Essential oils from Elaeoselinum asclepium∶Chemical composition,antimicrobial and antioxidant properties
Moufida Bouchekrit,Hocine Laouer,Mohamed Hajji,Moncef Nasri,Serkos Artin Haroutounian,Salah Akkal
1Laboratory of Natural Biological Resources Valorization,Faculty of Sciences,University of Setif,19000,Setif,Algeria
2Laboratory of Enzyme Engineering and Microbiology,National School of Engineers of Sfax,BP 1173-3038,Sfax,Tunisia
3Chemistry Laboratory,Agricultural University of Athens,Iera Odos 75,11855,Athens,Greece
4Valorization of Natural Resources,Bioactive Molecules and Biological Analysis Unit,Department of Chemistry,University of Mentouri Constantine1,25000,Constantine,Algeria
Essential oils from Elaeoselinum asclepium∶Chemical composition,antimicrobial and antioxidant properties
Moufida Bouchekrit1*,Hocine Laouer1,Mohamed Hajji2,Moncef Nasri2,Serkos Artin Haroutounian3,Salah Akkal4
1Laboratory of Natural Biological Resources Valorization,Faculty of Sciences,University of Setif,19000,Setif,Algeria
2Laboratory of Enzyme Engineering and Microbiology,National School of Engineers of Sfax,BP 1173-3038,Sfax,Tunisia
3Chemistry Laboratory,Agricultural University of Athens,Iera Odos 75,11855,Athens,Greece
4Valorization of Natural Resources,Bioactive Molecules and Biological Analysis Unit,Department of Chemistry,University of Mentouri Constantine1,25000,Constantine,Algeria
ARTICLE INFO
Article history:
in revised form 24 Feb,2nd revisedform27Apr,3rdrevisedform 27 Jun 2016
Accepted 15 Jul 2016
Available online 26 Aug 2016
Elaeoselinum asclepium Apiaceae
Essential oil
Antimicrobial activity Antioxidant activity
Reducing power
Objective:To evaluate the chemical composition of the essential oil isolated from Elaeoselinum asclepium(L.)Bertol.(E.asclepium),and test the efficiency of the essential oil as an antimicrobial and antioxidant agent.
Methods:Essential oil was obtained from the aerial parts of E.asclepium by hydro distillation and analyzed by gas chromatography and gas chromatography coupled with mass spectrometry.We study for the first time the chemical composition of the essential oil of E.asclepium,followed by the in vitro antimicrobial activities,which were evaluated by agar diffusion method against six Gram-positive bacteria,five Gram-negative bacteria,and two fungi.In addition,The antioxidant activities were also investigated using assays of 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity and ferricreducing capacity.
Results:The analyzed essential oil of the aerial parts of E.asclepium was rich inαpinene(43.9%),other compounds detected in appreciable amounts were sabinene(27.9%)andβ-pinene(16.0%).The essential oil yields 1.2%,the IC50values of essential oil in 1,1-diphenyl-2-picrylhydrazyl assay in the reducing power assay were 48.26 mg/ mL and at 1 mg/mL,respectively.The absorbance value of essential oil at 700 nm was 0.956.The antimicrobial effect was higher on Candida albicans ATCC 1024 strain with the inhibition zone 14.5 mm than bacteria and molds.
Conclusions:The essential oil of E.asclepium has antimicrobial and antioxidant activities.These species may be used as an important source of natural antimicrobial and antioxidant agents.
Original articlehttp://dx.doi.org/10.1016/j.apjtb.2016.07.014
1.Introduction
Aromatic plants,especially those from the Umbelliferae family are able to synthesize secondary metabolites,such as phenolic compounds,monoterpenes and sesquiterpenes[1]. Essential oil has been known to have antimicrobial and antioxidant proprieties[2-4].It is worth mentioning that several studies have been conducted using different bacteria and fungi[5,6].The activity of the chemical side was caused by the presence of terpenes and their oxygenated compounds,and each compound contributes to their biological activities[7-9].
The genus Elaeoselinum belongs to Umbelliferae family,Apioideae subfamily(Laserpitieae tribe)[10].According to Algerian flora[11],the genus Elaeoselinum includes two species:Elaeoselinumasclepiumsubsp.meoides(Koch.)(E.asclepium subsp.meoides)(synonym:Thapsia asclepium L.or Laserpitium asclepium L.)Fiori,called locally Afs or Klikha,and Elaeoselinum thapsioides(Desf.)Maire(synonym: Elaeoselinum fontanesii Boiss.),called locally Becibsa.
In Italy,Elaeoselinum asclepium(E.asclepium)has two subspecies[12]:Elaeoselinum asclepium subsp.asclepium(synonym:Elaeoselinumhispanicum(Lange)Pau),and E.asclepiumsubsp.meoides(Desf.)Fiori(synonym: Elaeoselinum meoides(Desf.)Koch ex DC).
In connection with the ongoing studies about secondary metabolites from Apiaceae,we are interested in the phytochemical investigation of the components of E.asclepium grown in Algeria.The aim of our work is to evaluate two biological activities of the aerial parts of these species:antibacterial and antioxidant activities.The antioxidant activity was evaluated using assays of 1,1-diphenyl-2-picrylhydrazyl(DPPH)radical scavenging activity and ferric-reducing capacity.On the other hand,the antimicrobial activity was evaluated against a wide range of different pathogenic microorganisms including bacteria,yeast and molds strains.
