ProductionofsecondarymetaboliteE2.2fromPhaleriamacrocarpaendophyticfungus
Beatrix Trikurnia Gasong,Raymond Rubianto Tjandrawinata
Dexa Laboratories of Biomolecular Sciences,Dexa Medica,Industri Selatan V Block PP no.7,Kawasan Industri Jababeka II,Cikarang 17550,Indonesia
ProductionofsecondarymetaboliteE2.2fromPhaleriamacrocarpaendophyticfungus
Beatrix Trikurnia Gasong,Raymond Rubianto Tjandrawinata*
Dexa Laboratories of Biomolecular Sciences,Dexa Medica,Industri Selatan V Block PP no.7,Kawasan Industri Jababeka II,Cikarang 17550,Indonesia
ARTICLE INFO
Article history:
in revised form 27 Aug 2015
Accepted 20 Sep 2015
Available online 15 Jan 2016
Endophytic fungi
Phaleria macrocarpa
Secondary metabolite
Colletotrichum gloeosporioides E2.2
Objective:ToisolatenewendophyticfungusfromPhaleriamacrocarpa(P.macrocarpa)that is able to produce E2.2 compound.
Methods:Endophytic fungi were isolated from P.macrocarpa.Morphological and molecular identification was done to determine the species of the endophytic fungus. High performance liquid chromatography was used to determine the ability of this fungus to produce E2.2 compound and to quantify the total yield of E2.2 from fungal fermentation.Fermentation process was optimized by observing suitable medium,pH and length of fermentation process.Phloroglucinol and gallic acid addition were examined to determine the effect of each compound on E2.2 production.
Results:One endophytic fungus was successfully isolated from P.macrocarpa plant. Morphological and molecular identification showed that it was a Colletotrichum gloeosporioides which belonged to Glomerellaceae family.This fungus showed highest production of E2.2 when incubated in potato dextrose broth with initial pH value of the medium at 5,and was incubated for 15 days.Phloroglucinol was found to better enhance E2.2 production.
Conclusions:Colletotrichum gloeosporioides found in P.macrocarpa plant is promising as a potential alternative source of E2.2.
Original articlehttp://dx.doi.org/10.1016/j.apjtb.2016.01.005
1.Introduction
A new way of generating natural compound is from endophytic fungi.Endophytic fungi are fungi that colonize living plant tissue without causing any immediate,overt negative effects[1].Mutualistic interaction between endophytic fungi and its host has generated a lot of interests regarding its broad potentialinresearch.Severalstudieshaveshownthat endophytic fungi are able to produce compounds that are similar to the secondary metabolites produced by its host[2]. Hence,endophytic fungus has high potential as a new source of bioactive compound.
Phaleria macrocarpa(P.macrocarpa)is a native Indonesian plant which has been used traditionally as herbal drink to treat many types of diseases such as cancer and diabetes[3].Recent studies also showed that P.macrocarpa exhibited numerous different bioactivity.A study by Hendra et al.showed that this plant exhibited antioxidative,anti-inflammatory and cytotoxic activity[4].Our previous study has successfully proven that P.macrocarpa has anti-proliferative activity against two types of cancer cells(MDA-MB-231 and MCF-7 human breast adenocarcinoma cell lines)[5].To further ascertain our findings,we isolated numerous different compounds from P.macrocarpa and found that E2.2 compound was responsible for the anticancer activity of P.macrocarpa[6].
Although our previous study has suggested that the use of E2.2 compound from P.macrocarpa as a treatment of cancer is promising,use of natural resources in pharmaceutical production still faces many challenges such as limited source of raw material and unstable qualityofraw materialdueto the environment[7].A large amount of P.macrocarpa fruit is needed to produce standardized P.macrocarpa extract.Consequently,a new alternative method to obtain E2.2 compound efficiently and effectively is required. Therefore,the present study is focused on obtaining E2.2compound from endophytic fungi of P.macrocarpa as a novel source of E2.2 compound.
