Anti-Candida and anti-Cryptococcus evaluation of 15 non-alkaloidal compounds from Pterogyne nitens
Caroline Sprengel Lima,Carlos Roberto Polaquini,Mariana Bastos dos Santos,Fernanda Patrícia Gullo,Fernanda Sangalli Leite,Liliane Scorzoni,Vanderlan da Silva Bolzani,Maria Josˊe Soares Mendes-Giannini,Ana Marisa Fusco-Almeida,Andrˊeia Alves Rezende,Luis Octavio Regasini*
1Laboratory of Green and Medicinal Chemistry,Department of Chemistry and Environmental Sciences,Institute of Biosciences,Letters and Exact Sciences,São Paulo State University(UNESP),São Josˊe do Rio Preto,Sao Paulo,Brazil
2Department of Clinical Analysis,School of Pharmaceutical Sciences,São Paulo State University(UNESP),Araraquara,São Paulo,Brazil
3Department of Organic Chemistry,Institute of Chemistry,São Paulo State University(UNESP),Araraquara,São Paulo,Brazil
4Department of Biology and Animal Sciences,Faculty of Engineering,São Paulo State University(UNESP),Ilha Solteira,São Paulo,Brazil
Anti-Candida and anti-Cryptococcus evaluation of 15 non-alkaloidal compounds from Pterogyne nitens
Caroline Sprengel Lima1,Carlos Roberto Polaquini1,Mariana Bastos dos Santos1,Fernanda Patrícia Gullo2,Fernanda Sangalli Leite2,Liliane Scorzoni2,Vanderlan da Silva Bolzani3,Maria Josˊe Soares Mendes-Giannini2,Ana Marisa Fusco-Almeida2,Andrˊeia Alves Rezende4,Luis Octavio Regasini1*
1Laboratory of Green and Medicinal Chemistry,Department of Chemistry and Environmental Sciences,Institute of Biosciences,Letters and Exact Sciences,São Paulo State University(UNESP),São Josˊe do Rio Preto,Sao Paulo,Brazil
2Department of Clinical Analysis,School of Pharmaceutical Sciences,São Paulo State University(UNESP),Araraquara,São Paulo,Brazil
3Department of Organic Chemistry,Institute of Chemistry,São Paulo State University(UNESP),Araraquara,São Paulo,Brazil
4Department of Biology and Animal Sciences,Faculty of Engineering,São Paulo State University(UNESP),Ilha Solteira,São Paulo,Brazil
ARTICLE INFO
Article history:
in revised form 2 Dec,2nd revised form 7 Dec 2015
Accepted 5 Jun 2016
Available online 26 Aug 2016
Candida
Cryptococcus
Antifungal
Pterogyne nitens
Flavonoid
Opportunistic fungi
Objective:To evaluate anti-Candida and anti-Cryptococcus activities of 15 nonalkaloidal compounds from Pterogyne nitens Tulasne(Leguminosae),a South American medicinal plant.
Methods:Compounds were submitted to antifungal assays,using microdilution method described by Clinical and Laboratory Standards Institute document,with minor modifications.Five species of Candida and two species of Cryptococcus,including clinical isolates were screened.Antifungal activity was expressed by minimum inhibitory concentration(MIC).Amphotericin B and fluconazole were used as standard antifungal drugs. Results:Among tested compounds,six substances presented fungal growth inhibition(MIC<31.2μg/mL)[threeflavonederivatives(1-3),aglycosylatedflavonolderivative(5)and two phenolic acids(10 and 12)].Sorbifolin(1),exhibited potent antifungal activity,demonstrating MIC value of 3.90μg/mL against Candida glabrata ATCC 90030,Cryptococcus gattii 118 and fluconazole-resistant clinical isolate of Cryptococcus neoformans var.grubii.Pedalin(2)and nitensoside B(3),two glycosylated flavone derivatives,were active against Cryptococcus neoformans ATCC 90012(MIC=7.80μg/mL).
