肌酸激酶的生理功能与运动
周多奇,龚 莉,钱振宇
(安庆师范大学 1.体育学院,2. 生命科学学院, 安徽 安庆 246133)
肌酸激酶的生理功能与运动
周多奇1,龚莉2,钱振宇1
(安庆师范大学 1.体育学院,2. 生命科学学院, 安徽 安庆 246133)
肌酸激酶(creatine kinase,CK)是催化磷酸肌酸和ATP间高能磷酸基团转移的关键酶,它保持ATP/ADP比例平衡和ATP的供给。肌酸激酶家族共有5种同工酶,分布于全身各种组织的需能部位。研究发现,CK酶在能量代谢中有重要作用,与肌肉收缩、神经功能、细胞膜的稳定性、细胞的能量反馈调节、有氧耐力有关。一些研究认为,CKM(肌型肌酸激酶,creatine kinase,muscle)基因多态性影响个体有氧运动能力和个体对耐力训练的敏感性。本文对这些成果作一综述,并对未来研究进行展望,以期为进一步的研究提供参考。
生物;肌酸激酶;能量代谢;有氧耐力;基因多态性
人类由4种不同的肌酸激酶(CK)亚基组成5种已知的同功酶,大都分布在需能的区域或结构附近。细胞质中CKB(脑型肌酸激酶,creatine kinase,brain)、CKM(肌型肌酸激酶,creatine kinase,muscle)亚基组成的3种同工酶均为二聚体,CKMM是骨骼肌中最富集的形式;CKBB在神经组织中表达最多,也在视网膜、听毛、精子、肾、皮肤和其他组织表达;心肌中CKMB活性最高,CKMM和CKBB也在心肌中表达。此外,线粒体内膜的外面还有2种CK同功酶,sMtCK(肌膜型线粒体肌酸激酶,sarcomeric mitochondrial creatine kinase)主要存在于肌肉;uMtCK(广泛型线粒体肌酸激酶,ubiquitous mitochondrial creatine kinase)普遍存在于身体各种组织,如血小板、精液,细胞中以2聚体或8聚体形式存在,活性形式可能是8聚体[1-2]。研究发现,CK在细胞能量代谢方面发挥重要作用, CKM基因多态性与个体有氧运动能力和个体对耐力训练的敏感性有关[3],前期研究发现,中国CKM基因A/G多态可能影响个体跑机能节省化(running economy,RE)对耐力训练的敏感性[4]。本文对CK的生理功能及其对运动的影响进行综述,以期为进一步研究参考。
1 肌酸激酶的生理功能
1.1CK酶与组织细胞的能量代谢
1.1.1CK-PCr空间能量缓冲系统
CK-PCr空间缓冲系统发挥转运高能磷酸化合物的作用主要通过分布于产能部位(线粒体和糖酵解)与需能部位的各种不同的CK酶的作用完成[8]。肌肉收缩时消耗能量的部位主要有3处:一是肌膜上Na+泵,影响动作电位的形成;二是肌球蛋白横桥,影响肌肉收缩;三是肌织网Ca2+泵,影响肌肉收缩。从图1可以看出,此3处均分布有CK酶。CKMM位于骨骼肌、心肌的M线附近[10]、糖分解较多的明带(I)附近[11]和肌质网(sarcoplasmic reticulum, SR)Ca2+ATP酶附近[12];CKBB多分布于脑和外周神经浆膜,少量分布于骨骼肌组织[13];2种线粒体CK位于线粒体内膜外面通过ATP/ADP的转运影响能量转移。MtCK(线粒体肌酸激酶,mitochondrial creatine kinase)和ATP合成酶、ANC(腺苷酸转运载体,adenine nucleotide translocase)、PIC(磷酸盐转运载体,phosphate carrier)、VDAC(电压依赖性阴离子通道,voltage-dependent anion channel)共同组成超级复合物,把线粒体产生的ATP转运到细胞质中[7,14](图2)。
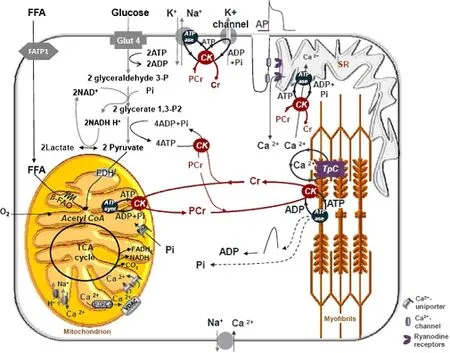
图1[1] 心肌和骨骼肌能量代谢图
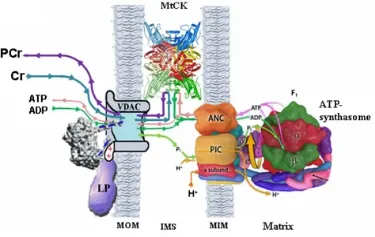