2.Materials and methods
2.1.Isolation and purification of endophytic fungi
Healthy P.macrocarpa plant was collected in August 2012 from West Java,Indonesia.Roots,stems and leaves of the plant were washed under tap water for 10-15 min and cut into small sizes around 1 cm×1 cm before being surface-sterilized.Surface sterilization was done in four steps.First,samples were rinsed in tap water.Then,the samples were immersed in 70% alcohol for 1 min,followed by 5.3%NaOCl for 5 min,70% ethanol for 30 s,and washed with sterile water until no trace of the previous solution was left.
Isolation process of endophytic fungi from P.macrocarpa was done using rose bengal chloramphenicol agar(BD Difco,New Jersey,USA).The surface-sterilized sample was placed in a Petri dish(Iwaki,Japan)containing the selective medium,and incubated for 1 week at 27°C.After 1 week of incubation,all fungi isolates were transferred into potato dextrose agar(Oxoid,Hampshire,United Kingdom).
2.2.Identification of E2.2-producing endophytic fungi
E2.2-producing endophytic fungi were identified based on its morphological and molecular characteristics.The fungi were cultivated in Petri dish containing potato dextrose agar(Oxoid,Hampshire,United Kingdom)and incubated for 1 week at 27°C. After1weekofincubation,macroscopic(colonyappearance)and microscopic(mycelia)characteristics were examined.Molecular identification was done using nucleotide sequencing of internal transcribed spacer(ITS)region of rRNA.DNA preparation and DNA sequencing were done using standardized method by Molecular Biology Service species barcoding from 1st Base(Singapore).Sequences obtained were then submitted to BLAST and NCBI to determine the strain of the fungi.
2.3.Fermentation and extraction
Fungalisolatewascultivatedandincubatedfor7daystoobtain the desired secondary metabolites.One plug(1 mm×1 mm)of mycelia was inoculated into 100 mL of potato dextrose broth(PDB)(BDDifco,NewJersey,USA)andincubatedinanInnova®40/40R Shaker Incubator(New Brunswick Scientific Co.,Inc.,USA)(Percival Scientific,USA)at 27°C,150 r/min.After incubation,mycelia and fermentation broth were separated using a 0.2μm filter(Merck Millipore,Darmstadt,Germany).The fermentation broth was then extracted two times with equal volumeofethylacetate(liquid-liquidextraction).Theresultingethyl acetate phase was then collected and evaporated under pressure until crude extract was obtained.After fermentation broth was extracted and crude extract was obtained,the extract was dissolved in methanol and filtered through 0.22μm membrane filter(Iwaki,Japan)prior to high performance liquid chromatography(HPLC)analysis.HPLC analysis was done using SunFire C18 column 4.6 mm×150 mm,5μm(Milford,USA).Methanol: 0.01%acetic acid was used as the mobile phase,flow rate was 1.0 mL/min,detection wave length was 320 nm,and injection volume was 20μL.
2.4.Optimization and quantification of E2.2 production
In order to obtain highest concentration of E2.2,optimization of fermentation condition was done.The fungi were incubated in two different medium with different incubation time and pH.All fungi isolates were incubated in PDB(Hampshire,United Kingdom)and malt extract broth(Hampshire,United Kingdom). The cultures were incubated in 4 different pH(4,5,7 and 8.5). The cultures were also incubated in different incubation time(4,12,and 15 h).After incubation,E2.2 concentration was measured using HPLC.
2.5.Effect of phloroglucinol and gallic acid on E2.2 production
The effects of phloroglucinol and gallic acid on the production of E2.2 compound were determined by adding 0.1 mg/mL of the substance in the fermentation medium.Each culture was then incubated for 7 days at 27°C,150 r/min.After incubation,E2.2 concentration was measured using HPLC.
3.Results
3.1.Isolation and purification of endophytic fungi
One fungal isolate was successfully isolated and purified which we later referred to as KP11.
3.2.Identification of E2.2-producing endophytic fungi
Early identification was done by examining the morphological characteristic of the fungus.Figure 1 shows the morphological character of the isolated fungus.Based on morphology analysis shown in Figure 1,the fungus could be described as filamentous fungi with white and orange colored velvety colony. It had conidia as vegetative spore and septate hyphae.
