Cytotoxic,genotoxic and apoptotic effects of naringenin-oxime relative to naringenin on normal and cancer cell lines
Abdurrahim Kocyigit,Ismail Koyuncu,Murat Dikilitas,Fatemeh Bahadori,Baki Turkkan
1Department of Medical Biochemistry,Faculty of Medicine,Bezmialem Vakif University,Istanbul,Turkey
2Department of Biology,Faculty of Arts and Sciences,Harran University,Sanliurfa,Turkey
3Department of Plant Protection,Faculty of Agriculture,Harran University,Sanliurfa,Turkey
4Department of Pharmaceutical Biotechnology,Faculty of Pharmacy,Bezmialem Vakif University,Istanbul,Turkey
5Department of Chemistry,Faculty of Arts and Sciences,Harran University,Sanliurfa,Turkey
Cytotoxic,genotoxic and apoptotic effects of naringenin-oxime relative to naringenin on normal and cancer cell lines
Abdurrahim Kocyigit1*,Ismail Koyuncu2,Murat Dikilitas3,Fatemeh Bahadori4,Baki Turkkan5
1Department of Medical Biochemistry,Faculty of Medicine,Bezmialem Vakif University,Istanbul,Turkey
2Department of Biology,Faculty of Arts and Sciences,Harran University,Sanliurfa,Turkey
3Department of Plant Protection,Faculty of Agriculture,Harran University,Sanliurfa,Turkey
4Department of Pharmaceutical Biotechnology,Faculty of Pharmacy,Bezmialem Vakif University,Istanbul,Turkey
5Department of Chemistry,Faculty of Arts and Sciences,Harran University,Sanliurfa,Turkey
ARTICLE INFO
Article history:
in revised form 25 Dec 2015
Accepted 18 Jan 2016
Available online 26 Aug 2016
Naringenin
Naringenin-oxime
Cytotoxic
Genotoxic
Apoptosis
Reactive oxygen species Comet assay
Objective:Toassessandcomparethecytotoxic,genotoxic,apoptoticandreactiveoxygen species(ROS)generating effects of naringenin(NG)and its new derived compound naringenin-oxime(NG-Ox)on MCF-7,HT-29,PC-12 cancer and L-929 normal cell lines. Methods:The cells were incubated with different doses of NG-Ox and NG(50-1000μmol/L)for 24 h.The cell viability was assessed based on ATP cell viability assay. Intracellular accumulation of ROS was determined using the fluorescent probes 2′7′-dichlorodihydrofluorescin diacetate.Genotoxic effects were evaluated by alkaline single cell gel electrophoresis assay(comet assay)and,the apoptotic effect was evaluated by acridine orange staining at below the IC50levels.
Results:Both NG-Ox and NG exhibited cytotoxic,genotoxic and apoptotic effects and resulted in increased ROS values in a dose-dependent manner.The effects were more pronounced on cancer cell lines.The cytotoxic,genotoxic and apoptotic effects of NG-Ox were higher than that of NG in all cell lines.Significant correlations were observed between cell viability,DNA damage,apoptosis and ROS,in all cell lines exposed to either NG-Ox or NG.
Conclusions:This study showed that both NG-Ox and NG possess cytotoxic,genotoxic and apoptotic activities through the production of ROS on cells,NG-Ox being the more effective one.Therefore,derived compound of NG might be used as antiproliferative agents for the treatment of cancer.
Original articlehttp://dx.doi.org/10.1016/j.apjtb.2016.08.004
1.Introduction
Cancer chemoprevention is defined as the use of substances of natural origin,biological agents,synthetic,or chemical compounds to reverse,suppress or prevent carcinogenic progression of invasive cancer[1].The main form of treatment at this point is chemotherapy,which consists of delivering drugs systemically so that they can reach and kill the tumor cells. But most of these drugs cause severe side effects in patients and need to be used at suboptimal levels.Therefore,studies have been focused on alternative chemopreventive agents such as some plant extracts,natural compounds obtained from plants or their derivatives.For example,flavonoids are found at high concentrations in citrus fruits in which the most common classes are flavanones,flavones,and flavonols[2,3]. These compounds exhibit a wide range of biological activities and positive health effects on mammalian cells,including antiinflammatory,antiatherogenic,and anticancer[4].Together withstrongantioxidantactivity,numerousstudieshave reported that flavonoids can show cytotoxic and apoptotic effects on various cancer cell lines[5,6].
Generally cytotoxic and antitumor agents are non-selective and kill normal proliferating cells.Identification of active cancer specific compounds remains a thrust area in drug screening and drug discovery mechanisms.Much emphasis has beenplaced on discovering new compounds that target tumor cells more efficiently and selectively with minimal toxic effects on normal cells[7,8].Naringenin(NG)is a member of flavonoids,which is also abundant in citrus fruits.It has been reported to have different biological activities such as antioxidant,free radical scavenging,anti-inflammatory and immunomodulatory effects[9-11].In addition to these potentials,NG has also been reported to induce cytotoxic and apoptotic activities in various cancer cell lines[12-14].Although,the molecular mechanisms of its antiproliferative and apoptotic effects have not been cleared[15],increasing evidence have supported that the increaseofreactiveoxygenspecies(ROS)generation contributes to the treatment of cancer cells[16].Khan et al.[17]demonstrated that the cytotoxic effect of NG is because of its pro-oxidant activity.
To seek for the derivatives of this compound with possible higher activity Turkkan et al.[18]synthesized,characterized and investigated the antioxidant properties of a new NG-derivatized compound called NG-Oxime(NG-Ox),and demonstrated that antioxidant and antigenotoxic properties of NG were significantly enhanced in its Ox form.Kocyigit et al.[19]demonstrated that both NG and NG-Ox are able to protect cells against oxidative damage and NG-Ox is more effective than NG.
¨Ozy¨urek et al.[20]also showed it's antiproliferative activity. However,there is no available report comparing cytotoxic,genotoxic,apoptotic potentials of NG-Ox relative to NG.The aim of the present study was to evaluate the cytotoxic,genotoxic,apoptotic and ROS generating effects of NG-Ox relative to NG in several cancer and normal cell lines to seek for a hope in cancer treatment.
2.Materials and methods
2.1.Chemicals
Naringenin[(±)-2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one],heat-inactivated fetal calf serum,Dulbecco's modified Eagle medium(DMEM),2′7′-dichlorodihydrofluorescin diacetate(H2DCF-DA),pencillin-streptomycin and ethidium bromide(EB)were purchased from Sigma-Aldrich(Seelze,Germany).NG-Ox[(±)-2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one oxime]was synthesized and characterized by Turkkan et al.[18].All other reagents used were of analytical grade unless otherwise stated.