Conclusions:Flavone derivatives from Pterogyne nitens can serve as prototypes for the design and development of innovative anti-Candida and anti-Cryptococcus hits.
Original articlehttp://dx.doi.org/10.1016/j.apjtb.2016.08.003
研究生师生关系主要包括教育关系、心理关系、伦理关系。教育关系是师生关系的核心,表现为导师与研究生的教学指导关系。心理关系是师生为完成共同的教学任务而产生的心理交往和情感交流。伦理关系处于师生关系体系中的最高层次,表现为导师与研究生构成一个特殊的道德共同体,各自承担和履行一定的伦理责任和伦理义务。当前研究生师生关系在上述各个层面均出现了不同程度的异化和失衡。
1.Introduction
In the last decades,there has been a significant increase in the incidence and prevalence of opportunistic fungi infections, includingcandidiasisandcryptococcosis.Thisincreaseisrelatedto the growing number of immunocompromised patients,including those with AIDS,cancer,transplant recipients and premature neonates[1,2].Seven Candida species are classified as having major clinical relevance,namely,Candida albicans(C.albicans),Candidatropicalis(C.tropicalis),Candidaglabrata(C. glabrata),Candida parapsilosis(C.parapsilosis),Candida krusei(C.krusei),Candida stellatoidea and Candida kyfer[3-6]. Candidiasis,the most common opportunistic yeast infection in the world has been in majority with C.albicans.This yeast is a causative agent of mucocutaneous and vulvovaginal infections,among other more invasive infections,such as septicemia,endocarditis,meningitis and peritonitis[3,4,7].Cryptococcosis is an important globally systemic mycosis and the third mostprevalent disease in AIDS patients[8].The most common clinical manifestation is cryptococcal meningitis,which has been mainly caused by Cryptococcus neoformans(C.neoformans)and Cryptococcus gattii(C.gattii).However,there are reports of human infections caused by C.albidus and Cryptococcus laurentii[9].
On the other hand,the inefficacy of conventional antifungal drugs against resistant strains,as well as their severe side effects,limited spectrum of action and drug-drug interactions justify the urgent search for novel antifungal compounds[10].In this way,natural products have long been used as prototypes for design of innovative drugs,which may be useful against infectious diseases,such as artemisinin,quinine,β-lactams,aminoglycosides,tetracyclines,echinocandins,griseofulvin,etc.[11].Several metabolites of diverse structural patterns have proven to be active against fungi,as well as the screening of plant extracts is a valid strategy being exploited to discover novel antifungal agents[12,13].
Pterogyne nitens Tulasne(Leguminosae)(P.nitens),popularly named as“b´alsamo”,“cocal”,“amendoim-bravo”,“amendoinzeiro”and“yvi-rar´o”isthesolememberofthegenus.Itisfoundin non-protected SouthAmerica areas,belonging tothelistofspecies recommendedforconservationgeneticsinBrazil.Also,P.nitensis admiredforthebeautyandodorofitsflowers,leavesandfruits[14]. Ethnopharmacological studies in Guarani communities revealed cold aqueous preparations from P.nitens stem barks have been used for the treatment of helminthic infestations,mainly against Ascaris lumbricoides[15].Chemically,P.nitens presented a variety of compounds,including guanidine alkaloids,flavonoids(flavones,flavonols,flavan-3-ols and catechins),phenolic acids,triterpenes and sterols[16-19].Guanidine alkaloids from P.nitens have demonstrated a broad spectrum of biological activities,including cytotoxic,pro-apoptotic,antibacterial and trypanocidal activity[20-25].Flavones and flavonols from P.nitens exhibited myeloperoxidase inhibitory and antioxidant activities[26-29].
In our previous study,we identified antimicrobial activity of P.nitens extracts and their four guanidine alkaloids against C.albicans,C.krusei,C.parapsilosis and C.neoformans[30]. Our goal with present work was to evaluate anti-Candida and anti-Cryptococcus activities of 15 non-alkaloidal compounds against five Candida species and two Cryptococcus species.