Figure 2 shows phylogeny tree of the isolated fungus based on neighbor-joining analysis compared to other similar fungi strains.Based on this analysis,this fungus was identified as Colletotrichum gloeosporioides(C.gloeosporioides)with 99% similarity.
3.3.Fermentation and extraction
The C.gloeosporioides was cultured in PDB and incubated for 14 days.After 7 days of incubation,fungi mycelia have formed and the medium appeared darker.The extracted fermentation broth was dark brown.Crude extract of the fermentation broth was examined with HPLC to determine whether the fungal isolate was able to produce E2.2.The HPLCresult showed that the fungal isolate turned out to be an E2.2-producing endophytic fungus(Figure 3).

Figure 1.Morphological characteristics of KP11(A)and microscopicmorphology of KP11(B and C).
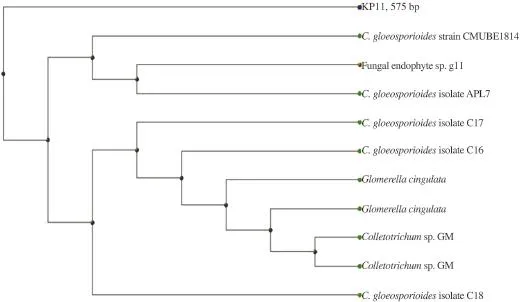
Figure 2.Phylogeny tree of KP11 based on ITS sequences of ribosomal DNA. C.gloeosporioides:Colletotrichum gloeosporioides.
3.4.Optimization and quantification of E2.2 production
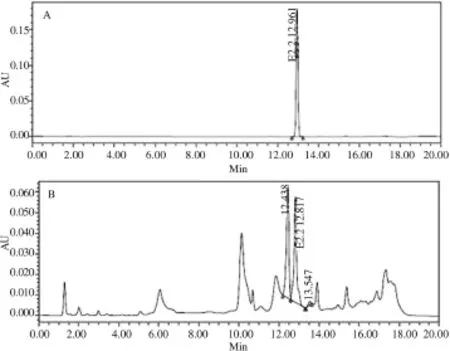
Figure 3.HPLC profile of E2.2 compound(A)and fermentation broth of KP11(B).

Table 1 The effect of some fermentation conditions on E2.2 concentration.
Table 1 shows the optimum fermentation condition for the C.gloeosporioides isolate.The result suggested that E2.2 production was optimum in starch-rich medium.Acidity did not cause significant difference in E2.2 production,although highest yield of E2.2 was obtained in pH 5.The result also suggested that longer incubation time resulted in higher concentration of E2.2.
3.5.Effect of phloroglucinol and gallic acid on E2.2 production
Figure 4 shows the effect of phloroglucinol and gallic acid addition on the fermentation broth visually.It is clearly seen that phloroglucinol or gallic acid addition resulted in less visible mycelia growth and also the darker fermentation broth obtained.Therefore,gallic acid and phloroglucinol should not be added more than 0.1 mg/mL as it would be toxic to the fungi's growth.
HPLC analysis was done to investigate the effect of phloroglucinolandgallicacidonE2.2productionbyC.gloeosporioides. The results showed that both phloroglucinol and gallic acid enhanced the amount of E2.2 produced by C.gloeosporioides.In the control medium,C.gloeosporioides produced 665.72μg/L of E2.2.However,in the presence of 0.1 mg/mL phloroglucinol or gallic acid,this endophytic fungus was able to produce more than two fold of E2.2 compound compared to the control.Phloroglucinol addition resulted in 2032.53μg/L of E2.2 compound whereas gallic acid addition resulted in 1736.989μg/L of E2.2 compound.

Figure 4.The effect of phloroglucinol and gallic acid addition into fermentation medium.
4.Discussion
Endophytic fungi have been known to produce compounds which are similar to its plant host[8].In this research,we use endophytic fungi from P.macrocarpa isolated using rose bengal agar to produce E2.2 compound.Rose bengal is often used for fungi isolation since it contains chloramphenicol as antibiotic to inhibit bacterial growth.The presence of rose bengal also helps the coloration of fungi colony,hence making the isolation process easier.