2.2.Samples
Both NG and NG-Ox were dissolved in dimethyl sulfoxide(DMSO)to prepare a stock solution(40 mmol/L).The stock solution was diluted with DMEM(contains no fetal bovine serum).The final concentration of DMSO in the NG and NG-Ox solution was<1%.Prior to start of experiments,we confirmed that this level of DMSO as well as the serum free media did not induce any DNA damages in the cells.Other reagents were prepared as fresh before each experiment.
2.3.Cell culture and maintenance
L-929 cells(as a standard cell line originated from mouse fibroblast cells)and human colorectal adenocarcinoma cell line(HT-29),were obtained from American Type Cell Culture Collection.Rat pheochromocytoma cell line(PC-12)and human breast cancer cell line(MCF-7)were kindly supplied as gifts from Prof.Dr.S.Ismet Delioglu Gurhan(Bioengineering Department,University of Ege,Turkey).All cells were cultured in DMEM equilibrated with 5%CO2atmosphere at 37°C.The medium was supplemented with 10%fetal calf serum,100 IU/mL of penicillin and 100 ng/mL of streptomycin.The number of viable cells was estimated by trypan blue exclusion test.
2.4.Cytotoxicity assay
Cytotoxic activities of NG-Ox and NG on HT-29,PC-12,MCF-7 and L-929 cells were determined by ATP levels using a luminescence test(Cell-Titer-Glo luminescent cell viability assay,Promega).Cells were seeded onto 96-well plates at a density of 5×103cells per well and incubated overnight at 37°C in 5%CO2.The medium was then replaced with fresh complete medium containing various concentrations of NG-Ox and NG(50-1000μmol/L).Control cells were treated with 1% DMSO.All the cells were incubated in a humidified 5%CO2and 95%O2at 37°C for 24 h.Then,the cells were rinsed with the culture medium and tested for ATP.Each of the samples was supplemented with 100μL of the prepared reagent(CellTiter-Glo luminescent cell viability assay,Promega),mixed for 2 min and incubated for 10 min at room temperature. The results were read using a luminometer(Varioskan Flash Multimode Reader,Thermo,Waltham,MA).The light emitted in the presence of ATP was quantitated in relative light units. The intensity of emitted light quants was directly related to ATP content in the tested sample.The cell viability was expressed as the percentage compared with the negative control group designated as 100%.IC50values were calculated from the concentration-response curves by non-linear regression analysis.All experiments were repeated three times and standard deviation was within 5%.
2.5.Measurement of ROS generation
Generation of ROS was assessed by using a cell-permeable fluorescent signal H2DCF-DA as an indicator for ROS[21,22].As described previously,H2DCF-DA is oxidized to a highly green fluorescent 2′7′-dichlorofluorescein(DCF)by the generation of ROS.Cancercelllineswerepretreatedwithvarious concentrations of NG and NG-Ox for 24 h.After 24 h incubation period,the cells were washed with cold phosphate buffer solution(PBS)and incubated with 100 mmol/L H2DCF-DA for another30minat37°C.DCFfluorescenceintensitywasmeasured using the fluorescence plate reader(Varioskan Flash Multimode Reader,Thermo,Waltham,MA)at excitation/emission=488/ 525 nm.The estimations were carried out thrice in triplicate,ensuring each time that the number of cells per treatment group were the same to ensure reproducibility.The values were expressed as%relative fluorescence compared to the control.
2.6.Genotoxic activity assays
Genotoxic effects of NG and NG-Ox on HT-29,PC-12,MCF-7 and L-929 cell lines were evaluated by using alkaline single cell gel electrophoresis assay(comet assay)according to Singh et al.[23]with slight modification.To determine thegenotoxic potential of NG-Ox and NG,three different cells were seededonto6-wellcellcultureplates(approximately 2×105cells per well)with cell culture medium and incubated at 37°C in 5%CO2for 24 h for cell establishment.After 24 h,below IC50concentrations of NG and NG-Ox(50-600μmol/L in 1%DMSO)were added to the cells and incubated for another 24 h at 37°C.DMSO(1%)was used as a negative control and 50μmol/L H2O2was used as a positive control.After incubation,the cells were washed with PBS,harvested using trypsin/ ethylene diamine tetraacetic acid(EDTA)and collected for centrifugation at 1750 r/min for 5 min at 4°C.The supernatant was discharged and the cell density was adjusted to 2×105cells/ mL using cold PBS.Re-suspended cells(10μL)were placed into centrifuge tubes for the comet assay as described below.All experiments were repeated in triplicate.
Cell suspensions(10μL)was mixed with 90μL of 0.6% low melting agarose and added to the slides pre-coated with 1%normal melting agarose[24].After solidification of the agarose,the slides were immersed in lysis solution(2.5 mol/ L NaCl,100 mmol/L EDTA,10 mmol/L Tris,1%Triton X-100 and 10%DMSO,pH 10)for 1 h at 4°C.After removing the slides from lysis solution,they were washed with cold PBS and placed in a horizontal electrophoresis tank side by side.DNA was allowed to unwind for 40 min in freshly prepared alkaline electrophoresis buffer containing 300 mmol/L NaOH and 10 mmol/L Na2EDTA(pH 13.0). After unwinding,electrophoresis was run at 300 mA for 25 min at 4°C under minimal illumination to prevent further DNA damage.The slides were washed three times with a neutralization buffer(0.4 mol/L Tris,pH 7.5)for 5 min at 4°C and then treated with ethanol for another 5 min before staining.Dried microscope slides were stained with EB(2μg/mL in distilled H2O;70μL/slide)covered with a coverslip and analyzed using a fluorescence microscope(Leica DM 1000,Solms,Germany)at a 200 magnification with epiflourescence equipped with a rhodamine filter(with an excitation wavelength of 546 nm;and a barrier of 580 nm).A hundred cells were randomly scored by eye in each sample on a scale of 0-4 based on fluorescence beyond the nucleus.The scale used was as follows:0,no tail;1,comet tail>half the width of the nucleus;2,comet tail equal to the width of the nucleus;3,comet tail longer than the nucleus and 4,comet>twice the width of the nucleus. Scoring cells in this manner have been shown to be as accurate and precise as using computerized image analysis. The individual scoring of the slides was blind,using coded slides.The visual score for each class was calculated by multiplying the percentage of cells in the appropriate comet class by the value of the class.The total visual comet score characterizing the degree of DNA damage in the entire study groups was the sum of the scores in the five comet classes. Thus,thetotalvisualscorecouldrangefrom0(all undamaged)to 400(all maximally damaged)arbitrary units(AU),as reported by Collins et al.[25].This method of measurement was proved to be valid and up-to-date[26].All oftheprocedureswerecompletedwiththesame biochemistry staff and DNA damage was detected using a single observer that was not aware of the subject's status. Cell viability measured with trypan blue exclusion test was above 95%for all treatments.Results of the triplicated tests are expressed in terms of arbitrary units recorded.All experiments were repeated in triplicate.
