Jatropha curcas L∶Phytochemical,antimicrobial and larvicidal properties
Sillma Rampadarath,Daneshwar Puchooa*,Rajesh Jeewon
1Department of Agriculture and Food Science,Faculty of Agriculture,University of Mauritius,R´eduit,Mauritius
2Department of Health Sciences,Faculty of Science,University of Mauritius,R´eduit,Mauritius
Jatropha curcas L∶Phytochemical,antimicrobial and larvicidal properties
Sillma Rampadarath1,Daneshwar Puchooa1*,Rajesh Jeewon2
1Department of Agriculture and Food Science,Faculty of Agriculture,University of Mauritius,R´eduit,Mauritius
2Department of Health Sciences,Faculty of Science,University of Mauritius,R´eduit,Mauritius
ARTICLE INFO
Article history:
Jatropha curcas Antimicrobial Phytochemical Larvicidal
Objective:To evaluate antimicrobial activities as well as the phytochemical and lavicidal properties of different parts of Jatropha curcas L.(J.curcas)growing in Mauritius. Methods:Determination of the presence of phytochemicals in the crude plants extracts by test tube reactions.Disc diffusion method and microdilution method were used to detect the antimicrobial sensitivity and activity(minimal inhibitory concentration).The crude solvent extracts were also tested on the larvae of two insects,Bactrocera zonata and Bactrocera cucurbitae(Diptera,Tephritidae).
Results:The antimicrobial activities were significantly dependent for the different crude plant extracts on the thirteen microorganisms tested.For the Gram-positive bacteria,the crude ethyl acetate extract was more efficient compared to the Gram-negative bacteria with both solvents being effective.The crude ethyl acetate extract of J.curcas bark and mature seed oil showed the highest efficacy.The highest mortality percentage was observed after 24 h for both Diptera flies with(66.67±2.89)%of Bactrocera cucurbitae larvae killed by ethyl acetate extract of J.curcas bark.
Conclusions:This paper compared the different J.curcas plant sections with respect to the effectiveness of the plant as a potential candidate for new pharmaceuticals.The larvicidal effect was also studied in order to demonstrate the dual purpose of the plant.
Original articlehttp://dx.doi.org/10.1016/j.apjtb.2016.01.019
1.Introduction
Plants derivatives have made a large contribution to human health as they have been used as source of preliminary compound of drugs.Widespread usages of drugs have led to the development of pathogen resistance,hence,urging research of new drugs for the treatment of diseases.Active compound present in the medicinal plants provide the bountiful resource of active compounds for the pharmaceutical,cosmetics and food industries,and more recently in agriculture for pest control[1].
Antimicrobial agents are substances that kill microorganisms or inhibit growth of the microorganisms.They are widely employed to cure bacterial diseases.Antimicrobial agents disrupt microbial processes or structures that differ from those of the host.They may damage pathogens by hampering cell wall synthesis,inhibiting microbial protein and nucleic acid synthesis,disrupting microbial membrane structure and function,or blocking metabolic pathways through inhibition of key enzymes[2-4].
Jatropha curcas L.(J.curcas)plant originated from Mexico andwasspreadtoAsiaandAfricabyPortuguesetradersasahedge plant and it belongs to the family Euphorbiaceae.In many subtropicalandsemi-aridregions,traditionally,J.curcasisusedforits medicinal properties and its seeds contain semi-dry oil which has been found to be useful for medicinal purposes.It has played a major role in the treatment of various diseases including bacterial and fungal infections.The seeds and leaves extracts of J.curcas,have shown molluscidal and insecticidal properties[5,6].The extracts of many Jatropha species including J.curcas have shown to display potent cytotoxic,anti-tumour and antimicrobial activities in different assays.The latex of J.curcas have shown to possess antibacterial activity against Staphylococcus aureus(S.aureus)[6],however,the antimicrobial activity of the other parts have not been fully investigated[7].
The objectives of this study were to investigate the effectiveness of whole plant of J.curcas plant against some selected human pathogens which are known to cause diseases and to compare the extent of antimicrobial properties of the different plant sections of J.curcas,hence determining the most active part of the plant.Furthermore,to investigate the dual purpose of the plant extracts,the larvicidal effect tests were carried on two chosen Bactrocera species namely Bactrocera zonata(B.zonata)and Bactrocera cucurbitae(B.cucurbitae).
2.Materials and methods
2.1.Plant material collection and miscellaneous experimental material
Barks,roots and immature,mature and fully mature leaves,pericarps and seeds of J.curcas plant(Accession number MAU 26484)were collected in Mauritius for the study.The analytical grade extraction solvents,media,standard antibiotics(Oxoid®),microplates(Merck®),Whatman filter papers and sterile Petridishes from Sigma®chemicals were used for the different assays.
