Pandanusamaryllifoliusleafextractincreasesinsulinsensitivityinhigh-fatdiet-induced obese mice
Suphaket Saenthaweesuk,Jarinyaporn Naowaboot,Nuntiya Somparn
1Division of Anatomy,Department of Preclinical Science,Faculty of Medicine,Thammasat University(Rangsit Campus),Pathum Thani 12120,Thailand
2Division of Pharmacology,Department of Preclinical Science,Faculty of Medicine,Thammasat University(Rangsit Campus),Pathum Thani 12120,Thailand
Pandanusamaryllifoliusleafextractincreasesinsulinsensitivityinhigh-fatdiet-induced obese mice
Suphaket Saenthaweesuk1,Jarinyaporn Naowaboot2*,Nuntiya Somparn2
1Division of Anatomy,Department of Preclinical Science,Faculty of Medicine,Thammasat University(Rangsit Campus),Pathum Thani 12120,Thailand
2Division of Pharmacology,Department of Preclinical Science,Faculty of Medicine,Thammasat University(Rangsit Campus),Pathum Thani 12120,Thailand
ARTICLE INFO
Article history:
Pandanus amaryllifolius Obesity
Insulin sensitivity
Fatty liver
Glucose transporter 4
Objective:To examine the effect of Pandanus amaryllifolius(P.amaryllifolius)leaf extract on the insulin resistance state in obese ICR mice.
Methods:Obesitywasinducedin micefedwith high-fat diet(45%fat)for12weeks.After the first six weeks on the diet,the obese mice were administered with the water extract of P.amaryllifolius leaf at 125 and 250 mg/kg/day,respectively for another six weeks.At the 5th week of treatment,oral glucose tolerance test was conducted.After six weeks of treatment,the levels of blood glucose,serum insulin,leptin,adiponectin,and lipid profiles were determined.The liver,muscle and epididymal fat tissues were removed for measuring the biochemical parameters and protein expression,as well as histological examination.
Results:Six weeks of treatment with P.amaryllifolius led to a significant reduction in the blood glucose level as well as improvement in the insulin resistance.P.amaryllifolius also increased the liver glycogen storage and serum adiponectin and decreased the serum leptin levels.A reduction in the serum and hepatic triglyceride,and non-esterified fatty acid levels was also observed.The histological examination showed that the obese mice treated with P.amaryllifolius reduced the lipid droplet in liver tissue and adipocyte size in epididymal fat tissue.The treatment also increased the protein expression of glucose transporter 4 in the muscle and fat tissues.
Conclusions:The treatment with P.amaryllifolius could decrease several parameters of impairedglucoseandlipidmetabolism.Tothebestofourknowledge,thisisthefirstreporton the role ofP.amaryllifolius leafextract in alleviating the insulin dysfunction in obesity state.
Original articlehttp://dx.doi.org/10.1016/j.apjtb.2016.08.010
1.Introduction
Obesity is one of the major risk factors for diabetes,hypertension,dyslipidemia,and atherosclerosis[1,2].The development of obesity is the etiology for inducing the status of insulin resistance,which is a primary risk factor for type 2 diabetes mellitus.The impaired insulin action in obesity can inhibit glucose output from the liver and glucose uptake into the fat and muscle cells[3].
Excessive lipid accumulation in the nonadipose tissues,especially in the liver,can lead to the development of fatty liver and insulin resistance.Fatty liver is a reversible condition that is marked by increased accumulation of triglyceride(TG)in the hepatocytes.The liver and adipose tissues have been found to remain dysfunctional in obesity condition[4-6].Adipose tissue playsanimportantroleintheregulationofenergy homeostasis and insulin resistance development.Long-term high-fat diet(HFD)feeding can cause adipocyte hypertrophy and its dysfunction,which is characterized by an excessive release of free fatty acids into the circulation[7].Adipose tissue produces adipokine,including adiponectin,which is responsiblefor controlling glucose and lipid metabolism.Studies on rodents and human subjects have shown that an increased plasma adiponectin level can improve the insulin resistance[8-12]. Moreover,theglucoseuptakeandinsulinsensitivityin peripheral tissues are involved in the stimulation of glucose transporter 4(GLUT4)signaling cascade[13-17].