2.7.Morphological evaluation by fluorescence microscopy
Morphological changes in cells were studied by acridine orange/ethidium bromide(AO/EB)double staining as described by McGahon et al.[27].Using this technique,the cells undergoing apoptosis are distinguished from the viable cells by the morphological changes of apoptotic nuclei.Ethidium bromide and acridine orange are DNA intercalating dyes. Acridine orange is taken up by both viable and dead cells and stains double-stranded and single-stranded nucleic acids.When acridine orange diffuses into double-stranded DNA,it emits green fluorescence upon excitation at 480-490 nm from viable cells[28].Ethidium bromide is taken up by dead cells and stains DNA orange.Briefly,the cells were cultured in six-well plates(2×105cells/well)and incubated overnight.Then,the cells were treated with NG and NG-Ox under doses of IC50determined by cytotoxicity assay for 24 h at 37°C.DMSO(1%)was used as a negative control.The cells were then harvested and washed twice with PBS.Finally,AO/EB solution was added to the cell suspension and the nuclear morphology was evaluated by fluorescence microscopy(Leica DM 1000,Solms,Germany). Multiple photos were taken at randomly-selected areas and a minimum of 100 cells were counted.According to the method,the live cells have normal green nuclei,apoptotic cells have green nuclei with fragmented chromatin,and dead cells have orange/red nuclei.Tests were done in triplicate.
2.8.Statistical analysis
The results are presented as the mean±SD of tree replicates.Data in all experiments were analyzed for statistical significance using analyses of variance(One-way ANOVA). The P value<0.05 was considered as statistically significant. IC50values of NG and NG-Ox over the cell lines were calculated by nonlinear regression analysis.Associations between ROS generation and cell viability parameters were analyzed by Pearson correlation coefficient.All statistical analyses were performed using SPSS package program for Windows(Version 20,Chicago,IL).
3.Results
3.1.Cytotoxicity of NG and NG-Ox
The effects of NG and NG-Ox on the viability of HT-29,PC-12,MCF-7 cancer cells and L-929 normal cells were determined via bioluminometric ATP cell viability assay.Our findings showed that NG-Ox and NG resulted in greater cellular death in HT-29,MCF-7 and PC-12 cancer cells than that of L-929 cells.The percentage of anti-proliferative activity progressively increased in a concentration dependent manner and,NG-Ox was more effective than NG in all cell lines(Figure 1).
While IC50of NG-Ox for MCF-7,HT-29,PC-12 and L-929 cells ranged between 470 and 790μmol/L,NG was less effective with the IC50values of 780-880μmol/L for MCF-7,HT-29 and PC-12 cells.IC50values of NG for L-929 cell lines could not be determined since the value was out of range.IC50values for NG-Ox and NG for the cell lines are given in Table 1.
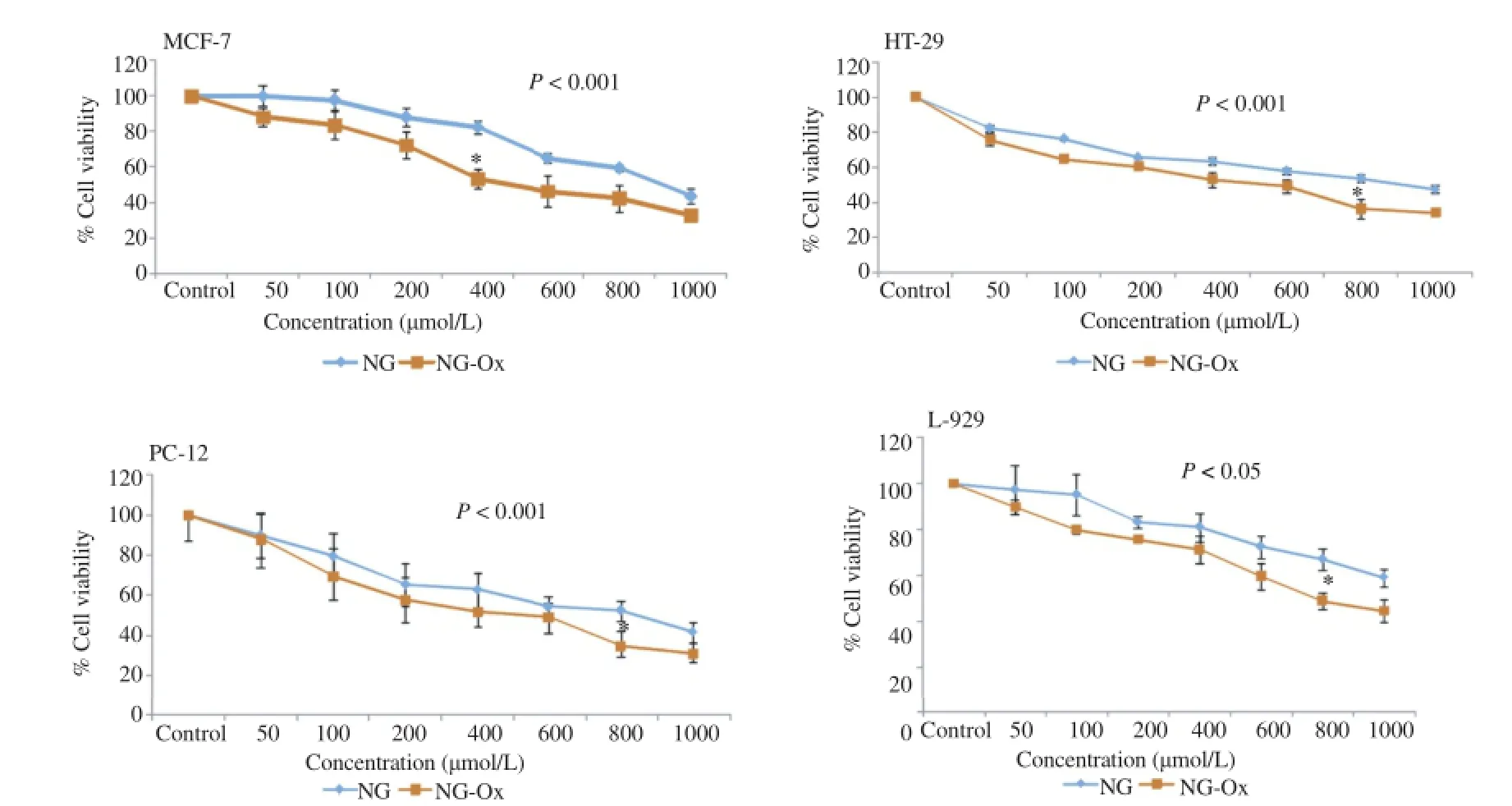
Figure 1.Effects of NG and NG-Ox on the viability of HT-29,PC-12,MCF-7 cancer cells and L-929 normal cells.