2.Materials and methods
2.1.Chemicals
DPPH,butylatedhydroxyanile(BHA)were purchased from Sigma-Aldrich(St Louis,MO,USA),potassium ferricyanide(LOBA chemicals),trichloroacetic acid(Scharlau chemicals,Espagne),dipotassium phosphate(ACROS,USA),Monopotassium phosphate(Panreac,Espagne)ferric chloride(Biomedicals)and other solvents,dimethylsulfoxide(DMSO)were used.
2.2.Plant material
The aerial part of E.asclepium(L.)Bertolis was collected in the region of Flifla(Skikda)on July 2013 during the period of blooming at 300 m above sea level.Then,they were freed from the impurities and dried in the shade at an ambient temperature. The collected plant was identified by Prof.Laouer Hocine,Laboratory of Natural and Biological Resources Valorization,Department of Biology and Plant Ecology.Voucher specimen was deposited in the herbarium of the same laboratory of University Ferhat Abbes,Setif 1(Voucher number 15-2016).
2.3.Essential oil extraction
The dried aerial parts of the studied plant were subjected for 3 h to hydro distillation using a type of Clevenger apparatus.The obtained essential oil was stored at 4°C until the time of test and analysis.
2.4.Essential oil analysis
2.4.1.Gas chromatography analysis
Chemical analysis of this essential oil was performed on Perkin-Elmer,Clarus 500 gas chromatograph,equipped with a flame ionization detector and HP 5MS 30 m×0.25 mm× 0.25μm film thickness capillary column.The detector,the injector and column temperatures were programmed at 300°C,230°C and(60-280)°C,respectively,the initial rate of this latter equals 3°C/min.Helium was employed as the carrier gas at rate of 1 mL/min.
2.4.2.GC-MS analysis
The GC-MS analyses were carried using a Hewlett Packard 5973-6890 GC-MS system operating on EI mode(fitted with a HP 5MS),using helium(1 mL/min)as the carrier gas.The first heating column was 60°C as initial temperature and then it was increased gradually up to 280°C with a 3°C/min rate.The different compounds were identified by the comparison of their retention indices[13]obtained by using various n-alkanes(C9-C24).Also,the electron ionization-mass spectra of this analysis was compared with the NIST/NBS,Wiley libraries spectra and literature[14,15].Furthermore,the marked phytochemicals was confirmed through comparing them with disposable authentic sample.
2.5.Antimicrobial assay
2.5.1.Microbial strains
Antibacterial activity of E.asclepium essential oil was tested against 11 strains of bacteria:Staphylococcus aureus ATCC 25923(S.aureus ATCC 25923),Micrococcus luteus ATCC 4698(M.luteus),Escherichia coli ATCC 25922(E.coli),Pseudomonas aeruginosa ATCC 27853(P.aeruginosa),Klebsiella pneumonia ATCC E47(K.pneumonia),Salmonella typhi ATCC 19430(S.typhi),Salmonella enterica ATCC 43972(S.enterica),methicillin-resistant Staphylococcus aureus ATCC 43300(MRSA),Listeria inocula CIP 74915(L.inocula),Bacillus cereus ATCC 11778(B.cereus)and Enterococcus faecalis ATCC 29212(E.faecalis).
Antifungal activity was tested using Aspergillus niger 2CA936(A.niger)andCandidaalbicansATCC1024(C.albicans).
2.5.2.Agar diffusion method
The study of the antimicrobial activity was carried out following the method of Berghe and Vlietinck[16].E.asclepium essential oil was dissolved at 100 mg/mL in 100%DMSO. Culture suspension(200μL)of the tested microorganisms 106CFU/mL of bacteria cells and 108spores/mL of fungal strains were dispersed on Luria-Burtani and Sabouraud agar medium,respectively.After that,with a sterile borer,holes(6-mm diameter)were made and each hole was filled up with 60μL of sample essential oil.The positive controls of bacteria and fungi were gentamycin and nystatine,respectively and DMSO as negative reference.The Petri dishes were placed in a cold room(4°C)for 3 h,and then incubated for 24 h at 37°C for bacteria,48 h at 30°C for C.albicans and 8 days for A.niger fungal strains.The evaluation of the antimicrobial activity was carried out by estimating the diameter of inhibition zones(mm). The work was repeated twice and the values are the average of two replicates.
2.6.Antioxidant assay
2.6.1.DPPH radical scavenging
The DPPH radical scavenging activity of E.asclepium essential oil was performed following the method of Kirby and Schmidt[17]with a few modifications.A volume of 500μL of essential oil at various concentrations(1-30 mg/mL)was mixed with 375μL of 99.8%ethanol and 125μL of DPPHsolution(0.02%)as a free radical source.After that,the preparation was incubated for 1 h in the dark at room temperature.At the end,scavenging capacity was estimated spectrophotometricallybycontrollingthereductionin absorbance at 517 nm.In its radical form(purple color),DPPH has an absorption band at 517 nm which disappears upon reduction by an antiradical molecule(yellow color).A good radical scavenging activity has been interpreted by decreasing it in mixture absorbance.Synthetic antioxidant,BHAwasusedaspositivereference.DPPHradical scavenging activity was calculated as:
DPPH radical scavenging activity%=[(Ac-As)/Ac]×100
where,Acis the absorbance of the control reaction,Asis the absorbance of E.asclepium essential oil.Tests were performed in duplicate.IC50values were estimated by a linear regression.