2.Materials and methods
2.1.Non-alkaloidal compounds from P.nitens
Flavonoids(flavone,flavonol and catechin derivatives)(1-8)and phenolic acids(9-13)were isolated and identified,using chemical procedures reported previously(Figure 1).Flavone derivatives,sorbifolin(1),pedalin(2)and nitensoside B(3),were isolated from leaves[26].Flavonol derivatives,quercetin(4),isoquercitrin(5),quercetin 3-O-sophoroside(6)and rutin(7)were obtained from fruits and flowers[27,31].Ourateacatechin(8)and the phenolic acids(9-13),such as caffeic acid(9),ferulic acid(10),sinapic acid(11),chlorogenic acid(12)and gallic acid(13)were isolated from flowers[18].
Triterpene acids(14)and(15)were purified from P.nitens leaves for the first time.Leaves of P.nitens were collected from Institute of Biosciences,Letters and Exact Sciences,São Paulo State University,São Josˊe do Rio Preto,Sao Paulo,Brazil(20°47′02.4′S,49°21′36.0′W)in July 2014 and a voucher specimen(10291)was deposited in the Herbarium of Ilha Solteira(HISA)of Faculty of Engineering,Ilha Solteira,São Paulo, Brazil.Shade-dried leaves(600 g)were ground and extracted with hexane(1.8 L×3,at room temperature).Dry hexane extract(10 g)was subjected to purification by successive chromatography columns over silica gel,eluted with mixtures of hexane and ethyl acetate,as well as furnishing betulinic acid(14 and 20 mg)and oleanonic acid(15 and 14 mg)(Figure 1). Structures of compound 14 and 15 were identified according to literature data,including13C nuclear magnetic resonance spectrum analysis[32].
2.2.Microorganisms
2.3.Minimum inhibitory concentration(MIC)
Dissolution of compounds was performed with dimethylsulfoxide on 96-well plates and their concentration ranged from 250.00 to 0.48μg/mL.Anti-Candida and anti-Cryptococcus activity experiments were carried out using reference broth microdilution method,as outlined in M27-A3 document produced by Clinical and Laboratory Standards Institute[35],with minor modifications[36].Amphotericin B and fluconazole(FCZ)were used as standard antifungal drugs.MIC values were determined as the lowest concentration of test samples which showed complete fungal growth inhibition.Some 96-well plates were analyzed visually and spectrophotometrically. All tests were performed in triplicate and in the three independent experiments.
3.Results
MIC values for all yeasts were given in Table 1.Out of 15 non-alkaloidal compounds(1-15),six substances presented fungal growth inhibition(MIC≤31.20μg/mL)including three flavone derivatives(1-3),a glycosylated flavonol derivative(5)and two phenolic acids(10 and 12).
Compound 1 demonstrated potent antifungal activity against both human opportunistic fungi,with MIC values ranging from 3.90 to 31.20μg/mL.In anti-Candida assays,the most potent effect of compound 1 was against C.glabrata(MIC=3.90μg/ mL),followed by C.krusei and C.parapsilosis(MIC=7.80μg/ mL).Thelowestpotencyofcompound1wasagainst C.albicans and C.tropicalis(MIC=31.20μg/mL).In the anti-Cryptococcus assays,compound 1 was active against three strains of C.neoformans var.grubii(MIC values of 3.90 and 7.80μg/mL),including fluconazole-resistant clinical isolate(CnR).For CnR strain,compound 1 exhibited a MIC value of3.90μg/mL,four times less potent than amphotericin B,which has been administered as gold standard for cryptococcosis treatment[37].Compound 1 was active against C.gattii(118),displaying a MIC value of 3.90μg/mL,four times less potent than amphotericin B.For C.neoformans var.grubii ATCC 90012 and CnS strains,compound 1(MIC=7.80μg/mL)was two times less potent than FCZ(MIC=4.00μg/mL).