Pandanus amaryllifolius(P.amaryllifolius)is a tropical plant found in India,South China,and Southeast Asia including Thailand.It exhibits several bioactivities such as antiviral[18],antioxidant[19],and antihyperglycemic activities[20].However,the effects of P.amaryllifolius leaf extract on the obesityinduced insulin resistant state have not been clearly demonstrated.Therefore,given the increasing incidence of obesity,the aim of this study was to investigate the effect of P.amaryllifolius leaf extract on impaired insulin sensitivity in HFD-fed mice.
2.Materials and methods
2.1.Plant extraction
The leaves of P.amaryllifolius were collected from Buriram,Thailand between July and September 2013.A voucher specimen(SKP 138 16 01 01)was deposited at the Faculty of Pharmaceutical Sciences,Prince of Songkla University,Thailand.The driedleaves(100g)wereextractedthreetimeswithdistilledwater(1 L)at 100°C for 30 min.This extract was concentrated and subsequently freeze-dried.By using this procedure,the yield obtained was 10.11%of the starting dry weight of the leaves.The P.amaryllifolius extract obtained during this procedure was kept at-20°C until further use.
2.2.Animals and induction of obesity
All experimental procedures involving animals were conducted in accordance with the standards of Association for Assessment and Accreditation of Laboratory Animal Care(Frederick,MD,USA)and approved by the Animal Ethics Committee,FacultyofMedicine,ThammasatUniversity,Thailand(Rec.No.AE 010/2014).Male ICR mice weighing 20-25 g were purchased from the National Laboratory Animal Center of Mahidol University,Nakhon Pathom,Thailand.They were maintained in an air-conditioned room[(25±2)°C]with a 12 h light:12 h dark cycle and fed with a low-fat diet and water ad libitum for a week.After random selection,the mice were fed with low-fat diet containing 10%fat(D12450H,Research Diets,New Brunswick,NJ,USA)with a total energy of 3.85 kcal/g or HFD containing 45%fat(D12451,Research Diets,New Brunswick,NJ,USA)with a total energy of 4.73 kcal/g for 12 weeks.
2.3.Treatment design
After the completion of the first sixweeks on diets,the animals weredividedintofourgroupswitheightmiceineachgroup.Group 1 consisted of normal control mice treated with 5%gum arabic;Group2comprisedobesecontrolmicetreatedwith5%gumarabic;and Groups 3 and 4 consisted of obese mice treated with 125 and 250 mg/kg of P.amaryllifolius extracts,respectively.The doses selection was based on our preliminary study,and the high blood glucose was reduced in the obese mouse model after one-week treatment with P.amaryllifolius extracts.The P.amaryllifolius extracts were dissolved in 5%gum arabic.The body weight and food intake of the mice were measured every week.After five weeks of treatment,the mice were fasted for 6 h and orally administered with glucose(2 g/kg)for oral glucose tolerance test(OGTT).The blood glucose levels were determined from the tail veinblood,beforeandaftertheglucoseloadingatregularintervals of 20,60,and 120 min.
2.4.Blood and tissue collection
After six weeks of treatment,the mice were fasted for 6 h and sacrificed with isoflurane anesthesia.Whole blood samples were collected from the heart for determining the blood glucose level,and the remaining blood samples were centrifuged at 3000 r/min for 10 min at 4°C.Subsequently,the serum was collected for measuring the lipid profiles,insulin,leptin,and adiponectin levels.The liver,gastrocnemius muscle,and epididymal fat tissues were then removed for the determination of biochemical parameters,protein expression,and histological examination.
2.5.Determination of serum insulin,leptin,and adiponectin levels
After six weeks of treatment,the fasting serum insulin,leptin,and adiponectin concentrations were measured using the ELISA kit(EMD Millipore,Billerica,MA,USA).
2.6.Determination of serum and liver lipid profiles
After six weeks of the treatment with P.amaryllifolius extracts,the serum total cholesterol(TC),TG and non-esterified fatty acid(NEFA),and liver TG levels were measured using the enzymatic colorimetric kit(Wako,Osaka,Japan).The protocols for liver TG and NEFA extractions were used as described previously[21].
2.7.Determination of hepatic glycogen synthesis
The glycogen content was determined as described previously[21].Briefly,the liver was homogenized in 30%KOH solution.The homogenized liver was then dissolved in a boiling water-bath(100°C)for 30 min and precipitated with 95%ethanol.The pellet was suspended in distilled water,mixed with 0.2%anthrone reagent,and absorbance was measured at 620 nm.