2.6.2.Ferric-reducing activity
The reducing power of E.asclepium essential oils was performed following the method of Yildirim et al.[18].Sample solutions(0.5 mL)with various concentrations(0.5-10.0 mg/ mL)of the essential oil added to 1.25 mL of 0.2 mol/L phosphate buffer(pH=6.6)and 1.25 mL of(10 g/L)C6N6FeK3solution.Then,the preparation was incubated for half an hour at 50°C.After this time,1.25 mL of(100 g/L)trichloroacetic acid was added.A 1.25-mL aliquot of the supernatant from each sample mixture was added to 1.25 mL of distilled water and 0.25 mL of(1.0 g/L)FeCl3solution in a test tube.The absorbance was estimated at 700 nm after 10 min of incubation at room temperature against a blank.
There is a direct relationship between the concentration of essential oil,BHA and reducing power,where the increase of concentration of essential oil and BHA causes an increase in the reducing capacity.EC50value(mg essential oil/mL)is the effective concentration at which the absorbance was 0.5 and it was obtained by interpolation from linear regression analysis[19].
3.Results
3.1.Essential oil analysis
Theessentialoilobtainedfromtheaerialpartof E.asclepium picked from the east of Algeria(Flifla-Skikda)was pale yellow with a pleasant and distinct odor for the flowering stage but colorless when the harvest is done in the last stage. The yield of this oil was 1.20%compared with the dry plant weight.Gas chromatography and gas chromatography-mass spectrometry(GC-MS)were used to analyze the essential oil produced.In Table 1,the different compounds of essential oil of E.asclepium(L.)Bertol.(quantity and quality)were found and arranged in order of elution in Rtx-1 column.Based on the results obtained,forty compounds are adopted which represent 99%of the full oil.
The highest percentages of compounds were monoterpenes such asα-pinene(43.9%),followed by sabinene(27.9%),βpinene(16.0%),limonene(2.0%)and 4-terpineol(1.4%). However,there is a small amount ofα-thujene(monoterpene bicyclique,0.9%),myrtenal(0.8%),3-carene(monoterpene bicyclique,0.7%),myrcene(monoterpene-hydrocarbon,0.7%),ρ-cymene(0.6%)and hydrocarbon C10H14(0.6%).
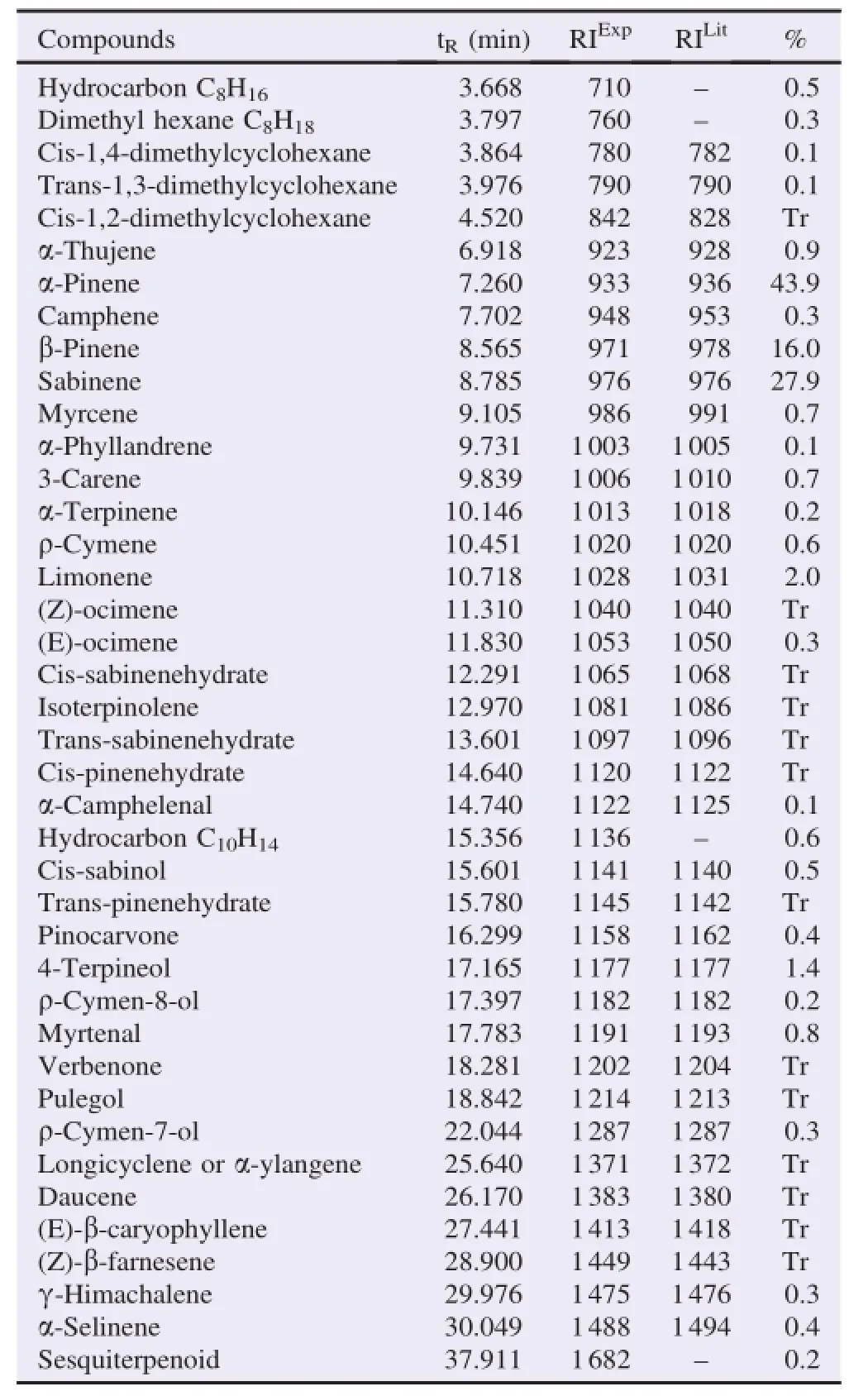
Table 1 Chemical composition of the essential oil of E.asclepium analyzed by GC-MS.