2.2.Plant and oil extraction methods
The plant crude extracts were obtained using the decoction method after the parts were thoroughly washed under running tap water,dried in shade.A total of 20 g of different plant parts were macerated in 40 mL of ethyl acetate(EA)and methanol(ME)moderately polar solvent systems for 48 h,respectively. The concentration process of the crude extracts were done after filtration on 15-18 mm pore size Whatman filter paper in a ventilated fume cupboard at room temperature and then stored at 4°C in dark bottles.The Soxhlet distillation steps were carried out for the seed oil extraction after the seeds were reduced to powder from the two different solvent systems and again stored at 4°C until further use.
2.3.Antibacterial and antifungal susceptibility test(disc diffusion method and microdilution method)
For the disc diffusion antimicrobial tests,the test pathogens(100μL/plate)were spread on Muller Hinton agar plates. Sterile paper discs of(6 mm diameter,0.09 mm thickness,5 discs/plate)were aseptically transferred on agar plates and were then soaked with equal volume 5μL of 0.2 g/mL(w/v)fixed concentration of crude plant extracts and seed oil.The zone of inhibition was measured(in mm diameter)after the plates were incubated at 37°C for 24-48 h.The pathogens were also tested with both a standard antibiotic tetracycline(30μg as positive control)and negative controls(solvents-EA and ME).The sterile discs were also solely tested on a sterile plate of Muller Hinton agar.
To determine the minimum inhibitory concentration(MIC)antibacterial activity of fractionated plant crude extracts and seed oil,the serial microdilution method was used.A total of 100μL of all tested pathogenic bacterial strains was diluted two-fold with 100μL sterile distilled water in a sterile 96-well microplate.EA and ME solvents were used as negative control and two-fold dilution of tetracycline(30μg/mL)was also used as positive control against each bacterium.After 24 h the MIC of the extracts inhibiting total bacterial growth was noted for the different microorganisms on the plates which were incubated at 37°C.Bacterial growth was assayed with the addition of 40μL of 0.2 mg/mL iodonitrotetrazolium violet to each well,and then incubated again at 37°C for 30 min.The experiment included negative controls the solvents ME and EA,and a positive control(tetracycline antibiotic).The plating for each microorganism was done in triplicate.
For the assays,sub-cultures of six Gram-positive[Bacillus algicola Acc.13/5(B.algicola),Bacillus cereus ATCC 11778(B.cereus),Listeria innocua ATCC 33090(L.innocua),S.aureus ATCC 29213,Staphylococcus epidermis ATCC 12228(S.epidermis),Viridibacillus arenosi LMG 22166(V.arenosi)],six Gram-negative[Escherichia coli ATCC 25922(E.coli ATCC 25922),Escherichia coli 0145:H28 Acc.No. CP006027.1(E.coli 0145:H28 Acc.No.CP006027.1),Klebsiella oxytoca ATCC 43086(K.oxytoca),Proteus mirabilis(P.mirabilis),PseudomonasaeruginosaATCC27853(P.aeruginosa),SalmonellatyphimuriumATCC14028(S.typhimurium)]and one fungus[Candida albicans ATCC 1023(C.albicans)]were done on sterile nutrient agar plates. After incubation of the bacterium strain in 10 mL of Mueller Hinton broth overnight at 37°C for 24-48 h,the cultures were standardised to an absorbance of 0.4-0.6 at 600 nm.
2.4.Larvae mortality assays The experiment was set in a completely randomised manner with 3 replicates of 20 larvae per treatment(food with extracts)and controls(food without extracts,and the two solvents)in
sterile Petri-dishes(9-cm diameter)and number of dead larvae per Petri-dishes were recorded after 24,48 and 72 h incubation period.Three-day-old larvae of B.cucurbitae and B.zonata were provided by the Entomology Division of the Ministry of Agro-Industry and Food Security.The crude extracts from different plant parts of ME and EA were tested for their effect on the larvae by spraying the natural food formulation with three different concentrations[200,400 and 800 mg/L(w/v)of plant crude and oil extracts].
2.5.Qualitative screening of the phytochemicals
The screening of secondary metabolites such as flavonoids,alkaloids,saponins,steroids,tannins,coumarins and phenols based on a series of test tube tests.
2.6.Experimentation statistical analysis
Minitab®17.2.1,NCSS®10.0 and Microsoft Excel 2010 were used for the statistical,tables and graphs analyses.The antimicrobial assays data were expressed as mean±SD with One/Two-way ANOVA at 5%and least significant difference test compared the differences between the means.For the mortality,log-probit was used to calculate LC50-lethal concentrations that kill 50%of the treated larvae andχ2test to compare the number of death recorded between the different extracts at the three tested concentrations.