2.8.Western blot
Plasma membrane proteins of skeletal muscle and epididymal adipose tissues were homogenized and extracted in TPER®mixed with Halt®protease inhibitor cocktail(Thermo Scientific,IL,USA).The plasma membrane GLUT4 protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes.The membranes were blocked and incubated with anti-GLUT4 and antiactin primary antibodies(dilution 1:200,Santa Cruz Biotechnology,Dallas,TX,USA).The membranes were incubated with horseradish peroxidase-conjugated secondary antibody(dilution 1:2000,Santa Cruz Biotechnology,Dallas,TX,USA),and immunoreactive bands were developed in Clarity™Western ECL substrate(Bio-Rad,CA,USA).Thebandintensitieswereanalyzedby densitometry using the Odyssey®Fc imaging system(LI-COR Bioscience,Lincoln,NE,USA).
2.9.Histological examination
Liver and epididymal fat tissues were fixed with 10% formalin and embedded in paraffin.The paraffin sections(3μm)were cut,mounted on glass slides and stained with hematoxylin and eosin.The area of adipocyte was calculated using an ImageJ software program(National Institute of Health,Bethesda,MD,USA).
2.10.Statistical analyses
Alldatawereexpressedasmean±SEM,andstatisticalanalysis was performed using One-way ANOVA,followed by Tukey's posthoctest.AvalueofP<0.05wasconsideredtobestatistically significant.The statistical analyses were performed using computer-based software SigmaStat(Systat Software,CA,USA).
3.Results
3.1.Glucose and lipid metabolic parameters
After six weeks of treatment,the body weight of obese control mice was found to be significantly higher than that of normal control mice(Figure 1A)(P<0.05).The body weight of obese mice treated with P.amaryllifolius extract at 125 and 250 mg/kg,respectively also followed an increasing trend.No significant difference in food intake was observed among the groups(Figure 1B).The energy intake in obese mice treated with 250 mg/kg P.amaryllifolius was significantly increased as compared to normal control mice(Figure 1C).However,the epididymal fat weight in all obese groups was significantly increased as compared to normal control group(Figure 1D)(P<0.05).No reduction in the fat weight was observed in obese mice treated with P.amaryllifolius extract.Interestingly,their adipocyte size was significantly smaller as compared to obese control mice(Figure 1E,F)(P<0.05).
After six weeks of treatment,the fasting blood glucose level of obesecontrolmicewasfoundtobesignificantlyhigherthanthatin normal control mice(Figure 2A)(P<0.05).Comparatively,a significant reduction in fasting blood glucose was observed in obese mice treated with P.amaryllifolius extracts at 125 and 250 mg/kg,respectively(P<0.05).Obese control mice showed a high serum insulin level as compared to normal control mice(Figure 2B),whereas a significant reduction in insulin level was notedinP.amaryllifolius-treatedobesemice(P<0.05).Asshown in Figure 2C,the P.amaryllifolius-treated obese mice showed increased adiponectin levels as compared to obese control mice. The serum leptin level of obese control mice was also found to be significantly higher than that of normal control mice(Figure 2D)(P<0.05)while obese mice treated with P.amaryllifolius at 125 and250mg/kgshowedasignificantreductionintheleptinlevel as compared to obese control mice(P<0.05).In comparison to normal control mice,the OGTT for obese control mice showed significantly higher levels of blood glucose in the fasting state and during120minaftertheglucoseloading(Figure2E)(P<0.05).In the case of P.amaryllifolius-treated obese mice,a significant reduction in the high blood glucose was observed at 60 and 120 min after glucose loading as compared to obese control mice(P<0.05).In addition,obese control mice showed a decreased liver glycogen content as compared to normal control mice;however,the treatment with P.amaryllifolius at 250 mg/kg restored this reduction(Figure 2F).
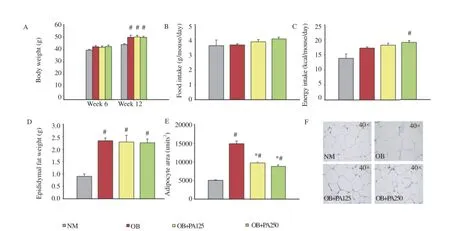
Figure 1.Effect of P.amaryllifolius on body weight(A),food intake(B),energy intake(C),epididymal fat weight(D),adipocyte size(E),and histology of epididymal fat tissues(F)in HFD-induced obese mice.Data are presented as mean±SEM(n=8).#:P<0.05 when compared to normal control mice.*:P<0.05 when compared to obese control mice.NM: Normal control mice;OB:Obese control mice;OB+PA125:Obese mice treated with P.amaryllifolius at 125 mg/kg;OB+PA250:Obese mice treated with P.amaryllifolius at 250 mg/kg.

