastids[J].Annu.Rev.Plant Physiol.Plant Mol.Biol,2000,51:111-140.doi:10.1146/annurev.arplant.51.1.111
[42]SMIRNOFF N,WHEELER G L.Ascorbic acid in plants:biosynthesis and function[J].Crit Rev Biochem mol Biol,2000,35(4):291-314.
AOX-Derived Signal Transduction Between M itochondria and Chlorop last
LIN Honghui,ZHANG Dawei,LIPengxu,ZHAIChenlei,HAN Xueying
(a.M inistry of Education Key Laboratory for Bio-Resource and Eco-Environment;b.College of Life Science,Sichuan University,Chengdu 610064,China)
Alternative oxidase(AOX)plays a key role in cyanide(CN)-resistant respiratory pathway,and thatchlorophyll accumulation will be largely delayed in AOX knockoutmutant.Recent studies showed that this delay of chlorophyll accumulation wasmore significant under the high light condition and mainly attributed to the blocked import of the concerned plastidial enzymes,a catalyzer of tetrapyrrole or phytol biosynthesis.Inhibition of cyanideresistant respiration was also accompanied by the increase of plastid NADPH/NADP+ratio,especially under the high light treatment.It thus suggests that light intensity affects chlorophyll synthesis during greening process by a metabolic signal,which may be attributed to the AOX-derived plastidial NADPH/NADP+ratio change.Therefore,AOX-derived signal between mitochondrion and chloroplast is essential for facilitating and sustaining the photosynthetic capacity.
alternative oxidase;metabolite signaling;NADPH/NADP+ratio;photosynthesis activity
Q946
A
10.16246/j.issn.1673-5072.2016.01.005
1673-5072(2016)01-0039-05
2015-12-22
国家基础研究计划(973计划,2015CB150100);国家自然科学基金项目(91417305,31470342,31400211)
林宏辉(1968—),男,四川仁寿人,博士,教授,博士生导师,主要从事作物呼吸代谢与光合作用、植物病理、作物抗性生理及其适应逆境的分子机理以及园林植物生物技术领域的研究工作。
林宏辉,E-mail:hhlin@scu.edu.cn
——有效的抗弓形虫药物靶标