3.Results
3.1.Antifungal activity of crude solvent extracts of different parts of local J.curcas plant
The different parts of J.curcas plant crude solvent extracts inhibited significantly(P<0.05)the growth of C.albicans with varying degrees of effectiveness as compared to the control with zone of inhibition values ranging from(8.40±0.55)to(12.60±1.52)mm.The results also showed that the crude EA extracts of immature and mature leaves prevented the growth of the fungus and its effect was only moderate.The following zone of inhibition results were observed for the both the crude EA mature seed oil[(10.60±1.14)mm]and ME immature[(11.20±0.84)mm]and mature[(10.60±0.89)mm]leaves,root ME[(10.20±1.10)mm]and immature pericarps ME[(11.20±1.64)mm]extracts.The remaining extracts also reduced the fungus growth with zone of inhibition less than 10 mm(Figure 1).
3.2.Antibacterial effect of crude solvent extracts of the different parts of the local J.curcas plant
Twelve bacterial stains were used to screen the possible antimicrobial activities of the different parts of J.curcas plant crude solvent extracts.The intensity of antimicrobial activity depends on the plant extract,and tested microorganisms.It was observed that tested plants exhibited better antibacterial activities than antifungal ones as higher zones of inhibition were observed for the different extracts of J.curcas.The antibacterial activity of J.curcas extracts was statistically highly significant(P<0.01)and the solvent extracts inhibited the growth of both Gram-positive and the Gram-negative bacteria(Figure 2).
The crude EA extracts proved to be a better solvent for the Gram-positive bacteria while for Gram-negative bacteria both solvents were effective.The most significant extracts were the crude EA of J.curcas bark and mature seed oil against S.aureus,B.algicola and E.coli 0145:H28 Acc.No. CP006027.1 with zone of inhibition of(23.40±2.19),(20.40±0.55)and(17.60±0.55)mm,respectively.Grampositive bacteria B.algicola,B.cereus,L.innocua and S.aureus were also susceptible to the following crude EA extracts of the immature and mature leaves,immature and mature seed oil and root with moderate inhibition zones lying between 13.00 and 14.60 mm.Methanol crude extracts of fully mature leaves and seed oil,immature leaves,seeds oil and pericarps,mature leaves pericarps,bark and root had a moderate antibacterial effect on Gram-negative bacteria E.coli ATCC 25922,E.coli0145:H28Acc.No.CP006027.1,K.oxytoca,P.mirabilis,P.aeruginosa,and S.typhimurium with zone of inhibition values ranging from 12.00 mm to 14.60 mm(Figure 2).
3.3.MIC assays
The antibacterial activity of the different parts of J.curcas was determined against both Gram-negative and Gram-positive bacteria and a fungus.The summarized results showed that the tested extracts displayed significant(P<0.05)selective antibacterial activities(Tables 1 and 2).Both ME and EA extractsinhibited the growth of the microorganisms.The MIC values for the Gram-positive and Gram-negative bacteria were between 3.75μg/mL and 100.00μg/mL and C.albicans between 17.80μg/mL and 83.30μg/mL.The very high values for several extracts indicated limited antibacterial efficacy.
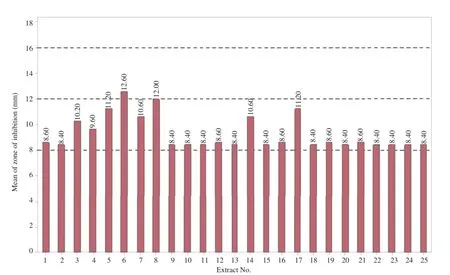
Figure 1.Antifungal effect of J.curcas extracts on C.albicans.
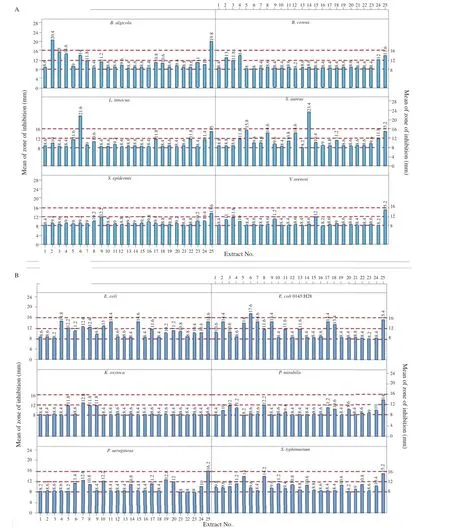
Figure 2.Antibacterial effect of J.curcas crude solvent extracts against both Gram-positive(A)and Gram-negative(B)bacteria.
The best activity was recorded with crude solvent extracts with the immature parts of J.curcas plants with MIC values ranging from 4.17μg/mL to 21.50μg/mL against 77%tested bacteria.MIC values below or equal to 10μg/mL were also recorded for several extracts against B.algicola ATCC No.13/5,S.aureus,E.coli ATCC 25922,E.coli 0145:H28 Acc.No. CP006027.1,K.oxytoca and S.typhimurium.The lowest MIC value was(3.75±0.00)μg/mL observed with both crude EA and ME of root,mature seed oil and leaves extracts against B.algicola,S.aureus,E.coli 0145:H28 Acc.No.CP006027.1 and E.coli ATCC 25922.The ME extracts exhibited stronger and broader spectrum of action compared to EA extracts and negative and positive controls.The Gram-negative strains were the most sensitive with MIC values up to 100.00μg/mL(Tables 1 and 2).