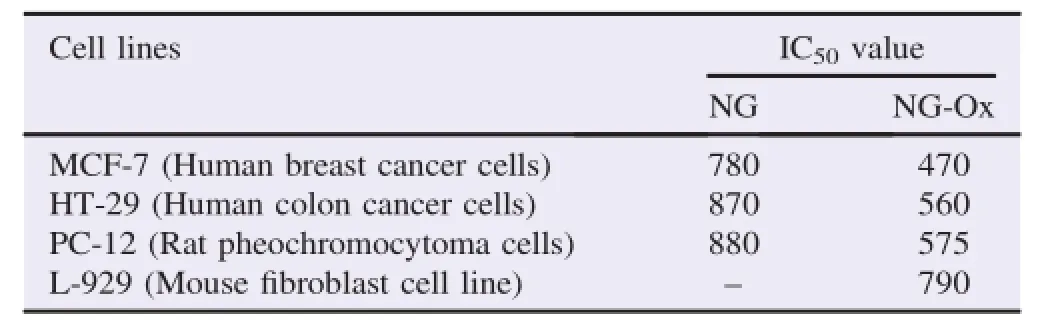
Table 1 The IC50values of NG and NG-Ox for MCF-7,HT-29,PC-12 and L-929 cells.μmol/L.
3.2.ROS generation ability of NG and NG-Ox
It has been entrusted that different ROS species are produced endogenously in many cell types in diverse cellular processes[29].It has also been proposed that ROS are active cellular signaling molecules[30],and have unabated role in the induction of apoptosis[31].In this context to check whether ROS production is associated with NG and NG-Ox induced apoptosis in cancer and normal cells,we evaluated intracellular ROS generation by fluorimetry using H2DCF-DA as a probe.We have observed that NG and NG-Ox could efficiently induce ROS generationinallcancer cellsinaconcentrationdependentmanner and,NG-Ox was more effective than that of NG(Figure 2).
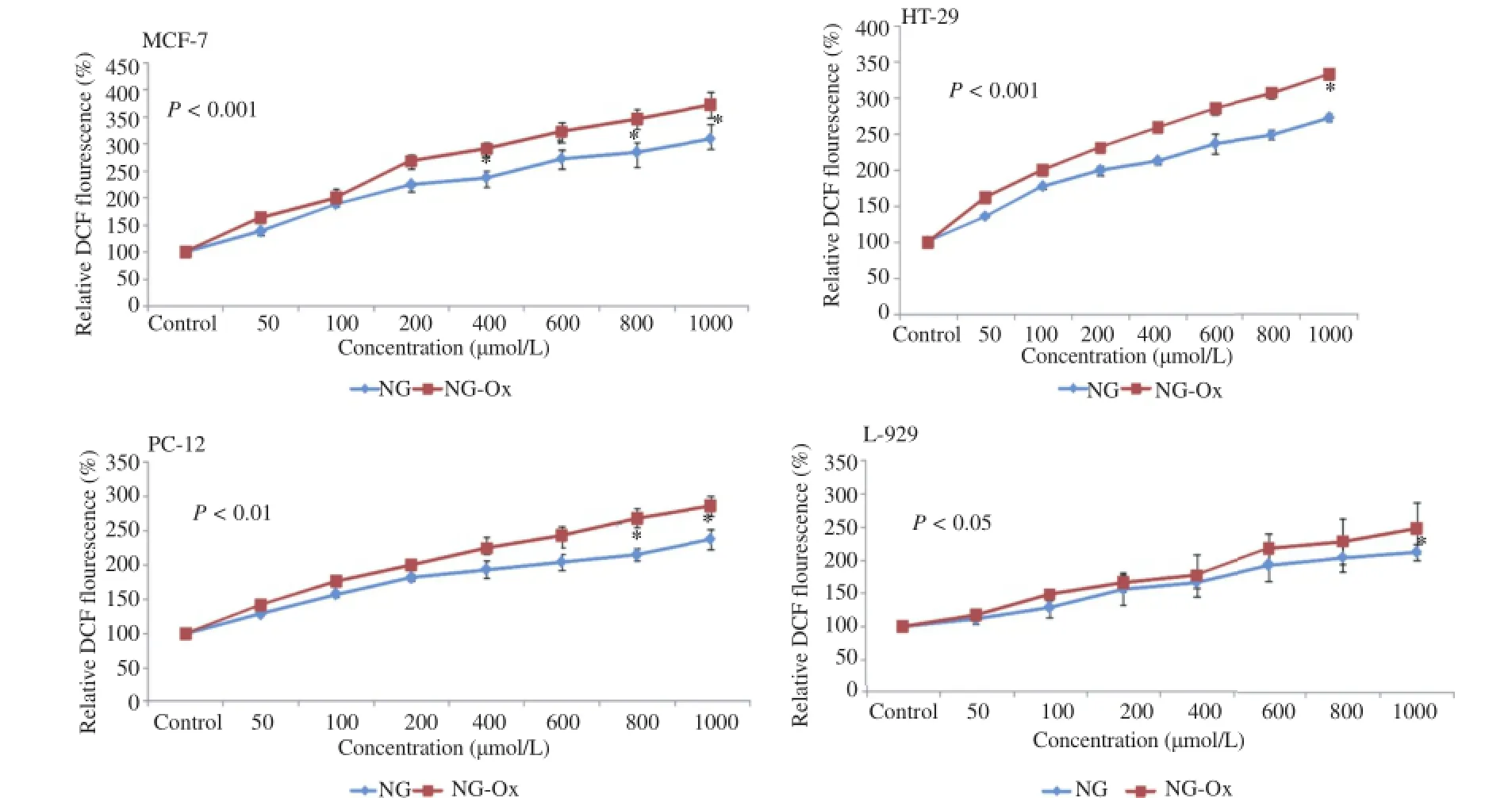
Figure 2.ROS generating effect of NG-Ox and NG on MCF-7,HT-29,PC-12,cancer cells and L-929 normal cells.
There was a significant negative relationship between ROS generatingactivityandcellviabilityinallcelllines(P<0.001).
3.3.Genotoxic activity
The genotoxic effects of the NG-Ox on cancer and normal cell lines were also investigated using comet assay(Figure 3).