图2[1] 线粒体CK复合物
1.1.2CK-PCr时间能量缓冲系统
CK-PCr作为一个能量池主要证据来自于几个方面[15]:(1)核磁分析发现,随着运动肌中的PCr浓度下降,ATP浓度只有微量降低;(2)转基因鼠研究发现,CKBB的过度表达能导致肝细胞ATP浓度显著增加,抗缺氧能力显著提高;(3)PCr浓度过低时出现肌肉疲劳;(4)补充肌酸、β-鸟氨酸等量混合物可以提高肌纤维柠檬酸合成酶的浓度,增强氧化耐力。同时,摄入外源肌酸能提高单次或重复力竭性运动的运动能力[16],运动耐力增加[17]。耐力能力的提高可能是由于细胞内肌酸浓度提高的结果。这反过来会促进MtCK活性,导致细胞质PCr和线粒体ADP浓度升高。线粒体ADP浓度越高,就越能刺激呼吸,从而增加摄氧量。
1.1.3CK-PCr能反馈调节细胞内的能量代谢
根据Vendelin-Aliev-Saks (VAS)模型和心肌能量代谢研究,肌肉周期性收缩会伴随ADP、PCr/Cr比例、 Pi和质子浓度周期性变化,这些变化会反馈调节细胞线粒体的能量代谢[18]。在肌肉CKMM反应时,磷酸没有被消耗,但可以自由扩散和通过磷酸盐转运载体进入线粒体;同时ADP由于非平衡状态向线粒体逐渐转移而浓度下降,从而用PCr磷酸化ADP,产生Cr,使PCr/Cr比例变化;这些信号可以通过CK-PCr缓冲系统转移到MtCK,ADP信号到达线粒体,增加ATP再合成。这样,通过MtCK-ANT复合物功能耦合促进了MtCK反应和ATP再合成[18]。
1.2CK酶影响神经系统功能
CKBB和uMtCK在神经系统能量代谢中起重要作用,研究表明,CKB和uMtCK基因敲除鼠空间学习能力、探索能力和体温调节能力下降;视网膜和内耳听毛的CKBB和uMtCK酶还可能影响视觉和听觉[19-21]。无论是记忆、视觉还是听觉都对运动技能的形成和运动能力有很大影响。
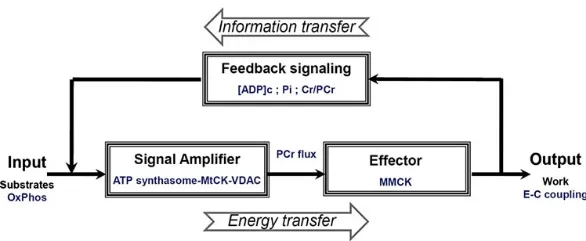
图3[1]CK 能量调节路径
1.3CK酶影响细胞稳定性,减少脂质过氧化
SAK等人报道细胞膜磷脂双分子层的维持与PCr有关,当缺氧和缺血时,心肌纤维膜稳定性下降,脂质过氧化增加[22]。体外注射PCr能减少心肌缺血造成的损害,其机制可能是膜表面的磷脂极性头部所带的两性电荷可以整合两性基团,导致膜从液相向固相过度(图4),减少磷脂转变成溶血磷脂[22];在缺氧和局部缺血时细胞内PCr 的快速下降是细胞膜不稳定的重要因素。
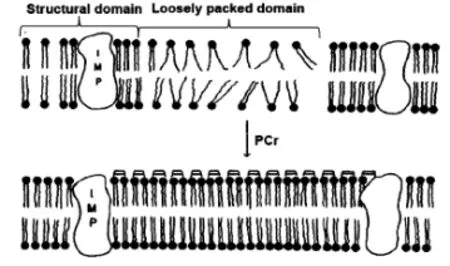
图4[1]PCr对磷脂双分子稳定性的影响
2 肌酸激酶对运动的影响
2.1CK酶对耐力运动的影响
研究表明,不同肌纤维类型中的CKMM活性有很大差异,I型肌纤维中CKMM活性较II型纤维至少低两倍[23],而低CKMM活性可能是耐力运动员工作肌群的典型特征。Van Deurse等发现,CKM基因敲除鼠在低强度的运动中,耐力有明显的提高,抗疲劳能力增强,肌肉组织合成ATP的能力也明显增强;同时,CKM缺陷鼠快肌纤维中线粒体体积增大,浓度增加,糖原浓度增加;柠檬酸合成酶和乳酸脱氢酶增加;氧化能力翻倍[24]。通过在鼠CKM基因的第2外显子插入一段无关序列[25],创造出一个新的变异体。与正常鼠相比,CKM I/I型鼠CKMM活性降低3倍,而CKM I/-型CKMM活性降低6倍;有趣的是这些变异体CKM mRNA随着CKMM活性的下降而成比例地减少,这说明CKMM酶的水平实际上受控于转录水平上。此外,CKMM酶的进一步降低伴随着线粒体Cytc氧化酶和柠檬酸合成酶适量增加。这说明CKMM和有氧能力负相关[15],且CKMM与有氧能力的负相关呈一定量的关系。