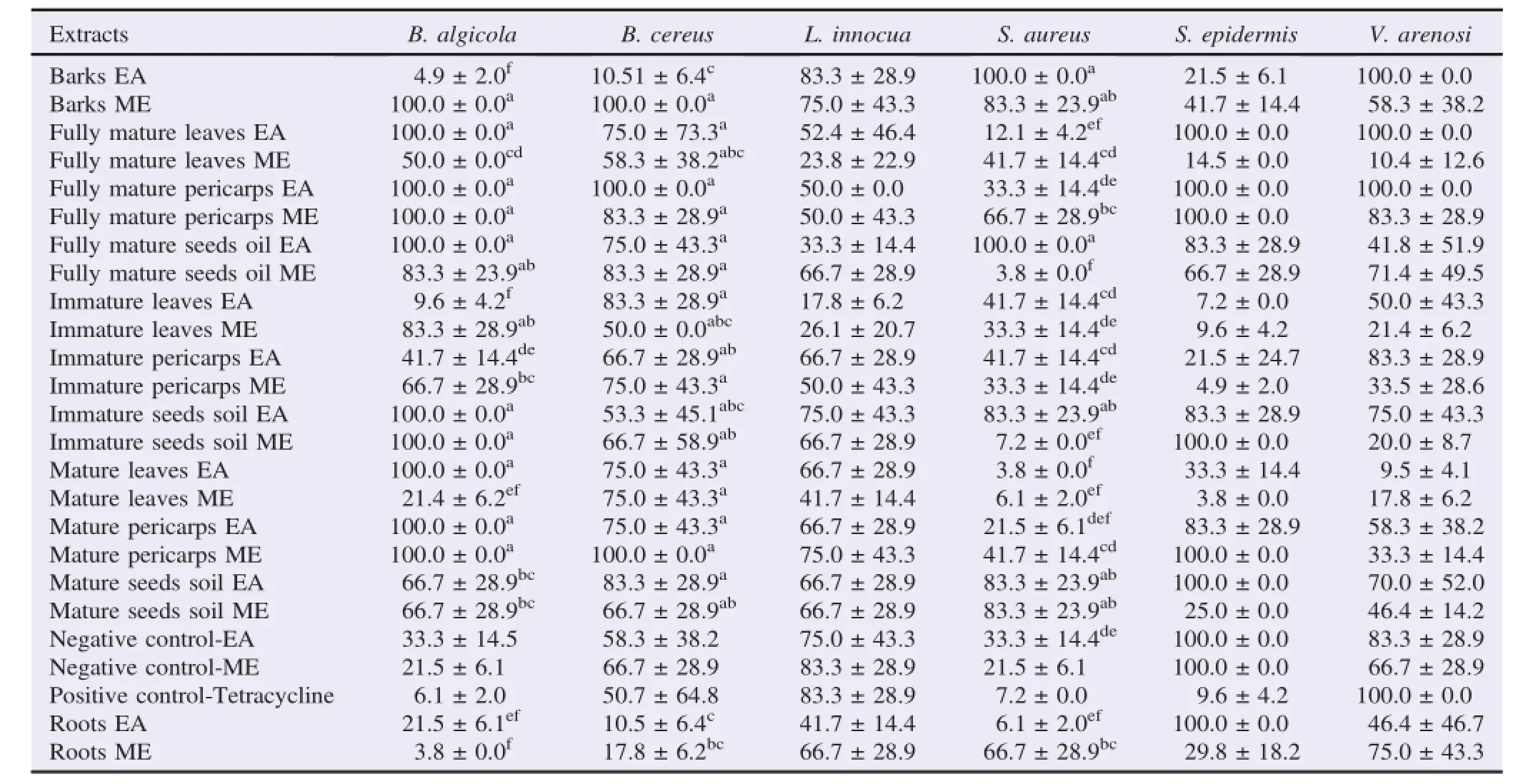
Table 1 Mean MIC of the aerial and roots parts of J.curcas L.plants crude and oil solvent extracts for the Gram-positive strains.μg/mL.