3.2.Antimicrobial activity
The results indicated that the essential oil of E.asclepium was active against the microorganisms assayed(Table 2).The essential oil tested showed various degrees of antibacterial and antifungal activities against most of bacteria and fungi tested,it was active against M.luteus ATCC 4698(10.0 mm)and B.cereus ATCC 11778(10.0 mm),however,no activity was observed on S.typhi ATCC 19430,E.faecalis ATCC 29212 and L.inocula CIP 74915 bacteria.The other bacterial strains(S.aureusATCC25923,S.enterica,P.aeruginosa,K.pneumonia ATCC E47)have a diameter of inhibition zones ranging from 8.0 to 9.0 mm.C.albicans was the most susceptible with an important inhibition zone of 14.5 mm.At this concentration,inhibition diameters shown by the essential oil were lower than those induced by gentamicin and nystatin.
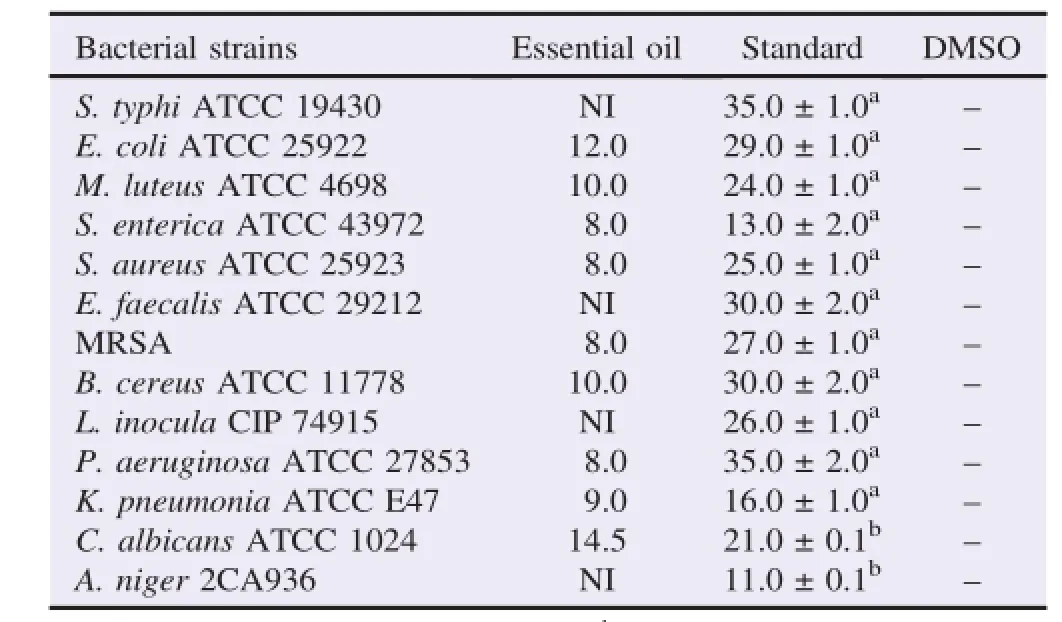
Table 2Inhibition diameters in mm of E.asclepium essential oil(mm).
3.3.Antioxidant activity
3.3.1.DPPH test
The examined essential oil could reduce the stable radical DPPH into DPPH-H.The IC50value of the essential oil of E.asclepium was 48.26 mg/mL,whereas the IC50of BHA was 12.80μg/mL.This activity was concentration-dependent.
3.3.2.Ferric-reducing activity
The EC50value of the essential oil of E.asclepium was 0.513 mg/mL,whereas the EC50of BHA was 22.24μg/mL.The essential oil exhibited low reducing potential in comparison with BHA.There is a direct relationship between the concentration of essential oil,BHA and reducing power,where the increase of concentration of essential oil and BHA causes an increase in the reducing capacity.