Compounds 2 and 3,two glycosylated flavone derivatives,were active against C.neoformans var.grubii(ATCC 90012),with MIC values of 7.80μg/mL,two times less potent than FCZ(MIC=4.00μg/mL).On the other hand,compounds 2 and 3 exhibited weak fungitoxicity against Candida species(MIC> 62.50μg/mL),except compound 2 which was moderately active against C.krusei(MIC=31.20μg/mL).
Interestingly,flavonols,compound 4 and its glycosylated derivatives(5-7)were significantly less fungitoxic than flavone derivatives(1-3).Among flavonol derivatives,compound 5 demonstrated potent anti-Cryptococcus activity against ATCC strain,displaying a MIC value of 15.60μg/mL,four times less potent than FCZ(MIC=4.00μg/mL).For this strain,compounds 4,6 and 7 were weakly active,exhibiting MIC values of 125.00,62.50 and 125.00μg/mL,respectively.The comparison of MIC values for compounds 4-7 indicated number of sugar units influenced anti-Cryptococcuseffect.Thus,orderofantifungal potency was monoglycosylated(5)>diglycosylated(6 and 7)>free aglycone(4).
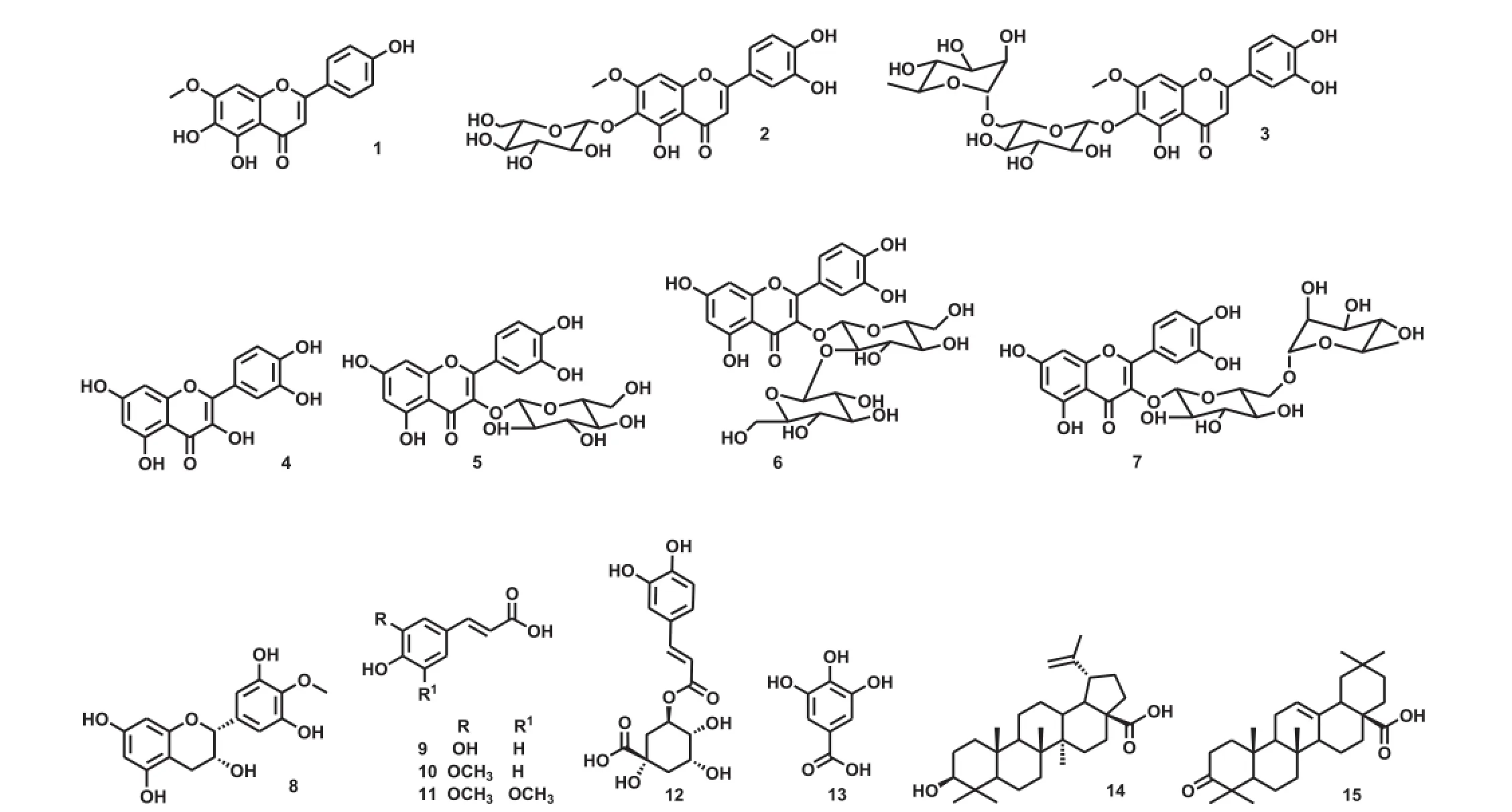
Figure 1.Structure of non-alkaloidal compounds from P.nitens.