Apple等杂交实验发现,小鼠经耐力训练,腓肠肌中CKB的mRNA含量增加40%,而CKM的mRNA减少42%。推测跑台训练使鼠腓肠肌CK酶活性的改变似乎很大程度上受控于在转录水平[26]。另一实验中,狗经耐力训练后,CKB mRNA含量增加2.6倍,而CKM mRNA下调30%[27]。此外,鼠经6周过度训练后,取比目鱼肌和腓肠肌,发现跖肌中CKM mRNA下降150%[28]。
2.2CK基因多态性与有氧运动能力的关联
此方向的研究集中于CKM基因的NcoI和TaqI多态。人的CKM基因位于19q13.2-13.3,长度约17.5 kb,含8个外显子和7个内含子,研究表明,在3'端不编码区NcoI多态,可能与耐力有关[29]。Rivera等[30]对加拿大80个家庭(80对父母和80位子女)进行了20周的耐力训练。结果发现,CKM基因NcoI多态GG纯合型的VO2max及其对耐力训练的敏感性显著低于AG或AA型。同时,在低反应群体中GG频率是其他两种基因型人数的3倍;在高反应群体中没有GG纯合子;同时父母中AG比AA或GG两种纯合子具有更高的VO2max。随后Rivera等[31]对加拿大495名受试者进行20周的耐力训练,进行连锁分析发现,CKM基因型影响个体VO2max对耐力训练的敏感性。 Fedotovskaia等[32]的研究也发现,CKM基因A/G多态性与有氧运动能力有关,AA和AG型个体具有更高的VO2max。但Rivera等对124名优秀的加拿大耐力运动员和115名加拿大男性普通人的CKM基因NcoI和TaqI多态进行分析,并未发现CKM基因NcoI多态在两组间有显著性差异[33]。Byung等对98名韩国优秀男运动员与64名对照组的case-control研究也未发现两组人群的CKM基因NcoI多态有显著性差异[34]。Robert[35]、Lucía[36]和Defoor[37]等也报道了类似结果。以上研究结果有较大的差异,可能是研究对象(种族、年龄、性别和项目等)、样本量、实验设计观察指标差异等原因造成的。
本课题组前期也对CKM基因NcoI多态与有氧运动能力的关系进行了探讨。让102名武警新兵完成18周5 000 m跑训练后,VO2max显著增加了1.50%,12 km/h下的VO2(l/min)下降了7.55%,表明,长跑明显提高了受试者有氧耐力水平,但表现出较大的个体差异,VO2max的变化范围是-13.64%~24.64%,VO2从-25%变到8%。这种个体差异与NcoI多态有关,携带AA和AG型的个体显著比GG型个体敏感,但GG型个体仅占2%。推测CKM基因NcoI多态可能与中国男子有氧运动能力有关[4]。
3 展 望
综上所述,CK家族酶在有氧运动能力的形成过程中的多个环节有重要作用,并且CKM基因多态性可能与运动训练效果有关,携带某一基因型的群体可能对运动训练有更高的敏感性,更易成为优秀运动员,但是目前的研究结果还有许多不确定性。结合当前该领域的研究现状,在CK家族基因多态性与有氧运动能力的关系研究中,存在以下几个需研究的问题:
(1) 在不同人群中结果不尽一致。即使对于同一SNP位点,如NcoI多态位点,在不同的研究,结果存在很大差异甚至完全相反。因此对于国外已有的阳性研究结果不能直接应用到中国运动员。
(2) 样本量较小。这也是目前所有杰出运动能力相关基因多态性研究所面对的问题,因为优秀运动员(健将和国际健将)的总体样本量有限,很多研究对象项目混杂。但按照统计学理论,可以按照case-control的1∶2的比例增加对照组的样本量来提高研究效度。
(3) 选用的SNP位点太少。CKM基因共有458个SNPs,目前的研究仅涉及NcoI和TaqI位点,而对于成员其他3个基因的相关研究鲜见报道。因此,应全面对CKM基因所有可能影响基因表达和基因功能的SNPs进行研究,尤其是分布于启动子区以及改变氨基酸编码的多态性。