4.Discussion
4.1.Chemical analysis
The essential oil's yield obtained in this study(1.2%)was relatively higher compared to the species yields in the same family:Daucus carota ssp.carota fruits ranged from 0.8%to 1.6%(v/w),roots up to 0.2%and leaves 0.3%from Ober-Sankt-Veit(v/w)[20],Astrodaucus persicus aerial parts 0.6%-0.9%(v/ w)while the roots 0.1%(v/w)[21],Anthemis pedunculata 0.10%(w/w)and Anthemis punctata 0.26%(w/w)[22],Daucus gracilis 0.68%(v/w)[23].However,the essential oil of Astrodaucus persicus roots gave a very low rate which was 0.1%(v/w)[21].Our result is in accordance with other analyses showing similar or low quantities of essential oils:Foeniculum vulgare fruit ranged from 1.1%to 2.9%(v/w)[24],Distichoselinum tenuifolium flowering umbels yielded 0.1%(v/w),ripe umbels 2.0%-2.6%(v/w)[25].AccordingtoFellahetal.[26],variations in yields could be attributed to several factors such as the extraction technique and the collection period of the plant material[23].In the study of Guido Flamini et al.[27],the yields of Daucus sahariensis Eos were different and this is based on the plant's growth phase.In fact,we have observed a lower yield of 0.27%for plants collected at the flowerbudding phase in comparison to the higher yields of 0.63% and 0.68%obtained for plants harvested at the flowering and fruiting phase,respectively.Bader et al.[10]reported that the highest essential oil of E.asclepium subsp.meoides growing in Sicily yield was obtained from the ripe fruits(3.8%),followed by the roots(2.2%)and the aerial parts(0.95%). Zheljazkov et al.[28]added the effect of the extraction time.
The results of essential oil's chemical composition are far from those of Evergetis et al.[29],which showed that the essential oil of E.asclepium is formed by:α-pinene(27.41%),β-pinene(5.23%),myrcene(5.98%)andβ-phellandrene(1.63%)are similar for the essential oil of Elaeoselinum gummiferum plant[30].In addition,sabinene,myrcene,αpinene,β-pinene and camphene were evaluated as main constituents,accordingtoessentialoilcontentofnine commercial varieties of Daucus carota ssp.sativus(fruits/ seeds)and Daucus carota ssp.major(flowers and fruits)[31]. On the other hand,the chemical analysis of the essential oil of Elaeoselinum W.D.J.Koch genus has initially shown the presenceoftrans-β-farnesene,α-phellandrene,α-copaene,germacrene-D,bicyclogermacrene andα-humulene[29].
Eitherα-pinene orα+β-pinene as the main constituent it is a common character for all the species,with the exception of Elaeoselinum tenuifolium fruits(where myrcene(47.9%)and sabinene(24.3%)were the main components),and one sample of Elaeoselinum fontanesii fruits,in which limonene(32.9%)was the principal compound.The analysis of the essential oil of E.asclepium subsp.meoides revealed the presence of kaurane and epi-13-manoyl oxide in very small amounts.The presence of manoyl oxide in Elaeoselinum gummiferum[30]confirms the ability of this genus to synthesize labdanediterpenes[10]. Distichoselinumtenuifolium,distributedwidelyonthe provinceofAlgarve,SouthPortugal,(=Elaeoselinum tenuifolium)istheuniquespeciesofthegenus.High percentage of myrcene(48%-85%)was not detected in the essential oil of Elaeoselinum species[25,30,32].
The study of the larvicidal activity of essential oil of Oenanthe pimpinelloides L.(Apiaceae),which essentially contains monoxygenated monoterpenes,indicated that this oil has a high activity against Culex pipiens larvae with IC50=40.26 mg/ L compared to the essential oil of E.asclepium(L.)Bertol,which contains pinenes and oxygenated monoterpenes,the activity of this oil was less with IC50=96.96 mg/L.The results of this study suggest that the monoxygenated monoterpenes own strong insecticidal activities against Culex pipiens L.[29]. According to Bakkali et al.[33],the extraction of essential oil under the same conditions from the same part of the plant which should be planted in the same soil,in the same climate and the same season of harvest gives an essential oil with constant composition.
4.2.Microbial test
The propagation of drug resistant pathogens is considered as one of the most dangerous threats to effective treatment of microbial infections.Over the ages,essential oils and other extracts of plants have shown their effectiveness as sources of natural products and mainly for their potential utilization as other remedies for numerous contagious diseases too[34].
Both E.asclepium and Daucus setifolius essential oils showed low antibacterial activity with all strains tested with varying zone of inhibition(from 6 mm to 13 mm)[35].The antibacterial activity of Daucus carota(D.carota)root oils may be slightly related with their main constituents,such as(Z)-a-santalol(14.1%),caryophylleneoxide(10.6%),andspathulenol(9.8%)[36].Whereas,the sensitive of the essential oilofDaucussetifoliusDesf,Bejaiapopulation,was dependent onβ-pinene content(41.1%)[35].P.aeruginosa showed natural resistance to many antibiotics[37,38].On the other side,Satrani et al.[39]reported that E.coli was more sensitive than S.aureus to the essential oils from Ammi visnaga(L.)Lam.(Maroc),this antibacterial activity was due to their major components:linalol(22.71%)and methyl-2-butyrate d'isoamyle(27.68%).C.albicans was also inhibited by the essential oil of Daucus gracilis and the susceptibility was probably due to the high content of the elemicin(35.3%)and the geranyl-acetate(26.8%)in the essential oil[23].Mileski et al.[5]haveestablishedthatthefractionscontainingthehigh concentrationofα-pineneandsabinenethatinhibited effectively the growth of microorganisms;especially against yeast C.albicans which confirm our results with the same strain.
Our work results were in the same axis with several studies which have shown that the essential oils are slightly less active against Gram-negative than Gram-positive bacteria[40,41].