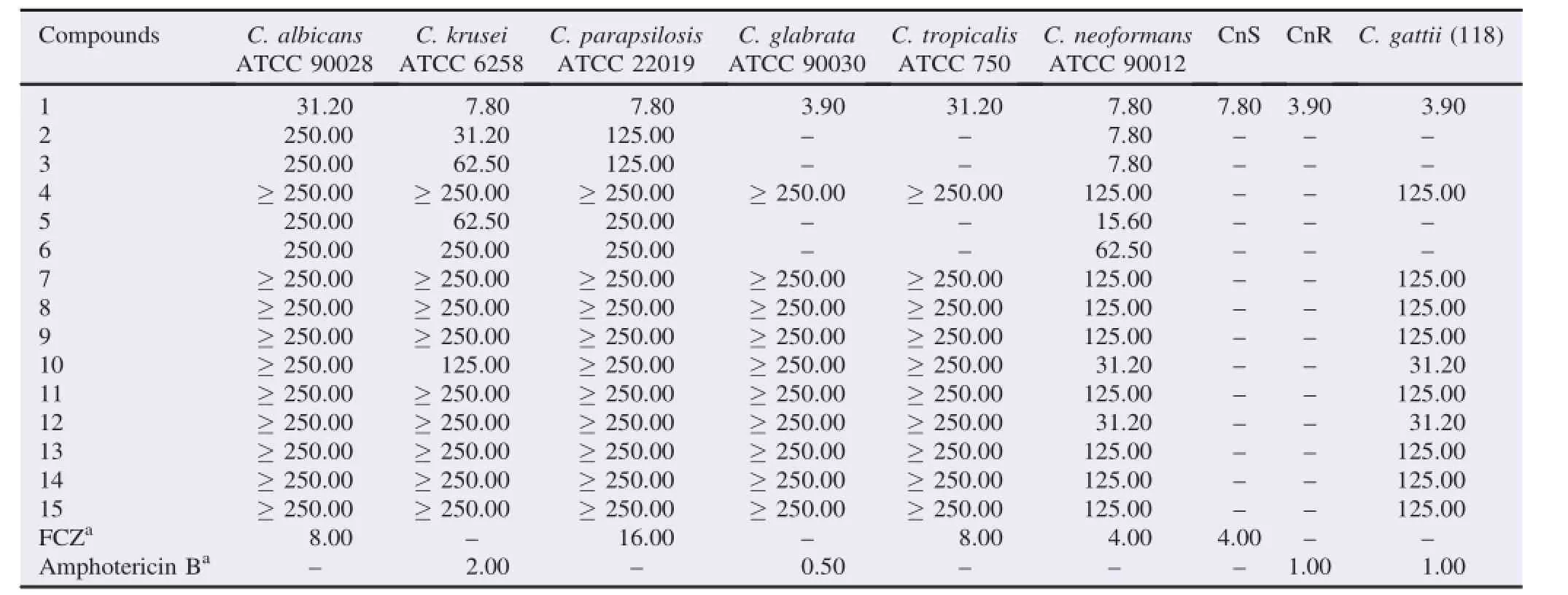
Table 1 MIC values of non-alkaloidal compounds(1-15)from P.nitens.μg/mL.