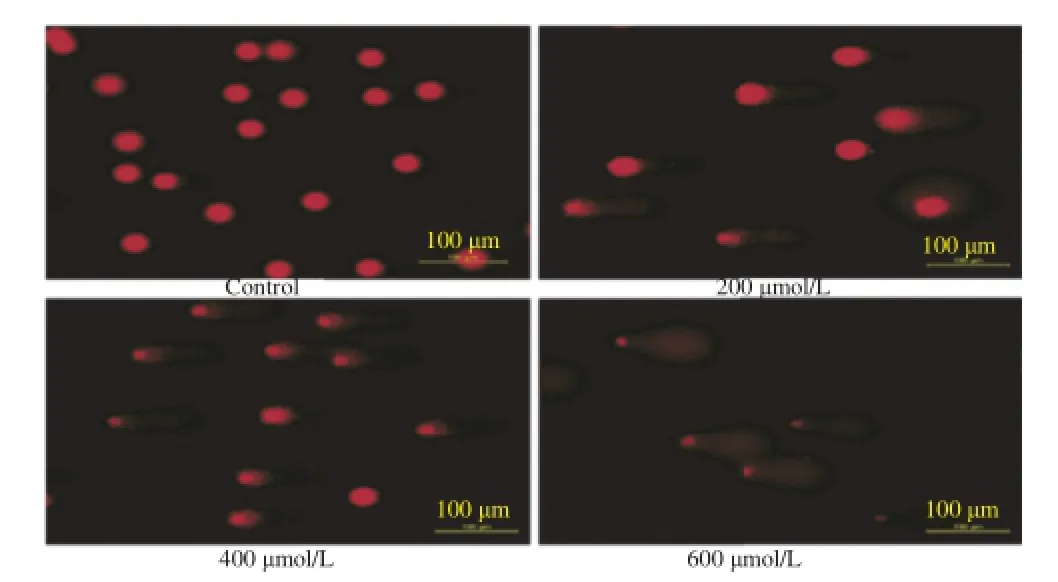
Figure 3.Genotoxic effect of NG-Ox(200-600μmol/L)on MCF-7 cancer cells after 24 h incubation.
For the evaluation of genotoxicity,the cells were treated with NG-Ox and NG at the under concentrations of their IC50.The results of genotoxic activity of NG-Ox and NG are presented in Figure 4.As seen in Figure 4,both NG-Ox and NG significantly induced DNA damages in MCF-7,HT-29,PC-12 and L-929 cell lines(above 50,100,200,400μmol/ L,respectively)and,NG-Ox was more effective than NG in all cell lines.
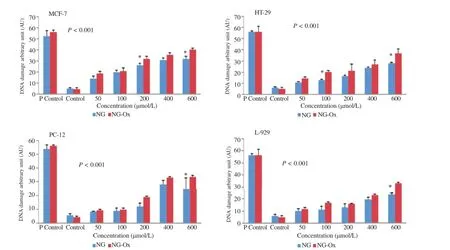
Figure 4.The comet assay results of NG and NG-Ox on HT-29,MCF-7,PC-12 and L-929 cell lines.
3.4.Morphological evaluation
For the evaluation of apoptosis,the cells were treated with NG and NG-Ox at various concentrations(50-600μmol/L)arranged according to their IC50values.After 24 h incubation,AO/EB staining was used to visualize nuclear changes and apoptotic body formation that are characteristic of apoptosis. Cells were viewed under a fluorescence microscope,and the images of apoptotic and control cells after AO/EB are presented in Figure 5.
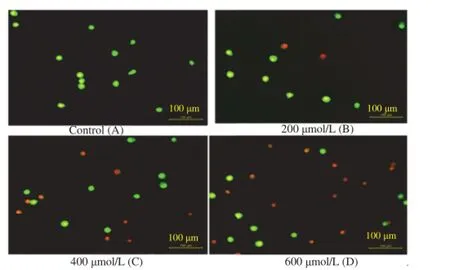
Figure 5.Different morphological patterns of apoptosis induced by NGOx determined by AO/EB double staining.
The apoptotic cells had orange particles in their nuclei whereas the viable cells were observed green.The results indicated that exposure of the cells below IC50doses of NG-Ox caused generally more apoptotic activity than those of NG inall cancer cell lines.As seen in Figure 6,both NG-Ox and NG significantly induced apoptosis in MCF-7,HT-29,PC-12 and L-929 cell lines and NG-Ox was more effective than NG in all cancer cells(Figure 6).
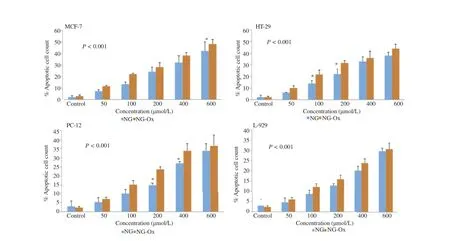
Figure 6.Apoptotic activity of NG and NG-Ox on HT-29,MCF-7,PC-12 and L-929 cell lines.
There were positive correlations between genotoxicity,apoptosis and ROS levels(P<0.001)and a negative relationship between cellviabilityand ROSgenerating activity(P<0.001)exposed to either NG-Ox or NG in all cell lines.
4.Discussion
Natural products or their derivatives have been demonstrated to have significant anticancer potentials due to their ability to inhibit tumor growth,angiogenesis and metastasis without many side effects[32-35].Much of these activities come from flavonoids,whichareprincipalcomponentsofmany phytochemicals and demonstrate the capacity to inactivate carcinogens,inhibit angiogenesis,and promote healthy cell proliferationandapoptosis.Inthepresentstudy,we demonstrated that treatment with NG-Ox showed more cytotoxic activity on several cancer and normal cells relative to NG in a dose-dependent manner.Since the cell death activity of NGOx was higher,we further evaluated to reveal the mechanism of its cytotoxicity.For this purpose,we determined genotoxic,apoptotic and ROS generating activities of NG-Ox and NG.
The possible antiproliferative effects of the NG-Ox and NG were evaluated using ATP cell viability assay and we also evaluated IC50values of these compounds.ATP is among the most sensitive luminometric tests to analyze cell culture viability.We observed that NG-Ox and NG resulted in great cellular death in HT-29,MCF-7 and PC-12 cancer cells and the percentage of antiproliferative activity progressively increased in adose-dependentmanner.Severalinvitrostudiesalso implanted mice.Similarly,Park et al.[15]showed that NG significantly induced antiproliferative activity via apoptosis in human leukemia THP-1 cells.However,the only report on cytotoxicity of NG-Ox is made byO¨zyu¨rek et al.[20]. However,thereisnoavailablereportcomparing antiproliferative activity between NG-Ox and NG.