Figure 2.Effect of P.amaryllifolius on fasting blood glucose(A),serum insulin(B),serum adiponectin(C),serum leptin(D),blood glucose in OGTT(E),and liver glycogen(F)in HFD-induced obese mice.Data are presented as mean±SEM(n=8).#:P<0.05 when compared to normal control mice.*:P<0.05 when compared to obese control mice.NM: Normal control mice;OB:Obese control mice;OB+PA125:Obese mice treated with P.amaryllifolius at 125 mg/kg;OB+PA250:Obese mice treated with P.amaryllifolius at 250 mg/kg.
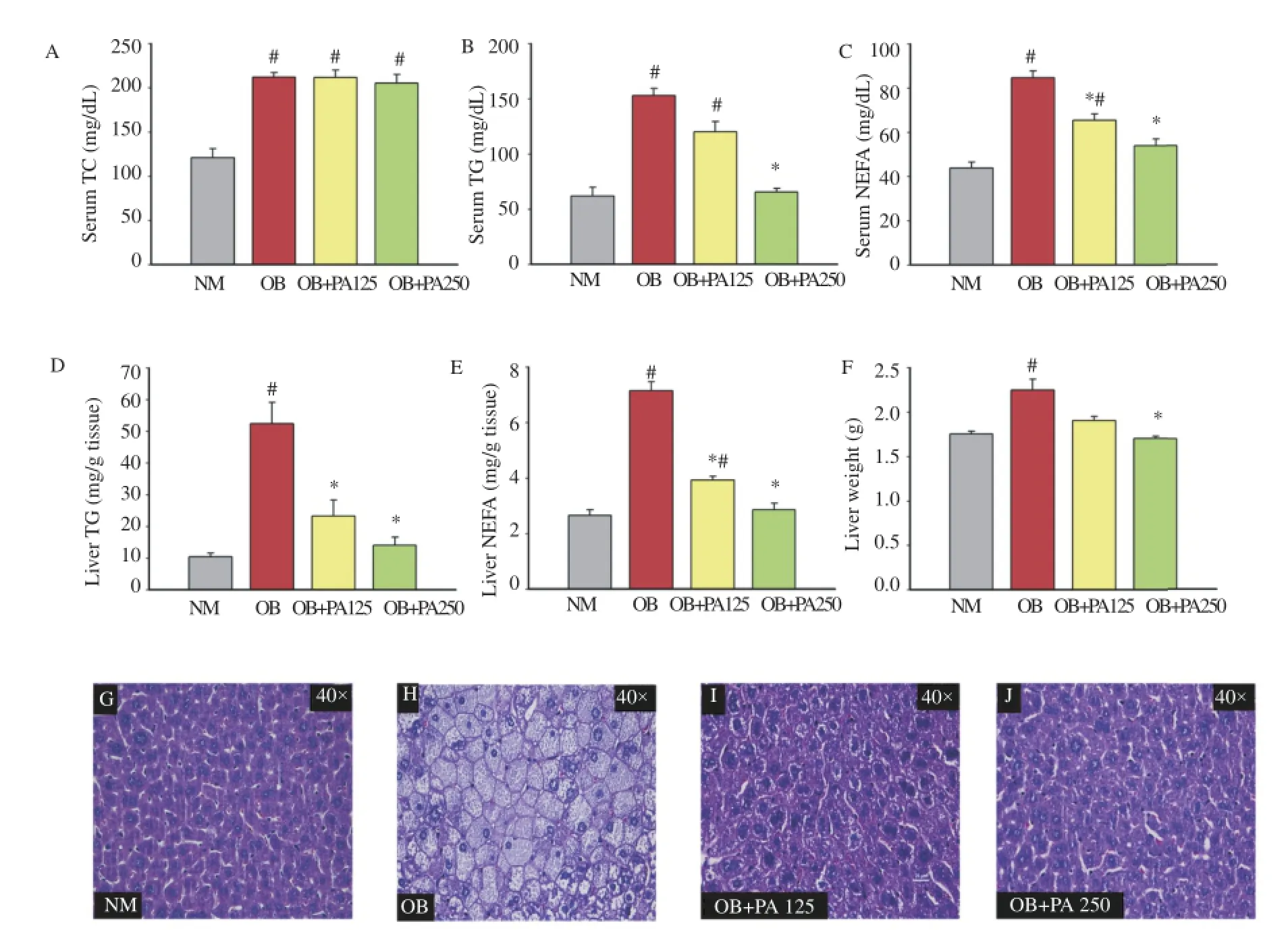
Figure 3.Effect of P.amaryllifolius on serum TC(A),serum TG(B),serum NEFA(C),liver TG(D),liver NEFA(E),liver weight(F),and histology of liver(G-J)in HFD-induced obese mice.Data are presented as mean±SEM(n=8).#:P<0.05 when compared to normal control mice.*:P<0.05 when compared to obese control mice.NM: Normal control mice;OB:Obese control mice;OB+PA125:Obese mice treated with P.amaryllifolius at 125 mg/kg;OB+PA250:Obese mice treated with P.amaryllifolius at 250 mg/kg.