Theinitialendophyticfungiisolationprocesson P.macrocarpa plant resulted in 28 endophytic fungi isolates. However,at the end of the purification step,there is only 1 remaining isolate that is successfully isolated and preserved.AccordingtoArnoldetal.,thediversityofendophyticfungiinahost plant is highly influenced by the characteristic of its host and growth condition or spatial factor of the host plant[9].Moricca et al.also reported that symbiotic relation between endophytic fungi and its host plant is controlled by the genes of both the fungi and the host plant,and is modulated by environmental factors[10].The different condition between its natural habitat and laboratory condition might be the cause of limited number of isolated endophytic fungi from P.macrocarpa plant as it fails to adapt to different environmental condition.
After we have obtained the endophytic fungus isolate,we identified this fungus based on its morphology and DNA sequence.TheresultsshowedthatKP11wasC.gloeosporioides,a common endophytic fungus that was present in a wide range of host plants.Several studies stated that this fungus may also be a plant pathogen[11,12].
Different studies have shown that C.gloeosporioides was found in several different plant hosts and therefore it is not hostspecific.However,C.gloeosporioides in different plants produce different metabolites specific to its host.For example,C.gloeosporioides that was isolated from Cryptocarya mandiocannaisabletoproduceantifungalcompound[13]. C.gloeosporioides from Piper nigrum was found to produce piperine[14].Furthermore,C.gloeosporioides from Forsythia suspense,Artemisia mongolica and Tectona grandis were able to produce phyllirin,colletotric acid,and taxol respectively[15-17].Inthisstudy,wehavesuccessfullyisolated C.gloeosporioides that is able to produce E2.2 compound found in P.macrocarpa.
HPLC was used to measure E2.2 concentration in the crude extract of C.gloeosporioides KP11 fermentation broth.Several peaks were seen in the HPLC result,which indicates that KP11 produces other compounds aside from E2.2.According to Tan and Zou,the ability of endophytic fungi to produce certain bioactive compounds originally characteristic of the host might be related to genetic recombination of the endophytic fungi with the host plant which occurs in evolutionary time[18].Since P.macrocarpa contains a wide range of compounds,we assume that C.gloeosporioides KP11 has evolved to produce severaldifferentcompoundssimilartothecompounds produced by P.macrocarpa.
InordertoachieveoptimumE2.2productionby C.gloeosporioides KP11,suitable fermentation condition must be applied during the fermentation process.Optimization was carried out on medium,pH,and length of incubation time. Table 1 shows the optimum fermentation condition for KP11. The result showed that PDB was more suitable for KP11 fermentation medium compared to MEB.In PDB,E2.2 concentration was almost three fold of the E2.2 concentration in MEB.This might be due to higher content of carbon source in PDB compared to MEB.Liquid state fermentation is known to depend mostly on soluble sugar in its medium for source of fermentation substrate which means that sugar content in the media highly affects the fermentation process[19].A study by Desmukh et al.showed that compared to other carbon sources,growth of C.gloeosporioides was at its highest when incubated in a starch-rich medium[20].PDB has a higher starch content compared to MEB,which explains the higher E2.2 concentration in PDB.
Table 1 also shows that E2.2 concentration increased from pH 4 to 5,and then gradually decreased from pH 7 to 8.5 where the optimum pH was at 5.This result is similar to a study by Drori et al.on external pectate lyase secretion by C.gloeosporioides[21].The study showed that pectate lyase production increases when the pH medium increases from 4 to 6 suggesting that there is an ambient pH signal transduction pathway in C.gloeosporioides.
Longer incubation time also showed higher yield of E2.2. The simple explanation to this is longer incubation time resulted in higher accumulation of E2.2 compound in the medium. Therefore,compared to 4 and 12 days,15 days of incubation showed higher E2.2 concentration.Further study is needed to determine at what stage of growth the KP11 fungus stop producing E2.2.