Among phenolic acids(9-13),compounds 10 and 12 exhibited moderate anti-Cryptococcus activity(MIC=31.20μg/ mL)against C.neoformans(ATCC 90012)and C.gattii(118). Compounds 8,14 and 15 were not active against both yeasts species(MIC≥125μg/mL).
4.Discussion
MIC values of compounds 1-3 corroborate antifungal potential of flavone derivatives,which have shown fungitoxicity against a broad spectrum of fungi species,including yeasts(Saccharomyces cerevisiae),halohyphomycetes(Aspergillus)and dermatophytes(Trichophyton and Epidermophyton)[38-40]. Nevertheless,our results to compound 1 were significantly opposite to those described by Taleb-Contini et al.,who reported absent growth inhibition until 500 mg/mL against C.albicans ATCC 1023 and C.tropicalis(clinical isolate from oral cavity),by using well diffusion assay[41].This difference may be related to strain types,susceptibility tests and/or purity grade of compounds.
Reviewofliteraturedataonanti-Candidaactivityofcompounds 4,10 and 13 is conflicting.A commercial sample of compound 4 presented higher anti-C.albicans activity(MIC=8μg/mL)than isolate samples from Buddleja salviifolia(MIC=125μg/mL)and Halimodendron halodendron(MIC=250μg/mL)[42-44].Similar behavior was observed for commercial gallic acid(MIC=8μg/ mL)in comparison to the one obtained from Lythrum salicaria(MIC=2500μg/mL),Paeonia rockii(MIC=30μg/mL)and Pelargonium reniforme subsp.reniforme(MIC=500μg/mL)[42,45-47].Also,commercialsampleofcompound10(MIC=20μg/mL)was quite different to sample obtained from Halimodendron halodendron(MIC=200μg/mL)[44,48].Our anti-C.albicans MIC value data were more similar to plant isolates than commercial samples.
Commercial samples of compounds 9 and 12 displayed MIC valuesof8and16μg/mL againstC.albicansandC.parapsilosis,respectively[42].Incontrast,ourMICvaluedataforcompounds9 and 12 against both yeasts were equal or superior to 250μg/mL. Compound 7 from P.nitens was not able to exhibit anti-C.albicans activity(MIC≥250μg/mL),on the other hand,rutincommercialsampledisplayedthepotenteffect(MIC=40μg/mL)[48].Martins et al.suggested that these differences could be probably assigned to purity grade of tested compounds[49].Additionally,we inferred this difference may be correlated to strain types.
In summary,15 non-alkaloidal compounds from P.nitens were evaluated against Candida and Cryptococcus species.Of these,compound 1 may be considered suitable template for design of innovative hits for the treatment of opportunistic yeast infections,including candidiasis and cryptococcosis.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This work was supported by the Support Foundation of São Paulo Research(FAPESP),National Council of Technological and Scientific Development(CNPq),and Office of Research of the São Paulo State University.The authors thank the Support Foundation of São Paulo Research(FAPESP),for fellowship to Sprengel Lima(Proc.2014/05445-3).
[1]de Oliveira RB,Atobe JH,Souza SA,de Castro Lima Santos DW. Epidemiology of invasive fungal infections in patients with acquired immunodeficiency syndrome at a reference hospital for infectious diseases in Brazil.Mycopathologia 2014;178(1-2):71-8.
[2]Vandeputte P,Ferrari S,Coste AT.Antifungal resistance and new strategies to control fungal infections.Int J Microbiol 2012;2012: 713687.
[3]Greenberg MS,Glick M.Burket's oral medicine:diagnosis and treatment.10th ed.Hamilton:BC Decker Inc.;2003.