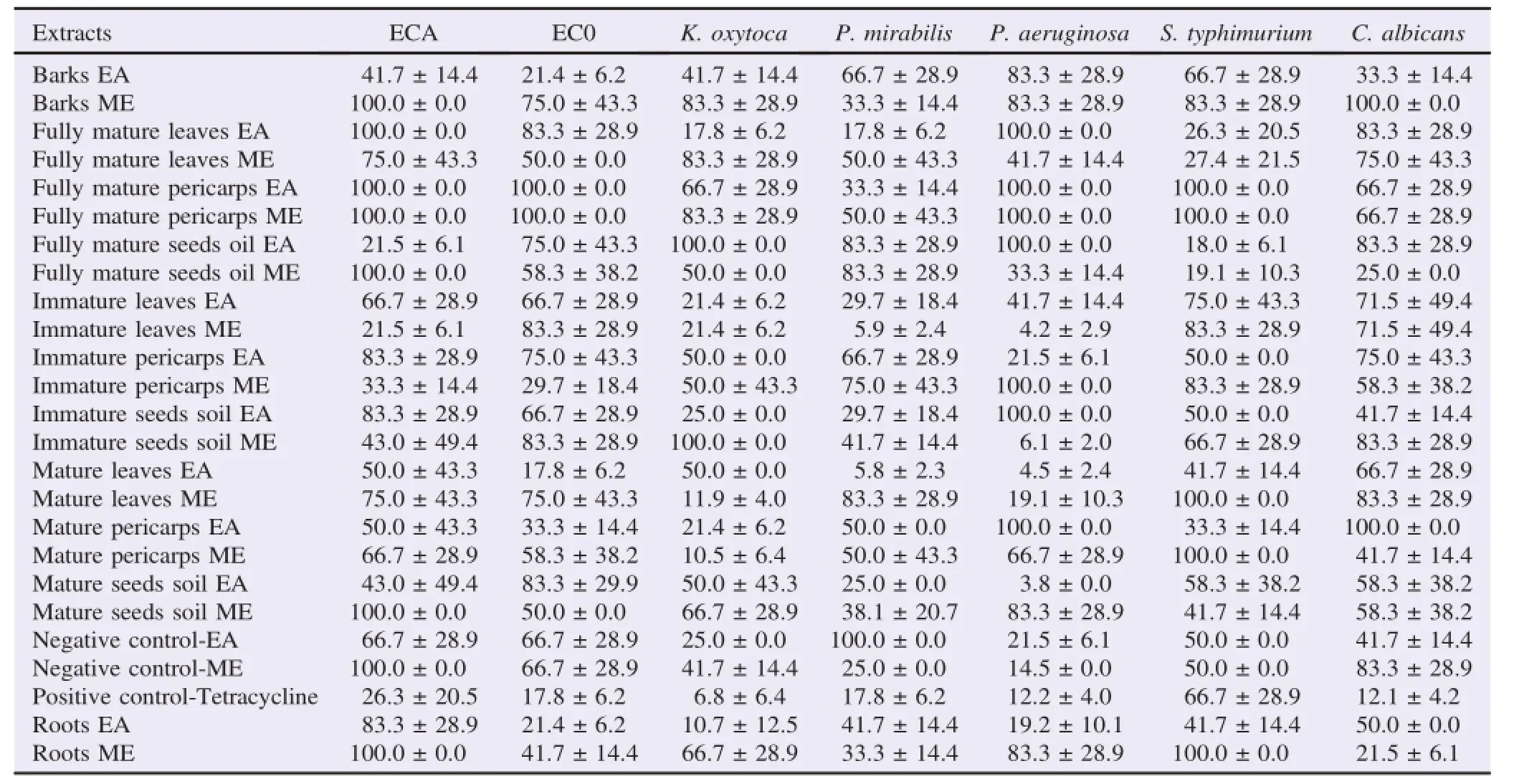
Table 2 Mean MIC of the aerial and roots parts of J.curcas L.crude and oil solvent extracts for the Gram-negative strains and one fungus.μg/mL.
3.4.Mortality bioassays
Twenty-two extracts from the different parts of J.curcas wereevaluatedfortheirlarvicidalpotenciesagainst B.cucurbitae(melon fruit fly larvae)and B.zonata(peach fly larvae).The mortality(LD50)bioassays for two Diptera species,were significant(P<0.05)for the crude solvent extracts of J.curcas plants.The calculated LD50values for B.cucurbitae and B.zonata larvae were between(290±5)and(1770±20)mg/L,and(180±5)and(3290±100)mg/L,respectively(Table 3).The lowest LD50determined was for crude ME fully mature leaves crude ME extract and the highest was for the bark ME extract.