Aside from optimization of growth medium,we also tried to enhance E2.2 production by adding phloroglucinol and gallic acid into the growth medium at the beginning of fermentation process.As previously shown in the results,phloroglucinol and gallic acid addition to the fermentation broth appeared to increase E2.2 production by KP11.The increase in E2.2 concentration might be due to the similar structure of E2.2 and phloroglucinol or gallic acid.E2.2 compound is known to be a benzophenone which is comprised of two benzene rings.Both phloroglucinol and gallic acid have a benzene structure.Because of the similar structure,it seems that phloroglucinol and gallic acid can be used as a precursor in E2.2 synthesis.Use of artificial precursor to increase production of a desired metabolite has been used in several experiments.One example is the use of tryptophan as a precursor for the production of indolediterpenoid[22].
Furthermore,phloroglucinol has been related to the synthesis of benzophenone although there is still no definite pathway. There are some studies that suggest phloroglucinol supports benzophenone synthesis in shikimate pathway whereas other studies suggest that benzophenones are derived from phloroglucinol[23].Thisrelationbetweenphloroglucinoland benzophenone synthesis might explain the increased E2.2 production after phloroglucinol addition.
In light of our discovery,C.gloeosporioides KP11 endophytic fungus is potential to be a new source of E2.2 compound. This method provides a more rapid,efficient,and controllable way of producing E2.2.However,further study is required to optimize the production of E2.2 compound in larger scale with this method.
C.gloeosporioides KP11 found in P.macrocarpa plant is promising as a potential alternative source of E2.2 compound.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors thank Audrey Clarissa and Hanna Christabel Rouli for careful reading of the manuscript.
[1]Stone JK,Polishook JK,White JF Jr.Endophytic fungi.In: Mueller GM,Bills GF,Foster MS,editors.Biodiversity of fungi:inventory and monitoring methods.Burlington:Elsevier Academic Press;2004,p.241-70.
[2]Zhao J,Shan T,Mou Y,Zhou L.Plant-derived bioactive compounds produced by endophytic fungi.Mini Rev Med Chem 2011;11(2):159-68.
[3]Abdullah N,Hassan NH,Rahman SSA,Ismail H,Khalid R,Yahya MF.In vitro propagation of Phaleria macrocarpa,God's Crown.E3 J Biotechnol Pharm Res 2014;5(2):18-23.
[4]Hendra R,Ahmad S,Oskoueian E,Sukari A,Shukor MY.Antioxidant,anti-inflammatory and cytotoxicity of Phaleria macrocarpa(Boerl.)Scheff fruit.BMC Complement Altern Med 2011;11:110.
[5]Tandrasasmita OM,Lee JS,Baek SH,Tjandrawinata RR.Induction of cellular apoptosis in human breast cancer by DLBS1425,a Phaleria macrocarpa compound extract,via downregulator of P13-kinase/AKT pathway.Cancer Biol Ther 2010;10(8):814-23.
[6]Aripin A,Arifin PF,Tjandrawinata RR,inventor;PT.Dexa Medica,assignee.Isolated compounds from Phaleria macrocarpa as anticancer agents.United States Patent US 08569382 B2.2013 Oct 29.
[7]Firenzuoli F,Gori L.Herbal medicine today:clinical and research issues.EvidBasedComplementAlternatMed2007;4(Suppl1):37-40.
[8]Kusari S,Pandey SP,Spiteller M.Untapped mutualistic paradigms linking host plant and endophytic fungal production of similar bioactive secondary metabolites.Phytocemistry 2013;91:81-7.
[9]Arnold AE,Maynard Z,Gilbert GS.Fungal endopytes in dicotyledonous neotropical trees:patterns of abundance and diversity. Mycol Res 2001;105(12):1502-7.
[10]Moricca S,Ginetti B,Ragazzi A.Species and organ specificity in endophytes colonizing healthy and declining Mediterranean oaks. Phytopathol Mediterr 2012;51:587-98.
[11]Abang MM,Abraham W,Asiedu R,Hoffmann P,Wolf G,Winter S.Secondary metabolite profile and phytotoxic activity of genetically distinct forms of Colletotrichum gloeosporoides from yam(Dioscorea spp.).Mycol Res 2009;113:130-40.