The antimicrobial agents such as essential oil and the majority of antibiotics can easily enter inside Gram-positive bacteria because the cell wall of this type of bacteria is rich on mucopolysaccharides and proteins but less on phospholipids
[41,42].
Hajji et al.[40]showed that the presence of oxygenated monoterpenes,monoterpene hydrocarbons and aldehydes in essential oils have the ability to inhibit the process of breathing and ions circulation and consequently the destruction of the bacterial cell.The biological activity of essential oil is often attributed to their major components,the moderate antibacterial activity of E.asclepium essential oil can be accorded to high amount of monoterpenes such asα-pinene(43.9%)and possibly because of the interaction effect between the different elements of oil[8,25,43].
So in our opinion,the essential oil's antimicrobial activity is thanks to the interactive effect between the major and minor compounds of this essential oil.Based on these results,it can be said that the essential oil of E.asclepium is able to inhibit the growth of yeast(C.albicans)and both Gram-negative(E.coli)and Gram-positive(S.aureus,M.luteus,B.cereus)bacteria.The used concentration of DMSO does not have any antimicrobial effect.
4.3.Antioxidative assay
The DPPH scavenging activity of E.asclepium essential oil(IC50=48.26 mg/mL)was weaker than BHA(IC50=12.80μg/ mL).However,the IC50of Algerian D.carota L.essential oil was 40.97 mg/mL[36]which is similar to the IC50of our essential oil.The works of Meliani et al.[36]have shown that the essential oils of Algerian D.carota L(stems/leaves)consist primarily of monoterpenes such asα-pinene,sabinene,β-pinene,limonene,myrcene,terpinene-4-ol.It is observed that the chemical composition of E.asclepium's essential oil(Table 1)is relative to D.carota L.which reflects the similar DPPH scavenging activity.
The antioxidant activity relies on the chemical composition. It seems that the essential oils which contain oxygenated sesquiterpenes,monoterpenesandphenoliccompoundshave greater antioxidant properties[9,36,44].
The essential oil's compounds have a direct association with the antioxidant activities.This may be the result of the presence of high ratio of the main constituents and also the result of the presence of other compounds in low concentration or the synergy between them.
4.4.Ferric-reducing activity
In general,the ferric-reducing test is mainly employed to see the capacity of natural antioxidant in giving an electron or hydrogen[45].According to various works,there is a direct relation between antioxidant ability and ferric-reducing of some bioactive molecules.For that reason,it is amply believed that the highest absorbance at 700 nm indicates a great reducing power[40].The reducing power of BHA was significantly more evident relating to the essential oils of aromatic plants.
High percentage of essential oil increased the antioxidant capacity,it means that there are certain elements in essential oil of E.asclepium which are electron donors and might react with free radicals to stabilize them and to end radical chain reactions[46].It can be concluded that this impact could be related to the presence of certain contents that have antioxidant capacity,and also to possible antagonistic and synergistic impact of contents and efficient groups in the essential oil[47].
It may be suggested that like all the medicinal plants,E.asclepium contains active substances(α-pinene andβ-pinene)and other bioactive substances potentially useful for medicinal properties and as natural food preservation.In the present study,it was found that the essential oil of the aerial part of E.asclepium possesses antimicrobial,antioxidant and radical scavenging activities.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors are thankful to Professor Moncef Nasri,Director of Laboratory of Enzyme Engineering and Microbiology,National School of Engineers of Sfax,Tunisia for his great contribution to this work.The authors would like to thank Professor Serkos A.Haroutounian of the Chemistry Laboratory,Agricultural University of Athens,Greece for the GC-MS analysis.This work was financially supported by Project Algero-Tunisienne with grant No.280/11/07/2012.
[1]Sahebkar A,Iranshahi M.Biological activities of essential oils from the genus Ferula(Apiaceae).Asian Biomed 2010;4(6):835-47.
[2]Hsouna AB,Trigui M,Mansour RB,Jarraya RM,Damak M,Jaoua S.Chemical composition,cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat.Int J Food Microbiol 2011;148(1):66-72.
[3]Hammoudi R,Dehak K,Mahammed MH,Ouldelhadj MD.[Chemical composition and antioxidant activity of essential oils from Deverra scoparia Coss.and Dur.(Apiaceae)].Leban Sci J 2015;16(2):27-36.French.
[4]Mileski KS,Dˆzamiˊcˊc AM,ˊCiriˊcˊc AD,Grujiˊc SM,Ristiˊcˊc MS,Matevski VS,et al.Radical scavenging and antimicrobial activity of essential oil and extracts of Echinophora sibthorpiana guss. from Macedonia.Arch Biol Sci 2014;66(1):401-13.
[5]Mileski KS,Dˆzamiˊc AM,ˊCiriˊc AD,Ristiˊcˊc MS,Grujiˊc SM,Matevski VS,et al.Composition,antimicrobial and antioxidant properties of endemic species Ferulago macedonica Micevski&E. Mayer Rec Nat Prod 2015;9(2):208-23.
[6]Shukla R,Singh P,Prakash B,Dubey NK.Antifungal,aflatoxin inhibition and antioxidant activity of Callistemon lanceolatus(Sm.)sweet essential oil and its major component 1,8-cineole against fungal isolates from chickpea seeds.Food Control 2012;25:27-33.
[7]Ebadollahi A.Plant essential oils from Apiaceae family as alternatives to conventional insecticides.Ecol Balkanica 2013;5(1): 149-72.