No reduction in serum TC was observed even after six weeks of treatment with P.amaryllifolius(Figure 3A).However,a significant reduction(P<0.05)in serum TG and NEFAwas observed in the case of P.amaryllifolius-treated obese mice(125 and 250 mg/kg)as compared to obese control mice(Figure 3B,C).In addition,the treatment with P.amaryllifolius significantly decreased the accumulation of TG and NEFA in the liver tissue(Figure 3D,E)(P<0.05).The liver weight of obese control mice increased as compared to normal control mice,and the increased liver weight was significantly reduced afterthetreatmentwithP.amaryllifolius(Figure3F)(P<0.05).In the case of liver histology,more lipid droplets were found in obese control mice compared to those in the P.amaryllifolius-treated obese mice(Figure 3G-J),which was consistent with the results of liver TG and NEFA.
3.2.Protein expressions of plasma membrane GLUT4
The expressions of plasma membrane GLUT4 proteins in the muscle and fat cells were significantly reduced in obese control mice(0.19-and 0.30-fold,respectively compared with the basal level)(Figure4A,B).However,treatmentwith 125and 250mg/kg P.amaryllifolius significantly increased the muscle GLUT4 proteinby0.78-and0.88-foldofcontrol,respectivelywhencompared with obese control group(Figure 4A).GLUT4 protein expression inadiposetissuewasalsoup-regulatedby0.68-and0.81-foldafter treatment with 125 and 250 mg/kg P.amaryllifolius,respectively(Figure 4B). level tends to be elevated and the adiponectin level is slightly reduced in dietary obese rodents[23].However,in this study,it was observed that the serum leptin was significantly reduced in obese mice treated with P.amaryllifolius.
Moreover,the treated obese mice showed a significant increase in the serum adiponectin level.These results suggest that this treatment may improve insulin resistance in HFD-induced obese mice.
GLUT4 is considered to be the most important glucose transporter in the peripheral tissues,such as the fat and muscle cells.Improved GLUT4 function can control the blood glucose level.However,the deficiency of insulin or insulin-resistant condition plays a crucial role in the suppression of GLUT4 translocation,leading to decreased uptake of glucose into the fat and muscle cells,which contributes significantly to the elevated glucose levels[24].Obesity is strongly related to insulin resistance that can lead to reduced GLUT4 expression in the insulin-sensitive tissues[14,16].Our results showed that the treatment with P.amaryllifolius restored the decreased GLUT4 protein expression leading to enhanced glucose uptake into the muscle and fat cells.This action could be useful in controlling the hyperglycemic condition.