[4]Sardi JC,Scorzoni L,Bernardi T,Fusco-Almeida AM,Mendes Giannini MJ.Candida species:current epidemiology,pathogenicity,biofilm formation,natural antifungal products and new therapeutic options.J Med Microbiol 2013;62(Pt 1):10-24.
[5]Silva F,Ferreira S,Duarte A,Mendonça DI,Domingues FC. Antifungal activity of Coriandrum sativum essential oil,its mode of action against Candida species and potential synergism with amphotericin B.Phytomedicine 2011;19(1):42-7.
[6]Bailly S,Maubon D,Fournier P,Pelloux H,Schwebel C,Chapuis C,et al.Impact of antifungal prescription on relative distribution and susceptibility of Candida spp.-trends over 10 years.J Infect 2016;72(1):103-11.
[7]Brunke S,Hube B.Two unlike cousins:Candida albicans and C.glabrata infection strategies.Cell Microbiol 2013;15(5):701-8.
[8]Gullo FP,Rossi SA,Sardi Jde C,Teodoro VL,Mendes-Giannini MJ,Fusco-Almeida AM.Cryptococcosis:epidemiology,fungal resistance,and new alternatives for treatment.Eur J Clin Microbiol Infect Dis 2013;32(11):1377-91.
[9]Khawcharoenporn T,Apisarnthanarak A,Mundy LM.Non-neoformans cryptococcal infections:a systematic review.Infection 2007;35(2):51-8.
[10]Perlin DS,Shor E,Zhao Y.Update on antifungal drug resistance. Curr Clin Microbiol Rep 2015;2(2):84-95.
[11]Butler MS,Robertson AA,Cooper MA.Natural product and natural product derived drugs in clinical trials.Nat Prod Rep 2014;31(11):1612-61.
[12]Abiodun OO,Sood S,Osiyemi OA,Agnihotri VK,Gulati A,Ajaiyeoba EO,et al.In vitro antimicrobial activity of crude ethanol extracts and fractions of Terminalia catappa and Vitex doniana.Afr J Med Med Sci 2015;44(1):21-6.
[13]Abubacker MN,Devi PK.In vitro antifungal potentials of bioactive compound oleic acid,3-(octadecyloxy)propyl ester isolated from Lepidagathis cristata Willd.(Acanthaceae)inflorescence.Asian Pac J Trop Med 2014;7:S190-3.
[14]Lorenzi H.Brazilian trees:manual of identification and cultivation of arboreal plants.2nd ed.Nova Odessa:Plantarum;1998.
[15]Crivos M,Martínez MR,Pochettino ML,Remorini C,Sy A,Teves L.Pathways as“signatures in landscape”:towards an ethnography of mobility among the Mbya-Guaraní(Northeastern Argentina).J Ethnobiol Ethnomed 2007;3:2.
[16]Ferreira FG,Regasini LO,de Oliveira AM,Campos JAD,Silva DHS,Cavalheiro AJ,et al.Evaluation of mutagenicity and antimutagenicity of different fractions of Pterogyne nitens(Leguminosae),using Tradescantia pallida micronuclei assay.Rev Bras Farmacogn 2009;19:61-7.
[17]Regasini LO,de Oliveira CM,Vellosa JCR,de Faria Oliveira OM,Siqueira Silva DH,da Silva Bolzani V.Free radical scavenging activity of Pterogyne nitens Tul.(Fabaceae).Afr J Biotechnol 2008;7(24):4609-13.
[18]Regasini LO,Fernandes DC,Castro-Gamboa I,Siqueira Silva DH,Furlan M,da Silva Bolzani V,et al.Chemical constituents of the flowers of Pterogyne nitens(Caesalpinioideae).Quim Nova 2008;31(4):802-6.
[19]RegasiniLO,Vieira-JúniorGM,FernandesDC,daSilva Bolzani VS,Cavalheiro AJ,Siqueira Silva DH.Identification of triterpenes and sterols from Pterogyne nitens(Fabaceae-Caesalpinioideae)using high-resolution gas chromatography.J Chil Chem Soc 2009;54(3):218-21.