Flavonoids are universally recognized antioxidants which can protect the cell from the oxidative stress,i.e.,neutralize the damaging effect of ROS.NG and NG-Ox have been well-known as antioxidant agents[18,20].However,at high concentrations,flavonoids and other polyphenols can show cytotoxic effects[37].The mechanism of dual protective-destructive behavior of flavonoids has not been elucidated yet.There are several mechanisms offered for the cytotoxicity of flavonoids including the inhibition of topoisomerases[38],kinases[39]and their prooxidant action[40].It is highly possible that the pro-oxidant effect is responsible for the selective antiproliferative activity of these compounds and ROS are key signaling molecules to modulate cell death[41].Accumulating evidence indicates that cancer cells produce high levels of ROS that lead to a state of increased basal oxidative stress.The increased production of ROS in cancer cells was observed in in vitro and in vivo studies[42].It has been found that NG had pro-oxidant potential[43].Ahamad et al.[44]demonstrated that NG leads to cell death in cancer cells via inducing ROS generation.We therefore investigated the effectiveness of NG and NG-Ox in generation of ROS.We found that exposure of cancer cells with NG and NG-Ox dramatically enhanced generation of intracellular ROS at different levels in a dose dependent manner and demonstrated that NG had antiproliferative effects in cancer cell lines.For example,Kanno et al.[36]found that NG caused cell death via apoptosis in various human cancer cell lines and had an inhibitory effect on tumor growth in sarcoma S-180-NG-Ox is more effective than that of NG in all cell lines.A negativesignificantcorrelationwasevidentbetweencell viability and ROS generating activity in all cancer cells.These findings demonstrated that both endogenous and exogenous ROS generation might induce killing of cancer cells with a synergistic effect[45].
ROS are constantly generated and eliminated in the biological system,and play important roles in a variety of normal biochemical functions and abnormal pathological processes. Consequently,humans have evolved antioxidant defense systems that limit their production.Generation of oxidants plays a vital role to initiate the cellular apoptotic cascade by disturbing the balance between cellular signals for survival and suicide. Growing evidence suggests that cancer cells exhibit increased intrinsic ROS,due in part to oncogenic stimulation,increased metabolic activity and mitochondrial malfunction[46].We hypothesize that different metabolic activity of cancer cells result in different levels of ROS generation and,there is a close relationship between ROS generating potentials and cytotoxic activity of cancer cells.It has also been reported that higher basal levels of ROS in leukemic cells are more sensitive to pro-oxidants as compared to their normal counterparts[47].Taken together,NG acting as pro-oxidant,effectively raised the cell's oxidative status beyond a threshold limit inducing apoptosis in leukemic cells.
Increased ROS generating and cytotoxic activity of NG-Ox compared to NG could be related to its molecular shape. Because,pro-oxidant activity is thought to be directly proportional to the total number of hydroxyl groups in a flavonoid molecule[48].Seriesofmonoanddihydroxy-flavonoids exhibited detectable pro-oxidant activity,while multiple hydroxyl groups,especially in the B-ring,significantly increased production of hydroxyl radicals in Fenton reaction[49].As illustrated in Figure 1,NG has three OH groups while NG-Ox carries four in the ring B.
In this study,we measured genotoxic effects of NG and NGOx on cancer cell lines with alkaline comet assay,using noncytotoxic concentrations of these two compounds.The comet assay is a sensitive method for detecting DNA strand breakage at the level of an individual cell.We observed that low concentrations of NG and NG-Ox caused no genotoxicity while the concentrations above 50μmol/L showed genotoxic activities in MCF-7 cells.Previous studies have demonstrated that DNA damage increases with the increasing concentration of NG together with other flavonoids in lymphocyte cells.Similarly,it has been shown that NG and Morin can induce peroxidation in nuclear membrane lipids along with the increase in DNA strand breakage.Unexpected genotoxic behavior of these compounds could explain,in part,by their pro-oxidant activity[50-53].It has been revealed that pro-oxidant compounds increase with the levels of H2O2[54].The H2O2can kill any living cells and abort their development at any stage and results in DNA damage by the hydroxyl radical generated via Fenton reaction.
Since the main goal of our study was to obtain agents that promote or induce apoptosis,NG-Ox and NG with low IC50against cancer and normal cells were selected to determine cell death mechanism(apoptosis or necrosis).Our results demonstrated that both NG-Ox and NG can show higher apoptotic activity on cancer cells.NG-Ox was more effective than NG in all cell types.
Apoptotic,necrotic and live cells can be distinguished using AO/EB staining.AO can penetrate living and dead cells and emits green fluorescence as a result of intercalation into doublestranded DNA while EB can only penetrate dead cells and emits red fluorescence after intercalation with DNA[55].Therefore,fluorescent DNA binding dyes AO and EB were used to detect the apoptotic and necrotic cells(Figure 5).In this figure,living cells are distinguished with a normal green nucleus,apoptotic cells show orange-stained nuclei with chromatin condensation or fragmentations,while necrotic cells are clearly observed with uniformly orange-stained cell nuclei with no condensed chromatin.Analysis of the AO/EB staining results revealed that NG-Ox and NG treated cancer cells clearly exhibited late-stage apoptotic events(chromatin condensation and nuclear fragmentation)with a significant decrease in cell viability.It has been demonstrated that NG and its synthetic derivatives induced apoptosis in cancer cell lines in different pathways[50-53].Apoptosis refers to programmed cell death.In the process of apoptosis,there are changes in the morphology of the cells,such as chromatin condensation and a reduction in cell volume,associatedwithfragmentationofDNAinto nucleosomal size fragments of 180-200 bp or multiples thereof in such systems apoptotic bodies are formed[56]. Induction of apoptosis has been considered to be the major mechanism of anticancer drug discovery,and the development of anti-cancer drugs that inhibit abnormal cancer cell proliferation and induce cell death through apoptosis is a fundamental objective of cancer research[57,58].
The present in vitro study demonstrated that both NG and its new derivatized compound NG-Ox have more cytotoxic,genotoxic and apoptotic activity through the production of ROS on cancer cells than in normal cells and NG-Ox was more effective than NG at equal doses.Therefore,derivatized compound of NG might be used as antiproliferative agents for the treatment of cancer.Since our data is based on in vitro experiments,further in vivo studies are required to consolidate the genotoxic and antiproliferative properties of NG and NG-Ox.
Conflict of interest statement
We declare that we have no conflict of interest.
[1]Fridlender M,Kapulnik Y,Koltai H.Plant derived substances with anti-cancer activity:from folklore to practice.Front Plant Sci 2015;6:799.
[2]Kaur J,Kaur G.An insight into the role of citrus bioactives in modulation of colon cancer.J Funct Foods 2015;13:239-61.
[3]Wang L,Wang J,Fang L,Zheng Z,Zhi D,Wang S,et al.Anticancer activities of citrus peel polymethoxyflavones related to angiogenesis and others.Biomed Res Int 2014;2014:453972.
[4]Lai CS,Wu JC,Ho CT,Pan MH.Disease chemopreventive effects and molecular mechanisms of hydroxylated polymethoxyflavones. Biofactors 2015;41(5):301-13.