[12]Rojas EI,Rehner SA,Samuels GJ,Van Bael SA,Herre EA,Cannon P,et al.Colletotrichum gloeosporoides s.l.associated with Theobroma cacao and other in Panama:multilocus phylogenies distinguish host-associated pathogens from asymptomatic endophytes.Mycologia 2010;102(6):1318-38.
[13]Inacio ML,Silva G,Teles H,Trevisan HC,Cavalheiro A,Bolzani VS,et al.Antifungal metabolites from Colletotrichum gloeosporoides,and endophytic fungus in Cryptocarya mandiocanna Nees(Lauraceae).Biochem Syst Ecol 2006;34:822-4.
[14]Chithra S,Jasim B,Sachidanandan P,Jyothis M,Radhakrishnan EK. Piperine production by endophytic fungus Colletotrichum gloeosporioides isolated from Piper nigrum.Phytomedicine 2014;21(4): 534-40.
[15]Zhang Q,Wei X,Wang J.Phillyrin produced by Colletotrichum gloeosporioides,and endophytic fungus isolated from Forsythia suspense.Fitoterapia 2012;83:1500-5.
[16]Zou WX,Meng JC,Lu H,Chen GX,Shi GX,Zhang TY,et al. Metabolites of Colletotrichum gloeosporioides,an endophytic fungus in Artemisia mongolica.J Nat Prod 2000;63(11):1529-30.
[17]Senthilkumar N,Murugesan S,Mohan V,Muthumary J.Taxol producingfungalendophyte,Colletotrichumgloeosporoides(Penz.)from Tectona grandis L.Curr Biotica 2013;7(1&2):8-15.
[18]Tan RX,Zou WX.Endophytes:a rich source of functional metabolites.Nat Prod Rep 2001;18:448-59.
[19]Raimbault M.General and microbiological aspects of solid substrate fermentation.Electron J Biotechnol 1998;1:1-15.
[20]Desmukh AJ,Mehta BP,Sabalpara AN,Patil VA.In vitro effect of various nitrogen,carbon sources and pH regimes on the growth and sporulation of Colletotrichum gloeosporioides Penz.and Sacc causing anthracnose of Indian bean.J Biopestic 2012;5:46-9.
[21]Drori N,Kramer-Haimovich H,Rollins J,Dinoor A,Okon Y,Pines O,et al.External pH and nitrogen source affects secretion of pectate lyase by Colletotrichum gloeosporioides.Appl Environ Microbiol 2003;69:3258-62.
[22]Mantle PG.The role of tryptophan as biosynthetic precursor of indole-diterpenoidfungalmetabolites:continuingadebate. Phytochemistry 2009;70:7-10.
[23]Rahman A.Studies in natural products chemistry.Amsterdam: Elsevier;2014.
17 Aug 2015
Raymond Rubianto Tjandrawinata,Metabolite Engineering Section,Dexa Laboratories of Biomolecular Sciences,Dexa Medica,Industri Selatan V Block PP no.7,Kawasan Industri Jababeka II,Cikarang 17550,Indonesia.
Tel:+62 21 89841901
Fax:+62 21 89841905
E-mail:Raymond@dexa-medica.com
Peer review under responsibility of Hainan Medical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Pacific Journal of Tropical Biomedicine2016年10期
Asian Pacific Journal of Tropical Biomedicine2016年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A review on promising phytochemical,nutritional and glycemic control studies on Moringa oleifera Lam.in tropical and sub-tropical regions
- A rare cause of acute abdomen-Spontaneous rectus sheath hematoma
- Evaluation of proline,chlorophyll,soluble sugar content and uptake of nutrients in the German chamomile(Matricaria chamomilla L.)under drought stress and organic fertilizer treatments
- Cytotoxic,genotoxic and apoptotic effects of naringenin-oxime relative to naringenin on normal and cancer cell lines
- Pandanusamaryllifoliusleafextractincreasesinsulinsensitivityinhigh-fatdiet-induced obese mice
- Jatropha curcas L∶Phytochemical,antimicrobial and larvicidal properties