(4) 目前的研究几乎只涉及CKM基因。CKM基因产物主要在骨骼肌细胞质,少量在心肌细胞质能量代谢中发挥作用,而对于有氧代谢更为重要的线粒体CK酶编码基因sMtCK和uMtCK、心肌、神经和其他组织的细胞质中CKB基因多态性研究几乎没有涉及。
[1] Wallimann T, Tokarska-Schlattner M, Schlattner U. The creatine kinase system and pleiotropic effects of creatine[J]. Amino Acids, 2011, 40:1271-1296.
[2] Wallimann T, Tokarska-Schlattner M, Neumann D, et al. The phospho-creatine circuit:molecular and cellular physiology of creatine kinases, sensitivity to free radicals and enhancement by creatine supplementation. In: Saks VA (ed) Molecular systems bioenergetics: energy for life [M]. Wiley, Weinheim, 2007: 195-264.
[3] Rankinen T RS, Bray MS,Loos R,et al. Advances in exercise, fitness, and performance genomics [J]. Med Sci Sports Exerc, 2010, 42(5):835-846.
[4] Zhou DQ, Hu Y, Liu G, et al. Muscle-specific creatine kinase gene polymorphism and running economy responses to an 18-week 5000-m training programme [J]. Br J Sports Med, 2006, 40(12):988-991.
[5] Kresge N, Simoni RD, Hill RL. Otto Fritz Meyerhof and the elucidation of the glycolytic pathway [J]. J Biol Chem, 2005, 280(4):e3.
[6] Guzun WR, Timohhina N, Tepp K, et al. Systems bioenergetics of creatine kinase networks: physiological roles of creatine and phosphocreatine in regulation of cardiac cell function [J]. Amino Acids, 2011, 40:1271-1296.
[7] Saks V, Monge C, Anmann T, et al. Integrated and organized cellular energetic systems: theories of cell energetics, compartmentation and metabolic channelling. In: Saks V (ed) Molecular system bioenergetics [M]. Energy for life,Wiley-VCH, 2007.
[8] Saks VA, Rosenshtraukh LV, Smirnov VN, et al. Role of creatine phospliokinase in cellular function and metabolism [J]. Can J Physiol Pharmacol, 1978,56 (5): 691-706.