[5]Hamedeyazdan S,Fathiazad F,Sharifi S,Nazemiyeh H.Antiproliferative activity of Marrubium persicum extract in the MCF-7 human breast cancer cell line.Asian Pac J Cancer Prev 2012;13(11):5843-8.
[6]Khan M,Rasul A,Yi F,Zhong L,Ma T.Jaceosidin induces p53-dependent G2/M phase arrest in U87 glioblastoma cells.Asian Pac J Cancer Prev 2011;12(12):3235-8.
[7]Benedekoviˊc G,Kovaˇceviˊc I,Popsavin M,Francuz JM,Kojiˊc V,Bogdanovic G,et al.Divergent total synthesis of crassalactones B and C and evaluation of their antiproliferative activity.Tetrahedron 2015;71(28):4581-9.
[8]Lavrard H,Salvetti B,Mathieu V,Rodriguez F,Kiss R,Delfourne E.Synthesis and in vitro antiproliferative activity of amidoandaminoanaloguesofthemarinealkaloidisogranulatimide.ChemMedChem 2015;10(4):607-9.
[9]Leonardi T,Vanamala J,Taddeo SS,Davidson LA,Murphy ME,Patil BS,et al.Apigenin and naringenin suppress colon carcinogenesis through the aberrant crypt stage in azoxymethane-treated rats.Exp Biol Med(Maywood)2010;235(6):710-7.
[10]Razo-Aguilera G,Baez-Reyes R,Alvarez-Gonz´alezb I,Paniagua-P´erez R,Madrigal-Bujaidar E.Inhibitory effect of grapefruit juice on the genotoxicity induced by hydrogen peroxide in human lymphocytes.Food Chem Toxicol 2011;49(11):2947-53.
[11]Sabarinathan D,Vanisree AJ.Naringenin,a flavanone alters the tumorigenic features of C6 glioma cells.Biomed Prev Nutr 2011;1(1):19-24.
[12]Meiyanto E,Hermawan A,Anindyajati.Natural products for cancer-targetedtherapy:citrusflavonoidsaspotentchemopreventive agents.Asian Pac J Cancer Prev 2012;13(2):427-36.
[13]Arul D,Subramanian P.Naringenin(citrus flavonone)induces growth inhibition,cell cycle arrest and apoptosis in human hepatocellular carcinoma cells.Pathol Oncol Res 2013;19(4):763-70.
[14]Matsuo M,Sasaki N,Saga K,Kaneko T.Cytotoxicity of flavonoids toward cultured normal human cells.Biol Pharma Bull 2005;28(2):253-9.
[15]Park JH,Jin CY,Lee BK,Kim GY,Choi YH,Jeong YK.Naringenin induces apoptosis through downregulation of Akt and caspase-3 activation in human leukemia THP-1 cells.Food Chem Toxicol 2008;46(12):3684-90.
[16]Gach K,Dlugosz A,Janecka A.The role of oxidative stress in anticancer activity of sesquiterpene lactones.Naunyn Schmiedebergs Arch Pharmacol 2015;388(5):477-86.
[17]Khan HY,Zubair H,Ullah MF,Ahmad A,Hadi SM.A prooxidant mechanism for the anticancer and chemopreventive properties of plant polyphenols.Curr Drug Targets 2012;13(14):1738-49.
[18]T¨urkkan B,Ozy¨urek M,Bener M,G¨uçl¨u K,Apak R.Synthesis,characterization and antioxidant capacity of naringenin-oxime. Spectrochim Acta A Mol Biomol Spectrosc 2012;85(1):235-40.
[19]Kocyigit A,Koyuncu I,Taskin A,Dikilitas M,Bahadori F,Turkkan B.Antigenotoxic and antioxidant potentials of newly derivatized compound naringenin-oxime relative to naringenin on human mononuclear cells.Drug Chem Toxicol 2016;39(1): 66-73.
[20]¨Ozy¨urek M,Akpınar D,Bener M,T¨urkkan B,G¨uçl¨u K,Apak R. Novel oxime based flavanone,naringin-oxime:synthesis,characterization and screening for antioxidant activity.Chem Biol Interact 2014;212:40-6.
[21]Min KJ,Jung KJ,Kwon TK.Carnosic acid induces apoptosis through reactive oxygen species-mediated endoplasmic reticulum stress induction in human renal carcinoma Caki cells.J Cancer Prev 2014;19(3):170-8.
[22]Wu T,Qiang L,Chen FH,Zhao Q,Yang Z,Zou MJ,et al.LFG-500,a newly synthesized flavonoid,induced a reactive oxygen species-mitochondria-mediated apoptosis in hepatocarcinoma cells. Biomed Prev Nutr 2011;1(2):132-8.
[23]Singh NP,McCoy MT,Tice RR,Schneider EL.A simple technique for quantitation of low levels of DNA damage in individual cells.Exp Cell Res 1988;175(1):184-91.
[24]Inaba K,Inaba M,Romani N,Aya H,Deguchi M,Ikehara S,et al. Generation of large numbers of dendritic cells from mouse bone marrowculturessupplementedwithgranulocyte/macrophage colony-stimulating factor.J Exp Med 1992;176(6):1693-702.
[25]Collins AR,Ma AG,Duthie SJ.The kinetics of repair of oxidative DNA damage(strand breaks and oxidised pyrimidines)in human cells.Mutat Res 1995;336(1):69-77.
[26]Dikilitas M,Kocyigit A.Assessment of computerized and manual analysis of slides processed in single cell gel electrophoresis assay. Fresenius Environ Bull 2012;21(10):2981-7.
[27]McGahon AJ,Martin SJ,Bissonnette RP,Mahboubi A,Shi Y,Mogil RJ,et al.The end of the(cell)line:methods for the study of apoptosis in vitro.Methods Cell Biol 1995;46:153-85.
[28]Ebrahim K,Shirazi FH,Vatanpour H,Zare A,Kobarfard F,Rabiei H.Anticancer activity of cobra venom polypeptide,cytotoxin-II,against human breast adenocarcinoma cell line(MCF-7)via the induction of apoptosis.J Breast Cancer 2014;17(4): 314-22.
[29]Davis Volk AP,Moreland JG.ROS-containing endosomal compartments:implications for signaling.Methods Enzymol 2014;535:201-24.
[30]Ray PD,Huang BW,Tsuji Y.Reactive oxygen species(ROS)homeostasis and redox regulation in cellular signaling.Cell Signal 2012;24(5):981-90.
[31]Chaube SK,Shrivastav TG,Tiwari M,Prasad S,Tripathi A,Pandey AK.Neem(Azadirachta indica L.)leaf extract deteriorates oocyte quality by inducing ROS-mediated apoptosis in mammals. Springerplus 2014;3:464.