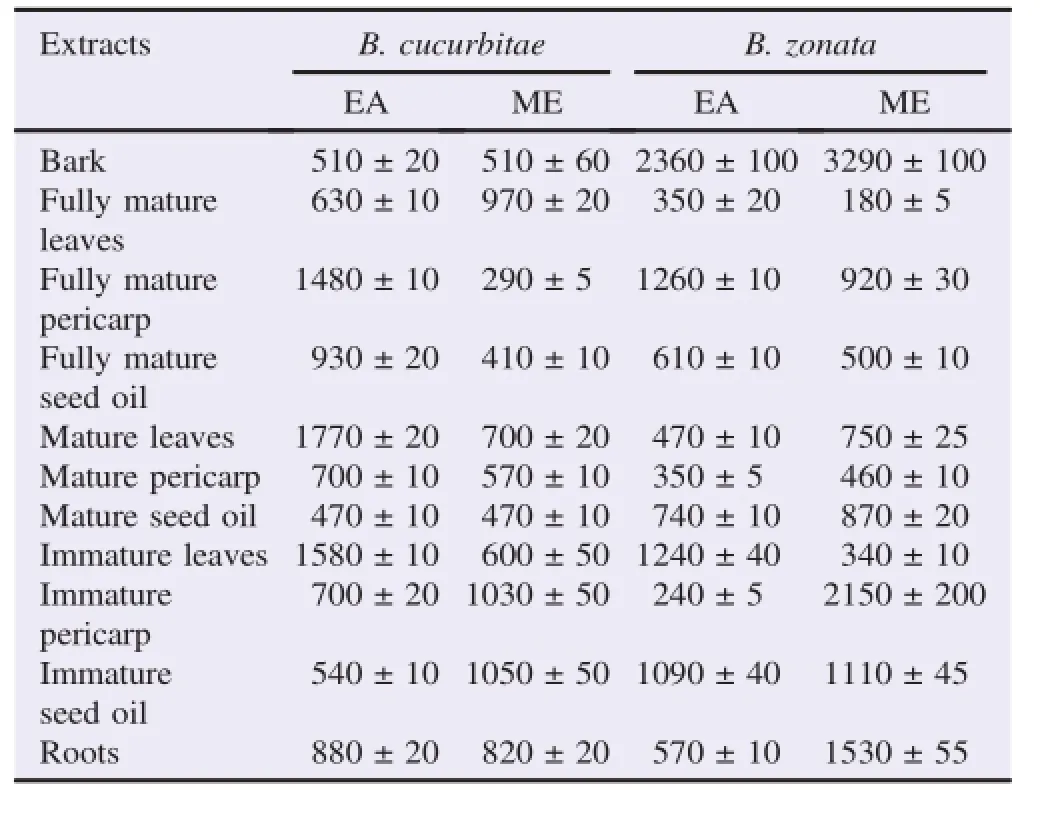
Table 3 LC50of J.curcas crude solvent extracts against Diptera fruit flies.mg/L.
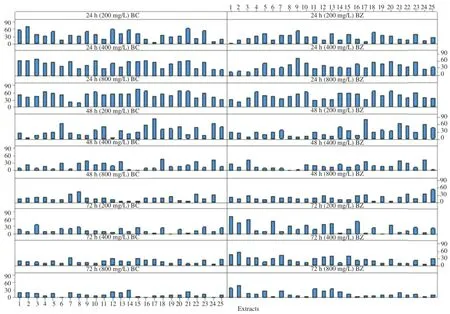
Figure 3.Anti-insecticidal activity of crude solvent extracts of different J.curcas parts on larvae of B.cucurbitae and B.zonata at the concentration of 200,400 and 800 mg/L for 24 h,48 h and 72 h.
The tested solvent plant extracts exerted promising significant larvicidal activity(P<0.05)after 24 h,48 h and 72 h(Figure 3)periods of the different concentration(200,400 and 800 mg/L)crude extracts exposure.The highest mortalitypercentage was observed for the most of the crude solvents extracts after 24 h for both Diptera flies.After 24 h,the highest percentage of larvae killed(66.67±2.89)%was obtained for B.cucurbitae and EA bark extract and similarly(70.00±8.66)% for B.zonata and ME fully mature leaves extract,and(75.00±5.00)%for B.cucurbitae and ME fully mature seed oil extract at 200,400 and 800 mg/L concentration.
After 72 h,crude EA of bark and immature pericarp extracts,the cumulative percentage of killed larvae observed was 96.97% at 200 mg/L,100%for crude EA of mature pericarp extract at 400 mg/L and 93.33%and 95.00%for EA of mature seed oil and ME of fully mature pericarp extracts at 800 mg/L for B.cucurbitae and B.zonata respectively.The cumulative lowest mortality was 66.67%for EA immature and seed oil extracts.
3.5.Preliminary phytochemical test for J.curcas
The main phytochemicals constituents detected from the different parts of J.curcas plants were alkaloid,steroids,tannins,flavonoids,phenol and coumarins(Table 4).All the six secondary metabolites were present in mature pericarp ME of J.curcas.Twelve crude solvent extracts:bark EA,roots EA,immature leaves EA,mature leaves ME,fully mature leaves ME,fully mature leaves EA,mature seed oil ME,mature seed oil EA,fully mature seed oil EA,immature pericarp ME,mature pericarp EA,fully mature pericarp ME had five of the metabolites.Ethyl acetate solvent was better than ME in extracting most of the tested secondary metabolites.Only alkaloids and coumarins were noted in mature leaves EA extract. as a medicine[8].Numerous biologically active substances have been isolated and characterised from all parts of the Jatropha plant.Their action mechanisms have been studied in associate to a great number of applications of J.curcas in traditional medicines.However,the different parts of the whole J.curcas plant have been for the first time reported as a promising candidate for their effects as potential antimicrobial and larvicidal agents.