[8]BenMarzougHN,RomdhaneM,LebrihiA,MathieuF,Couderc F,Abderraba M,et al.Eucalyptus oleosa essential oils: chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts(stems,leaves,flowers and fruits).Molecules 2011;16(2):1695-709.
[9]Ksouri A,Dob T,Belkebir A,Krimat S,Chelghoum C.Chemical composition and antioxidant activity of the essential oil and the methanol extract of Algerian wild carrot Daucus carota L.ssp. carota.(L.)Thell.J Mater Environ Sci 2015;6(3):784-91.
[10]Bader A,Cioni PL,Flamini G.GC-MS analysis of the essential oils of ripe fruits,roots and flowering aerial parts of Elaeoselinum asclepium subsp.meoides growing in Sicily.Nat Prod Commun 2010;5(7):1111-4.
[11]Quezel P,Santa S.New flora of Algeria and the southern desert regions.Vol.2.Paris:CNRS Editions;1963.
[12]Pignatti S.Flora d'Italia.Vol.2.Bologna:Edagricole;1982,p.241-2.
[13]Van den Dool H,Kratz PD.A generalization of the retension index system including linear temperature programmed gas-liquid partition chromatography.J Chromatogr 1963;11:463-71.
[14]Massada Y.Analysis of essential oil by gas chromatography and spectrometry.New York:Wiley;1976.
[15]Adams RP.Identification of essential oil components by gas chromatography/mass spectroscopy.Illinois:Allured;1995.
[16]Berghe VA,Vlietinck AJ.Screening methods for antibacterial and antiviral agents from higher plants.Meth Plant Biochem 1991;6: 47-68.
[17]Kirby AJ,Schmidt RJ.The antioxidant activity of Chinese herbs for eczema and of placebo herbs.J Ethnopharmacol 1997;56(2): 103-8.
[18]Yildirim A,Mavi A,Kara AA.Determination of antioxidant and antimicrobial activities of Rumex crispus L.extracts.J Agric Food Chem 2001;49(8):4083-9.
[19]Gaamoune S,Harzallah D,Kada S,Dahamna S.Evaluation of antioxidant activity of flavonoids extracted from Galium tunetanum Poiret.Res J Pharm Biol Chem Sci 2014;5(2):341-8.
[20]Chizzola R.Composition of the essential oil from Daucus carota ssp.carota growing wild in Vienna.J Essent Oil Bear Plant 2010;13(1):12-9.
[21]Goodarzi S,Hadjiakhoondi A,Yassa N,Khanavi M,Tofighi Z. Essential oils chemical composition,antioxidant activities and total phenols of Astrodaucus persicus.Iran J Basic Med Sci 2016;19: 159-65.
[22]Laouer H,El Kolli M,Boulaacheb N,Akkal S.Chemical composition and antibacterial activity of the essential oil of Anthemis pedunculata and Anthemis punctata.Yanbu J Eng Sci 2014;9:76-83.
[23]El Kolli M,Laouer H,El Kolli H,Akkal S,Sahli F.Chemical analysis,antimicrobial and anti-oxidative properties of Daucus gracilis essential oil and its mechanism of action.Asian Pac J Trop Biomed 2016;6(1):8-15.
[24]Mota AS,Martins MR,Arantes S,Lopes VR,Bettencourt E,Pombal S,et al.Antimicrobial activity and chemical composition of the essential oils of Portuguese Foeniculum vulgare fruits.Nat Prod Commun 2015;10(4):673-6.
[25]Tavares AC,Goncalves MJ,Cruz MT,Cavaleiro C,Lopes CM,Canhoto J,et al.Essential oils from Distichoselinum tenuifolium: chemicalcomposition,cytotoxicity,antifungalandantiinflammatory properties.J Ethnopharmacol 2010;130:593-8.
[26]Fellah S,Romdhane M,Abderraba M.[Extraction and a study of essential oils of Salvia officinalis from two different regions in Tunisia].J Soc Alger Chim 2006;16(2):193-202.French.
[27]Flamini G,Smaili T,Zellagui A,Gherraf N,Cioni PL.Effect of growth stage on essential-oil yield and composition of Daucus sahariensis.Chem Biodivers 2013;10:2014-20.
[28]Zheljazkov VD,Cantrell CL,Astakie T,Jeliazkova E.Distillation time effect on lavender essential oil yield and composition.J Oleo Sci 2013;62(4):195-9.
[29]EvergetisE,MichaelakisA,KioulosE,KoliopoulosG,Haroutounian SA.Chemical composition and larvicidal activity of essential oils from six Apiaceae family taxa against the West Nile virus vector Culex pipiens.Parasitol Res 2009;105:117-24.
[30]Pala-PaulJ,Perez-AlonsoMJ,Velasco-NegueruelaA. A contribution to the knowledge of the oil of Elaeoselinum gummiferum(Desf.)Tutin.J Essent Oil Res 2011;13:362-3.
[31]Flamini G,Cosimi E,Cioni PL,Molfetta I,Braca A.Essential-oil composition of Daucus carota ssp.major(Pastinocello Carrot)and nine different commercial varieties of Daucus carota ssp.sativus fruits.Chem Biodivers 2014;11:1022-33.