The cholesterol absorption from the small intestine shows increased cholesterol level in HFD feeding[25].The HFD model can increase the risk of hypertriglyceridemia possibly due to the
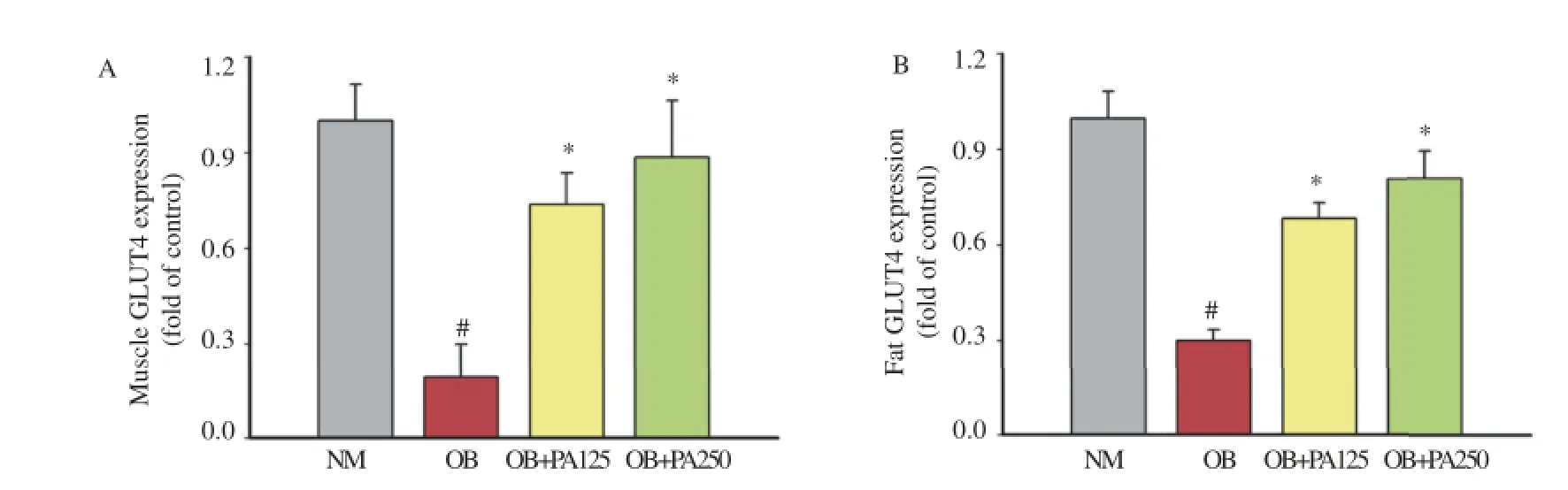
Figure 4.Effect of P.amaryllifolius on muscle(A)and fat(B)plasma membrane GLUT4 protein expressions in HFD-induced obese mice.Data are presented as mean±SEM(n=8).#:P<0.05 when compared to normal control mice.*:P<0.05 when compared to obese control mice.NM: Normal control mice;OB:Obese control mice;OB+PA125:Obese mice treated with P.amaryllifolius at 125 mg/kg;OB+PA250:Obese mice treated with P.amaryllifolius at 250 mg/kg.
4.Discussion
In the present study,HFD-induced insulin resistant state with decreased glucose tolerance and insulin sensitivity and increased insulin level in mice.This model also showed dyslipidemia and increased hepatic fat accumulation.Furthermore,this model was found to have reduced hepatic glycogen contents.Interestingly,the treatment with P.amaryllifolius extract can significantly improve the conditions of hyperinsulinemia,glucose intolerance,hyperlipidemia,and fatty liver in obese mice.
Insulin resistance is a characteristic feature of type 2 diabetes mellitus development.This condition is related to hyperglycemia and decreased glycogen synthesis in type 2 diabetes mellitus[22]. The present study showed a significant reduction in hyperglycemia and improved insulin sensitivity in the P.amaryllifoliustreated obese mice.Moreover,the treatment with P.amaryllifolius alsoimprovedglucosemetabolismbyrestoringglycogen synthesis.Adipokines,such as leptin and adiponectin,are recognized as systemic factors affecting insulin sensitivity.The leptin increased absorption and formation of TG and decreased TG uptake into the fat tissues[26].Other studies showed that the increased fat accumulation in the liver tissue and the elevated circulating FFA are the indexes in insulin resistance cases[27,28].Although treatment with P.amaryllifolius could not decrease the elevated serum TC,all doses of the treatment effectively reduced the levels of serum TG and NEFA.Obese mice treated with P.amaryllifolius also showed reduced liver TG and NEFA levels with decreased fat accumulation in the liver.The reduction of hyperlipidemia during treatment with P.amaryllifolius could be the result of stimulated lipid storage in fat tissue and suppressed TG and NEFA concentrations in the serum and liver.