Furthermore,solvents used for extraction played an important role in the extraction of the phytochemicals.The plant section that exhibited the best antimicrobial properties was those extracted using EA extracts from the bark and seed oil.This could be due to the higher presence of biologically active metabolites in the EA extracts than the ME[9].This is in agreement with the findings of Srinivasan et al.[8]who reported that different solvents have different extraction capacities and different spectrum of solubility for the phyto-constituents which are known to be biologically active.
The susceptibility of several fungi to J.curcas extracts was found significant,but in this study the antibacterial properties of the plant extracts were better than the antifungal activity.These secondary metabolites exert antibacterial and antifungal activity through different mechanisms.The production of secondary metabolites by plants is dependent on environmental conditions and the secondary plant metabolites are affected by gene level and the genetic diversity of plant metabolites.Therefore,this could be a few reasons explaining the difference of previous and present reported results of the antibacterial/antifungal activities.
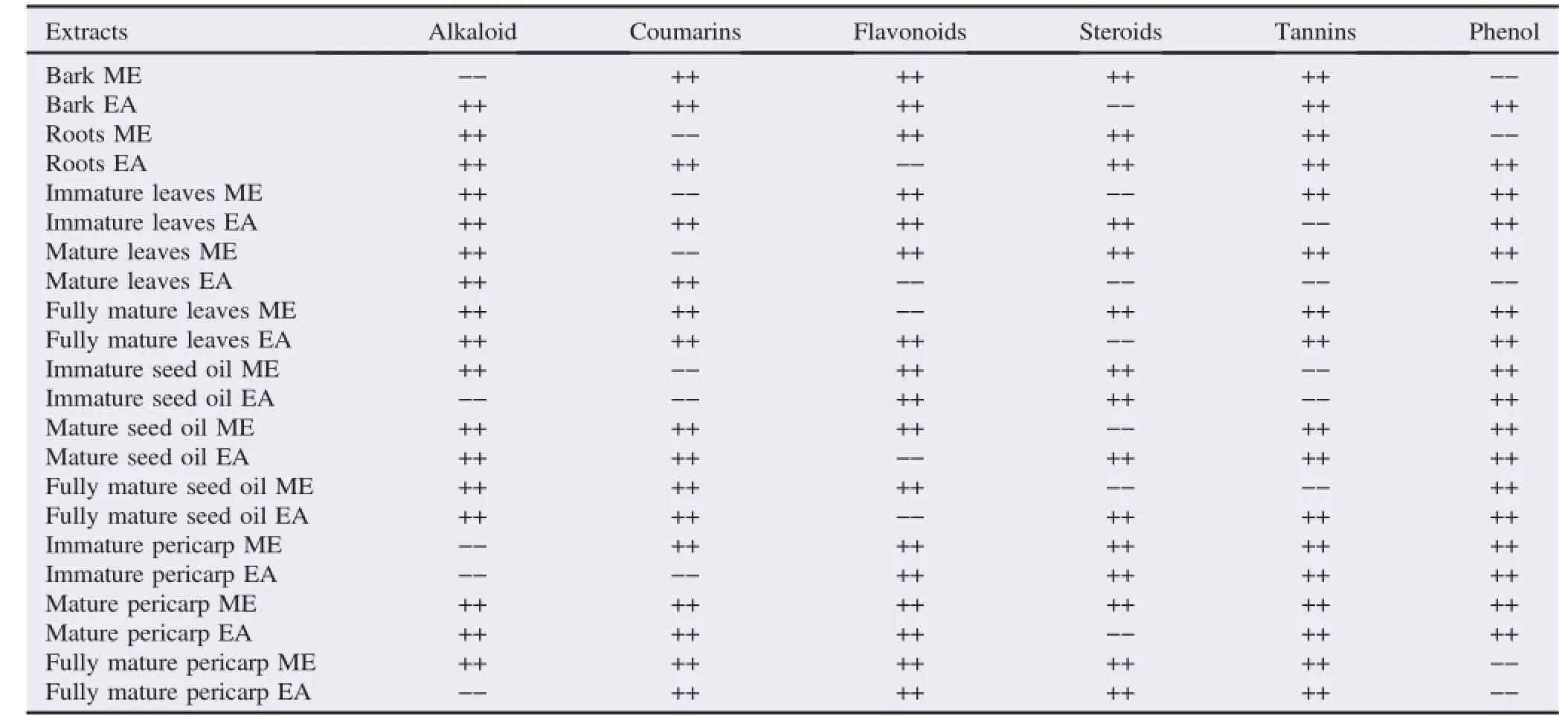
Table 4 Phytochemical constituents of J.curcas extracts.