[32]Ortega T,Carretero ME,Bermejo P,Pardo P.[Essential oils in Umbellifers.Study of essential oil of Elaeoselinum asclepium(L.)Bertol.subsp.asclepium].An Jardin Bot Madrid 1986;43:121-4. Spanish.
[33]Bakkali F,Averbeck S,Averbeck D,Idaomar M.Biological effects of essential oils-a review.Food Chem Toxicol 2008;46: 446-75.
[34]Tepe B,Daferara D,Sokmen M,Polissiou M,Sokmen A.In vitro antimicrobial and antioxidant activities of the essential oils and various extracts of Thymus eigii M.Zohary et P.H.Davis.J Agric Food Chem 2004;52(5):1132-7.
[35]LaouerH,BouhedaA,HaroutounianS,EvergetisE,Bouchekrit M,Sahli F,et al.Chemical and biological study of essential oils of two populations of Algerian Daucus setifolius Desf.Pharmacogn Commun 2013;3(1):7-11.
[36]Meliani N,Dib MA,Bendiabdellah A,Djabou N,Chikhi I,Allali H,et al.Evaluation of antioxidant activity of essential oil and extracts from Algerian Daucus carota L.aerial parts.Glob J Pharm Res 2012;1(5):1121-9.
[37]Henwood CJ,Livermore DM,James D,Warner M.Pseudomonas Study Group.Antimicrobial susceptibility of Pseudomonas aeruginosa:results of a UK survey and evaluation of the British society forantimicrobialchemotherapydiscsusceptibilitytest. J Antimicrob Chemother 2001;47(6):789-99.
[38]Aboaba S,Akande A,Flamini G.Chemical composition,toxicity and antibacterial activity of the essential oils of Garcinia mangostana from Nigeria.J Essent Oil Bear Plant 2014;17(1):78-86.
[39]Satrani B,Farah A,Fechtal M,Talbi M,Bouamrani ML.[Chemical composition and antibacterial and antifungal activity of the essential oil of Ammi visnaga(L.)Lam.from Marocco].Acta Bot Gallica 2004;151(1):65-71.French.
[40]Hajji M,Masmoudi O,Souissi N,Triki Y,Kammoun S,Nasri M. Chemical composition,angiotensin I-converting enzyme(ACE)inhibitory,antioxidant and antimicrobial activities of the essential oil from Periploca laevigata root barks.Food Chem 2010;121(3): 724-31.
[41]Torbati M,Nazemiyeh H,Lotfipour F,Nemati M,Asnaashari S,Fathiazad F.Chemical composition and in vitro antioxidant and antibacterial activity of Heracleum transcaucasicum and Heracleum anisactis roots essential oil.Bioimpacts 2014;4(2): 69-74.
[42]Fraternalea D,Flaminib G,Bisioc A,Albertinid MC,Ricci D. Chemical composition and antimicrobial activity of Salvia x jamensis essential oil.Nat Prod Commun 2012;7(9):1237-40.
[43]Mansouri N,Satrani B,Ghanmi M,El Ghadraoui L,Guedira A,Aafi A.[Chemical composition,antimicrobial and antioxidant activity of the essential oil of Juniperus communis from Morocco]. Bull Soc Roy Sci Li`ege 2011;80:791-805.French.
[44]Proestos C,Lytoudi K,Mavromelanidou OK,Zoumpoulakis P,Sinanoglou VJ.Antioxidant capacity of selected plant extracts and their essential oils.Antioxidants(Basel)2013;2:11-22.
[45]Shimada K,Fujikawa K,Yahara K,Nakamura T.Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion.J Agr Food Chem 1992;40:945-8.
[46]Merghache D,Boucherit-Otmani Z,Merghache S,Chikhi I,Selles C,Boucherit K.Chemical composition,antibacterial,antifungal and antioxidant activities of Algerian Eryngium tricuspidatum L.essential oil.Nat Prod Res 2014;28(11): 795-807.
[47]ObameLC,KoudouJ,ChalchatJC,Bassol´eI,EdouP,Ouattara AS,et al.Volatile components,antioxidant and antibacterial activities of Dacryodes buettneri H.J.Lam Engl.essential oil from Gabon.Sci Res Essay 2007;2(11):491-5.
20 Jan 2016
Moufida Bouchekrit,Laboratory of Natural Biological Resources Valorization,Faculty of Sciences,University of Setif,19000,Setif,Algeria.
Tel:+213 558932784
E-mail:bouchekritmoufida@ymail.com
Foundation Project:Supported by Project Algero-Tunisienne with grant No.280/ 11/07/2012.
Peer review under responsibility of Hainan Medical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Pacific Journal of Tropical Biomedicine2016年10期
Asian Pacific Journal of Tropical Biomedicine2016年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A review on promising phytochemical,nutritional and glycemic control studies on Moringa oleifera Lam.in tropical and sub-tropical regions
- A rare cause of acute abdomen-Spontaneous rectus sheath hematoma
- Evaluation of proline,chlorophyll,soluble sugar content and uptake of nutrients in the German chamomile(Matricaria chamomilla L.)under drought stress and organic fertilizer treatments
- ProductionofsecondarymetaboliteE2.2fromPhaleriamacrocarpaendophyticfungus
- Cytotoxic,genotoxic and apoptotic effects of naringenin-oxime relative to naringenin on normal and cancer cell lines
- Pandanusamaryllifoliusleafextractincreasesinsulinsensitivityinhigh-fatdiet-induced obese mice