Inconclusion,theadministrationofP.amaryllifolius improved the insulin resistance in HFD-induced obese mice by reducing the blood glucose level,stimulating insulin sensitivity,decreasing serum and hepatic TG and NEFA,stimulating protein expression of GLUT4,and reducing adipocyte size in epididymal fat tissues.Therefore,these results support the usefulaction of treatment with P.amaryllifolius leaf extracts in the improvement of insulin action in the obesity condition.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This research was supported by the research grant from the Faculty of Medicine,Thammasat University(Contract number: GEN2-22/2015).
[1]Rios-HoyoA,CortesMJ,Rios-OntiverosH,MeaneyE,Ceballos G,Gutierrez-Salmean G.Obesity,metabolic syndrome,and dietary therapeutical approaches with a special focus on nutraceuticals(polyphenols):a mini-review.Int J Vitam Nutr Res 2014;84:113-23.
[2]Ghoorah K,Campbell P,Kent A,Maznyczka A,Kunadian V. Obesity and cardiovascular outcomes:a review.Eur Heart J Acute Cardiovasc Care 2016;5(1):77-85.
[3]Saltiel AR,Kahn CR.Insulin signalling and the regulation of glucose and lipid metabolism.Nature 2001;414:799-806.
[4]Tveden-Nyborg P,Birck MM,Ipsen DH,Thiessen T,de Bie Feldmann L,Lindblad MM,et al.Diet-induced dyslipidemia leads to nonalcoholic fatty liver disease and oxidative stress in guinea pigs.Transl Res 2016;168:146-60.
[5]Reid DT,Eksteen B.Murine models provide insight to the development of non-alcoholic fatty liver disease.Nutr Res Rev 2015;28:133-42.
[6]Xiao W,Ren M,Zhang C,Li S,An W.Amelioration of nonalcoholic fatty liver disease by hepatic stimulator substance via preservation of carnitine palmitoyl transferase-1 activity.Am J Physiol Cell Physiol 2015;309:C215-27.
[7]Bargut TC,Mandarim-de-Lacerda CA,Aguila MB.A high-fish-oil diet prevents adiposity and modulates white adipose tissue inflammation pathways in mice.J Nutr Biochem 2015;26:960-9.
[8]R¨uhl R,Landrier JF.Dietary regulation of adiponectin by direct and indirect lipid activators of nuclear hormone receptors.Mol Nutr Food Res 2016;60:175-84.
[9]Chakraborti CK.Role of adiponectin and some other factors linking type 2 diabetes mellitus and obesity.World J Diabetes 2015;6:1296-308.
[10]Chang E,Choi JM,Park SE,Rhee EJ,Lee WY,Oh KW,et al.Adiponectin deletion impairs insulin signaling in insulin-sensitive but not insulin-resistant 3T3-L1 adipocytes.Life Sci 2015;132:93-100.
[11]Medina-Urrutia A,Posadas-Romero C,Posadas-S´anchez R,Jorge-Galarza E,Villarreal-Molina T,Gonz´alez-Salazar Mdel C,et al. Role of adiponectin and free fatty acids on the association between abdominal visceral fat and insulin resistance.Cardiovasc Diabetol 2015;14:20.
[12]Liu Y,Palanivel R,Rai E,Park M,Gabor TV,Scheid MP,et al. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high-fat diet feeding in mice. Diabetes 2015;64:36-48.
[13]Xu PT,Song Z,Zhang WC,Jiao B,Yu ZB.Impaired translocation of GLUT4 results in insulin resistance of atrophic soleus muscle. Biomed Res Int 2015;2015:291987.
[14]Llanos P,Contreras-Ferrat A,Georgiev T,Osorio-Fuentealba C,Espinosa A,Hidalgo J,et al.The cholesterol-lowering agent methyl-beta-cyclodextrin promotes glucose uptake via GLUT4 in adult muscle fibers and reduces insulin resistance in obese mice. Am J Physiol Endocrinol Metab 2015;308:E294-305.
[15]Gannon NP,Conn CA,Vaughan RA.Dietary stimulators of GLUT4 expression and translocation in skeletal muscle:a minireview.Mol Nutr Food Res 2015;59:48-64.
[16]Favaretto F,Milan G,Collin GB,Marshall JD,Stasi F,Maffei P,et al.GLUT4 defects in adipose tissue are early signs of metabolic alterations in Alms1GT/GT,a mouse model for obesity and insulin resistance.PLoS One 2014;9:e109540.