[9] Miller K. Halow J, Koretsky AP. Phosphocreatine protects transgenic mouse liver expressing creatine kinase from hypoxia and ischemia [J]. Am J Physiol, 1993,265: C1544-C1551.
[10] Turner DC, Wallimann T, Eppenberger HM. A protein that binds specifically to the M-line of skeletal muscle is identified as the muscle form of creatine kinase [J]. Proc Natl Acad Sci USA, 1973, 70:702-705.
[11] Wegmann G, Huber R, Zanolla E, et al. Differential expression and localization of brain-type and mitochondrial creatine kinase isoenzymes during development of the chicken retina: Mi-CK as a marker for differentiation of photoreceptor ceils [J]. Differentiation, 1991,46 (2): 77-87.
[12] Korge P, CamphcIl KB. Local ATP regeneration is important for sarcoplasmic rcticulum Ca2+pump function [J]. Am J Physiol, 1994,267: C357-C366.
[13] Saks VA, Kuznetzov AV, Kupriyanov VV, et al. Creatine kinase of rat heart mitochondria: the demonstration of functional coupling to oxidative phosphorylalion in an inner membrane preparation [J] . J Biol Chcm, 1985, 260: 7757-64.
[14] Saks V, Beraud N, Wallimann T. Metabolic compartmentation— a system level property of muscle cells [J]. Int J Mol Sci , 2008, 9:751-767.
[15] Echegaray M, Rivera MA. Role of creatine kinase isoenzymes on muscular and cardiorespiratory endurance [J]. Genetic and Molecular Evidence, 2001,31(13):919-934.
[16] Guerrero-Ontiveros ML, WaIliman T. Creatine supplementation in health and disease. Effects of chronic creatine ingestion in vivor:down regulation of the expression of creatine kinase transporter isoiforms in skeletal muscle [J]. Mol Cell Biochem, 1998,184:427-37.
[17] Rico Sanz J, Mendez, Marco MT. Creatine enhances oxygen uptake and performance during alternating intensity exercise [J]. Med Sci Sports Exerc, 2000,32(2): 379-85.
[18] Guzun R, Timohhina N, Tepp K, et al. Regulation of respiration controlled by mitochondrial creatine kinase in permeabilized cardiac cells in situ. Importance of system level properties [J]. Biochim Biophys Acta, 2009, 1787:1089-1105.
[19] Carolina RJ, Catharina EE, Vander Z,et al.Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility [J].European Journal of Neuroscience, 2002,15:1692-1706.
[20] Femke S, Carolina RJ, Frank O, et al.Mice lacking the UbCKmit isoform of creatine kinase reveal slower spatial learning acquisition, diminished exploration and habituation, and reduced acoustic startle reflex responses [J].Molecular and Cellular Biochemistry, 2004, 256:305-318.
[21] Femke S, Frank O, Bart A,et al.Structural and behavioural consequences of double deficiency for creatine kinases BCK and UbCKmit [J].Behavioural Brain Research, 2005, 157: 219-234.
[22] Saks V, Strumia E. Phosphocreatine: Molecular and cellular aspects of the mechanism of cardioprotective action [J]. Curr Therapeut Res, 1993, 53:565-598.
[23] Yamashita, K, Yoshioka T. Profiles of creatine kinase isoenzyme compositions in single muscle fibers of different types [J]. J Muscle Res Cell Motil, 1991, 12:37-44.
[24] Van Deursen J, Heerschap A, Oerlemans F. Skeletal muscles of mice deficient in M-creatine kinase lack burst activity [J]. Cell, 1993,74:621-631.
[25] VAN Deursen J, Ruitenbeek W, Heerschap A, et al. Creatine kinase in muscle energy metabolism: a study of mouse mutants with graded reduction in CK expression [J]. Proc Natl Acad Sci USA, 1994,91:9091-9095.
[26] Apple P S, Billardello JJ. Expression ol'creatine kinase M and B mRNAs in treadmill trained rat skeletal muscle [J]. Life Sci, 1994,55 (8): 585-92.