[32]Dall'Acqua S.Natural products as antimitotic agents.Curr Top Med Chem 2014;14(20):2272-85.
[33]Biersack B,Schobert R.Indole compounds against breast cancer:recent developments.Curr Drug Targets 2012;13(14): 1705-19.
[34]Negi AS,Gautam Y,Alam S,Chanda D,Luqman S,Sarkar J,et al. Natural antitubulin agents:importance of 3,4,5-trimethoxyphenyl fragment.Bioorg Med Chem 2015;23(3):373-89.
[35]Vindya NG,Sharma N,Yadav M,Ethiraj KR.Tubulins-the target for anticancer therapy.Curr Top Med Chem 2015;15(1): 73-82.
[36]Kanno S,Tomizawa A,Hiura T,Osanai Y,Shouji A,Ujibe M,et al.Inhibitory effects of naringenin on tumor growth in human cancer cell lines and sarcoma S-180-implanted mice.Biol Pharm Bull 2005;28(3):527-30.
[37]Marozien.e A,Nemeikait.e-ˇC.enien.e A,Vidˆzi¯unait.e R,ˇC.enas N. Correlation between mammalian cell cytotoxicity of flavonoids and the redox potential of phenoxyl radical/phenol couple.Acta Biochim Pol 2012;59(2):299-305.
[38]Russo P,Del Bufalo A,Cesario A.Flavonoids acting on DNA topoisomerases:recent advances and future perspectives in cancer therapy.Curr Med Chem 2012;19(31):5287-93.
[39]Hou DX,Kumamoto T.Flavonoids as protein kinase inhibitors for cancer chemoprevention:direct binding and molecular modeling. Antioxid Redox Signal 2010;13(5):691-719.
[40]Yen GC,Duh PD,Tsai HL,Huang SL.Pro-oxidative properties of flavonoids in human lymphocytes.Biosci Biotechnol Biochem 2003;67(6):1215-22.
[41]Wu CC,Bratton SB.Regulation of the intrinsic apoptosis pathway by reactive oxygen species.Antioxid Redox Signal 2013;19(6): 546-58.
[42]Halliwell B.Oxidative stress and cancer:have we moved forward?Biochem J 2007;401:1-11.
[43]Zhang C,Cao S,Toole BP,Xu Y.Cancer may be a pathway to cell survival under persistent hypoxia and elevated ROS:a model for solid-cancer initiation and early development.Int J Cancer 2015;136(9):2001-11.
[44]Ahamad MS,Siddiqui S,Jafri A,Ahmad S,Afzal M,Arshad M. Induction of apoptosis and antiproliferative activity of naringenin in human epidermoid carcinoma cell through ROS generation and cell cycle arrest.PLoS One 2014;9(10):e110003.
[45]L´opez-L´azaro M.Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy.Cancer Lett 2007;252(1):1-8.
[46]Pelicano H,Carney D,Huang P.ROS stress in cancer cells and therapeutic implications.Drug Resist Updat 2004;7(2):97-110.
[47]Doering M,Ba LA,Lilienthal N,Nicco C,Scherer C,Abbas M,et al.Synthesis and selective anticancer activity of organochalcogen based redox catalysts.J Med Chem 2010;53(19):6954-63.
[48]Cao G,Sofic E,Prior RL.Antioxidant and prooxidant behavior of flavonoids:structure-activity relationships.Free Radic Biol Med 1997;22(5):749-60.
[49]Heim KE,Tagliaferro AR,Bobilya DJ.Flavonoid antioxidants: chemistry,metabolism and structure-activity relationships.J Nutr Biochem 2002;13(10):572-84.
[50]Alam MA,Subhan N,Rahman MM,Uddin SJ,Reza HM,Sarker SD.Effect of citrus flavonoids,naringin and naringenin,on metabolic syndrome and their mechanisms of action.Adv Nutr 2014;5(4):404-17.
[51]Mir IA,Tiku AB.Chemopreventive and therapeutic potential of“naringenin,”a flavanone present in citrus fruits.Nutr Cancer 2015;67(1):27-42.
[52]Orhan IE,Nabavi SF,Daglia M,Tenore GC,Mansouri K,Nabavi SM.Naringenin and atherosclerosis:a review of literature. Curr Pharm Biotechnol 2015;16(3):245-51.
[53]Torricelli P,Ricci P,Provenzano B,Lentini A,Tabolacci C. Synergic effect ofα-tocopherol and naringenin in transglutaminaseinduced differentiation of human prostate cancer cells.Amino Acids 2011;41(5):1207-14.
[54]Xie CL,Pan YB,Hu LQ,Qian YN.Propofol attenuates hydrogenperoxide-induced apoptosis in human umbilical vein endothelial cells via multiple signaling pathways.Korean J Anesthesiol 2015;68(5):488-95.
[55]Ribble D,Goldstein NB,Norris DA,Shellman YG.A simple technique for quantifying apoptosis in 96-well plates.BMC Biotechnol 2005;5(1):12.
[56]Prokhorova EA,Zamaraev AV,Kopeina GS,Zhivotovsky B,Lavrik IN.Role of the nucleus in apoptosis:signaling and execution.Cell Mol Life Sci 2015;72(23):4593-612.
[57]Jiang QL,Zhang S,Tian M,Zhang SY,Xie T,Chen DY,et al. Plant lectins,from ancient sugar-binding proteins to emerging anticancer drugs in apoptosis and autophagy.Cell Prolif 2015;48(1): 17-28.
[58]Khan M,Maryam A,Qazi JI,Ma T.Targeting apoptosis and multiple signaling pathways with icariside II in cancer cells.Int J Biol Sci 2015;11(9):1100-12.
2 Dec 2015
AbdurrahimKocyigit,DepartmentofMedical Biochemistry,Faculty of Medicine,Bezmialem Vakif University,34093,Istanbul,Turkey.
Tel:+90 212 5232288
Fax:+90 212 6217578
E-mail:abdurrahimkocyigit@yahoo.com
Peer review under responsibility of Hainan Medical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Pacific Journal of Tropical Biomedicine2016年10期
Asian Pacific Journal of Tropical Biomedicine2016年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A review on promising phytochemical,nutritional and glycemic control studies on Moringa oleifera Lam.in tropical and sub-tropical regions
- A rare cause of acute abdomen-Spontaneous rectus sheath hematoma
- Evaluation of proline,chlorophyll,soluble sugar content and uptake of nutrients in the German chamomile(Matricaria chamomilla L.)under drought stress and organic fertilizer treatments
- ProductionofsecondarymetaboliteE2.2fromPhaleriamacrocarpaendophyticfungus
- Pandanusamaryllifoliusleafextractincreasesinsulinsensitivityinhigh-fatdiet-induced obese mice
- Jatropha curcas L∶Phytochemical,antimicrobial and larvicidal properties