[17]Govers R.Molecular mechanisms of GLUT4 regulation in adipocytes.Diabetes Metab 2014;40:400-10.
[18]Ooi LS,Sun SS,Ooi VE.Purification and characterization of a new antiviral protein from the leaves of Pandanus amaryllifolius(Pandanaceae).Int J Biochem Cell Biol 2004;36:1440-6.
[19]Ghasemzadeh A,Jaafar HZE.Optimization of reflux conditions for total flavonoid and total phenolic extraction and enhanced antioxidant capacity in Pandan(Pandanus amaryllifolius Roxb.)using response surface methodology.Sci World J 2014;2014:523120.
[20]ChiabchalardA,NooronN.Antihyperglycemiceffectsof Pandanus amaryllifolius Roxb.leaf extract.Pharmacogn Mag 2015;11:117-22.
[21]Naowaboot J,Somparn N,Saentaweesuk S,Pannangpetch P. Umbelliferone improves an impaired glucose and lipid metabolism in high-fat diet/streptozotocin-induced type 2 diabetic rats.Phytother Res 2015;http://dx.doi.org/10.1002/ptr.5392.
[22]Samuel VT,Shulman GI.Mechanisms for insulin resistance: common threads and missing links.Cell 2012;148:852-71.
[23]Buettner R,Scholmerich J,Bollheimer LC.High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity(Silver Spring)2007;15:798-808.
[24]Burcelin R,Crivelli V,Perrin C,Da Costa A,Mu J,Kahn BB,et al. GLUT4,AMP kinase,but not the insulin receptor,are required for hepatoportal glucose sensor-stimulated muscle glucose utilization. J Clin Invest 2003;111:1555-62.
[25]Chandak PG,Obrowsky S,Radovic B,Doddapattar P,Aflaki E,Kratzer A,et al.Lack of acyl-CoA:diacylglycerol acyltransferase 1 reduces intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E knockout mice.Biochim Biophys Acta 2011;1811:1011-20.
[26]Khalifeh-Soltani A,McKleroy W,Sakuma S,Cheung YY,Tharp K,Qiu Y,et al.Mfge8 promotes obesity by mediating the uptake of dietary fats and serum fatty acids.Nat Med 2014;20: 175-83.
[27]Bermudez JA,Velasquez CM.[Profile of free fatty acids(FFA)in serum of young Colombians with obesity and metabolic syndrome].Arch Latinoam Nutr 2014;64:248-57.Spanish.
[28]Neuman MG,Cohen LB,Nanau RM.Biomarkers in nonalcoholic fatty liver disease.Can J Gastroenterol Hepatol 2014;28:607-18.
26 Jan 2016
Jarinyaporn Naowaboot,Division of Pharmacology,Department of Preclinical Science,Faculty of Medicine,Thammasat University(Rangsit Campus),Pathum Thani 12120,Thailand.
Tel:+66 2 9269732
Fax:+66 2 9269710
E-mail:naowaboot@yahoo.com
All experimental procedures involving animals were conducted in accordance with the standards of Association for Assessment and Accreditation of Laboratory Animal Care and approved by the Animal Ethics Committee,Faculty of Medicine,Thammasat University,Thailand(Rec.No.AE 010/2014).
Foundation Project:Supported by the research grant from the Faculty of Medicine,Thammasat University(Contract number:GEN2-22/2015).
Peer review under responsibility of Hainan Medical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
Receivedinrevisedform16Feb2016 Accepted 22 Apr 2016
Available online 1 Sep 2016
 Asian Pacific Journal of Tropical Biomedicine2016年10期
Asian Pacific Journal of Tropical Biomedicine2016年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A review on promising phytochemical,nutritional and glycemic control studies on Moringa oleifera Lam.in tropical and sub-tropical regions
- A rare cause of acute abdomen-Spontaneous rectus sheath hematoma
- Evaluation of proline,chlorophyll,soluble sugar content and uptake of nutrients in the German chamomile(Matricaria chamomilla L.)under drought stress and organic fertilizer treatments
- ProductionofsecondarymetaboliteE2.2fromPhaleriamacrocarpaendophyticfungus
- Cytotoxic,genotoxic and apoptotic effects of naringenin-oxime relative to naringenin on normal and cancer cell lines
- Jatropha curcas L∶Phytochemical,antimicrobial and larvicidal properties