[27] Alam M,Vaynhlat M, Goswami SK, et al. Activation of creatine kinase-B and phospliolanihan gene expression in transformed latissinius dorsi muscle: evaluation of mRNA by polymerase chain reaction [J]. J Mol Cell Carcliol, 1996, 28(9): 1901-1901.
[28] Tsika RW, Hauschka SD, Gao L. M-crcatinc kinase gene expression in mechanically overloaded skeletal muscle of transgenic mice [J]. Am J Physiol, 1995, 269: C665-C674.
[29] Bouchard C, Wolfarth B, Rivcra MA, et al. Genetic determinants of endurance performance. In: Shephard R.I, Astrand PO. editor. Endurance in sports [M]. 2nd ed. London: Blackwell Science, 2000,223-243.
[30] Rivera MA, Dionne FT, Simoneau JA, et al. Muscle-specific creatine kinase gene polymorphism and VO2maxin the Heritage family study [J]. Med Sci Sports Exerc, 1997, 29 (10):1311-1317.
[31] Rivera MA, Perusse JA , Simoneau J,et al. Linkage between a muscle-specific CK gene marker and VO2maxin the HERITAGE Family Study [J]. Med Sci Sports Exerc, 1999, 31:698-701.
[32] Fedotovskaia ON, Popov DV, Vinogradova OL,et al. Association of the muscle-specific creatine kinase (CKMM) gene polymorphism with physical performance of athletes [J]. Fiziol Cheloveka, 2012, 38(1):105-109.
[33] Rivera M A, Dionne F T, Wolfarth B, et al. Muscle-specific creatine Kinase gene polymorphisms in elite endurance athletes and sedentary controls [J]. Med Sci Sports Exerc,1997,29(11): 1444-1447.
[34] Byung, Yong Kang, et al. Muscle specific creatine kinase gene polymorphisms in korean elite athletes[J]. J Toxicol Pub Health, 2003, 19(2): 115-121.
[35] Robert S, Cristina M, Barreda M, et al. Association between CKMM genotype and endurance performance level in hispanic marathon runners[J]. Med Sci Sports Exerc, 2004,36(5) Supplement: S260.
[36] Lucía A, Gómez-Gallego F, Chicharro JL, et al. Is there an association between ACE and CKMM polymorphisms and cycling performance status during 3-week races? [J]. Int J Sports Med, 2005, 26(6):442-447.
[37] Defoor J, Martens K, Matthijs G, et al. The caregene study: muscle-specific creatine kinase gene and aerobic power in coronary artery disease[J]. Eur J Cardiovasc Prev Rehabil, 2005,12(4):415-417.
Physiological Function of Creatine Kinase and Sport
ZHOU Duo-qi1, GONG Li2,QIAN Zhen-yu1
(1.Department of Physical Education;2.Department of biology, Anqing Normal University, Anqing, Anhui 246133, China)
The creatine kinase (CK) is a key enzyme which catalyzes the transfer of high-energy phosphates between PCr and ATP, thus keeping cellular ATP-to-ADP ratios balanced and the ATP pool highly charged. Five isoenzymes in CK family were expressed in regions consuming energy all over different tissues. Many experimental studies have revealed that CK may play important role during energy metabolism, and they were associated with muscle contraction, neurological function, stability of membranal, feedback regulation of energy metabolism and aerobic endurance performance. Other studies found that CKMM gene polymorphisms may contribute to individual aerobic endurance and its’ sensibility to endurance training. The purpose of this work is to provide a new background for the people who are studying in this field by attempting to analyze these results, and inspire future researches.
biology; creatine kinase; energy metabolism; aerobic Endurance; gene polymorphism
2016-02-02
安徽省自然科学基金面上项目(1308085MH161)。
周多奇,男,安徽定远人,硕士,安庆师范大学体育学院教授,硕士生导师,研究方向为运动生理生化。E-mail: duoqizhou@163.com
时间:2016-8-17 11:31
http://www.cnki.net/kcms/detail/34.1150.N.20160817.1131.029.html
G804
A
1007-4260(2016)03-0113-06
10.13757/j.cnki.cn34-1150/n.2016.03.029