4.Discussion
J.curcas is a versatile plant having potential uses in the medicinal field.These medicinal uses of different plant parts had been intensively investigated and studied by several researchers.The plant contains mixtures of different chemical compounds that may act individually,additively or in synergy
Anothersecondarymetabolitecompoundobservedin J.curcas was alkaloid,widely present in the plant.One of the most common biological properties of alkaloids is their toxicity against cells of foreign organisms[9].Alkaloids which are one of the largest groups of phytochemicals in plants have amazing effects,hence explaining the effectiveness of J.curcas on both Gram-negative and Gram-positive bacteria.The absence ofalkaloids in J.curcas leaf extracts had been reported by Kubmarawa et al.[10],whereas this work report the presence of alkaloids in the three different stages of the leaves,irrespective of solvents used for extraction.Different parts of J.curcas contain the toxic alkaloids curcin and phorbol ester which prevent animals from feeding on it.Hence,the presence of these compounds in J.curcas corroborates with both the antimicrobial activities and larvicidal effect observed.
The inhibitory effect of the extracts of J.curcas against pathogenic bacterial strains and larval development can promote the plant as a potential candidate for the treatment of ailments caused by these pathogens.The role of J.curcas in medicinal uses should be taken into consideration as it shows promising future in the pharmaceutical field.Commercializing on the medicinal product derived from J.curcas may turn out to be more profitable than using Jatropha as fuel substitution.The economics of making and marketing for these products should be further explored and encouraged.
Conflict of interest statement
We declare that we have no conflict of interest.
[1]Rachana S,Tarun A,Rinki R,Neha A,Meghna R.Comparative analysis of antibacterial activity of Jatropha curcas fruit parts. J Pharm Biomed Sci 2012;15(12):1-4.
[2]Namuli A,Abdullah N,Sieo CC,Zuhainis SW,Oskoueian E. Phytochemical compounds and antibacterial activity of Jatropha curcas Linn.extracts.J Med Plants Res 2011;5(16): 3982-90.
[3]Dada EO,Ekundayo FO,Makanjuola OO.Antibacterial activities of Jatropha curcas(Linn)on coliforms isolated from surface waters in Akure,Nigeria.Int J Biomed Sci 2014;10(1): 25-30.
[4]Nyembo K,Kikakedimau N,Mutambel H,Mbaya N,Ekalakala T,Bulubulu O.In vitro antibacterial activity and phytochemical screening of crude extracts from Jatropha curcas Linn.Eur J Med Plants 2012;2(3):242-51.
[5]Omoregie EH,Folashade KO.Broad spectrum antimicrobial activity of extracts of Jatropha curcas.J Appl Pharm Sci 2013;3(4): 083-7.
[6]Afzal M,Kazmi I,Khan R,Singh R,Chauhan M,Bisht T,et al. Bryophyllum pinnatum:a review.Int J Res Biol Sci 2012;2(4): 143-9.
[7]Sharma A,Saxena S,Rani U,Rajore S,Batra A.Broadspectrumantimicrobialpropertiesofmedicinallyimportant plant Jatropha curcas L.Int J Pharm Sci Rev Res 2010;4(3): 11-4.
[8]Srinivasan D,Nathan S,Sures T,Lakshmana Perumalsamy P. Antimicrobial activity of certain Indian medicinal plants used in folkloric medicine.J Ethnopharmacol 2001;74:217-20.
[9]Warra AA.Cosmetic potentials of physic nut(Jatropha curcas Linn.)seed oil:a review.Am J Sci Ind Res 2012;3(6): 358-66.
[10]Kubmarawa D,Ajoku GA,Enwerem NM,Okorie DA.Preliminary phytochemical and antimicrobial screening of 50 medicinal plants from Nigeria.Afr J Biotechnol 2007;6(14): 1690-6.
23 Nov 2015
Daneshwar Puchooa,Department of Agriculture and Food Science,Faculty of Agriculture,University of Mauritius,R´eduit,Mauritius.
Tel:+230 4541041
Fax:+230 4655743
E-mail:sudeshp@uom.mu
Peer review under responsibility of Hainan Medical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
Received in revised form 6 Jan 2016 Accepted 25 Jan 2016
Available online 26 Aug 2016
 Asian Pacific Journal of Tropical Biomedicine2016年10期
Asian Pacific Journal of Tropical Biomedicine2016年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A review on promising phytochemical,nutritional and glycemic control studies on Moringa oleifera Lam.in tropical and sub-tropical regions
- A rare cause of acute abdomen-Spontaneous rectus sheath hematoma
- Evaluation of proline,chlorophyll,soluble sugar content and uptake of nutrients in the German chamomile(Matricaria chamomilla L.)under drought stress and organic fertilizer treatments
- ProductionofsecondarymetaboliteE2.2fromPhaleriamacrocarpaendophyticfungus
- Cytotoxic,genotoxic and apoptotic effects of naringenin-oxime relative to naringenin on normal and cancer cell lines
- Pandanusamaryllifoliusleafextractincreasesinsulinsensitivityinhigh-fatdiet-induced obese mice