[20]Regasini LO,Castro-Gamboa I,Siqueira Silva DH,Furlan M,Barreiro EJ,Ferreira PMP,et al.Cytotoxic guanidine alkaloids from Pterogyne nitens.J Nat Prod 2009;72(3):473-6.
[21]Tajima Y,Nakagawa H,Tamura A,Kadioglu O,Satake K,Mitani Y,et al.Nitensidine A,a guanidine alkaloid from Pterogyne nitens,is a novel substrate for human ABC transporter ABCB1. Phytomedicine 2014;21(3):323-32.
[22]Tajima Y,Murase H,Satake K,Mitani Y,Regasini LO,da Silva Bolzani V,et al.Nitensidine A,a guanidine alkaloid from Pterogyne nitens,induces osteoclastic cell death.Cytotechnology 2015;67:585-92.
[23]Duarte RA,Mello ER,Araki C,Bolzani Vda S,Siqueira e Silva DH,Regasini LO,et al.Alkaloids extracted from Pterogyne nitens induce apoptosis in malignant breast cell line.Tumour Biol 2010;31(5):513-22.
[24]Coqueiro A,Regasini LO,Stapleton P,da Silva Bolzani V,Gibbons S.In vitro antibacterial activity of prenylated guanidine alkaloids from Pterogyne nitens and synthetic analogues.J Nat Prod 2014;77(8):1972-5.
[25]Siqueira MC,Silva MTA,Regasini LO,Silva DHS,Cicarelli RMB. Trypanocidal activity of pterogynidine and nitensidine E using two distinct Trypanosoma cruzi strains.Planta Med 2009;75:945.
[26]Fernandes DC,Regasini LO,Vellosa JC,Pauletti PM,Castro-Gamboa I,Bolzani VS,et al.Myeloperoxidase inhibitory and radical scavenging activities of flavones from Pterogyne nitens. Chem Pharm Bull(Tokyo)2008;56(5):723-6.
[27]Regasini LO,Vellosa JC,Silva DH,Furlan M,de Oliveira OM,Khalil NM,et al.Flavonols from Pterogyne nitens and their evaluation as myeloperoxidase inhibitors.Phytochemistry 2008;69(8):1739-44.
[28]Okumura LL,Regasini LO,Fernandes DC,da Silva DH,Zanoni MV,Bolzani Vda S.Fast screening for antioxidant properties of flavonoids from Pterogyne nitens using electrochemical methods.J AOAC Int 2012;95(3):773-7.
[29]Vellosa JC,Regasini LO,Bell´o C,Schemberger JA,Khalil NM,de Araújo Morandim-Giannetti A,et al.Preliminary in vitro and ex vivo evaluation of afzelin,kaempferitrin and pterogynoside action over free radicals and reactive oxygen species.Arch Pharm Res 2015;38:1168-77.
[30]Regasini LO,Pivatto M,Scorzoni L,Benaducci T,Fusco-Almeida AM,Giannini MJS,et al.Antimicrobial activity of Pterogyne nitens Tul.,Fabaceae,against opportunistic fungi.Rev Bra Farmacogn 2010;20(5):706-11.
[31]Regasini LO,Lopes AA,Silva DHS,Furlan M,Young MCM,Maria DA,et al.Antiproliferative effect of Pterogyne nitens on melanoma cells.Rev Ciˆenc Farm B´asica Apl 2007;28:335-40.
[32]Mahato SB,Kundu AP.13C NMR spectra of pentacyclic triterpenoids-a compilation and some salient features.Phytochemistry 1994;37(6):1517-75.
[33]Matsumoto MT,Fusco-Almeida AM,Baeza LC,Melhem Mde S,Medes-Giannini MJS.Genotyping,serotyping and determination of mating-type of Cryptococcus neoformans clinical isolates from São Paulo State,Brazil.Rev Inst Med Trop São Paulo 2007;49(1): 41-7.
[34]Raso TF,Werther K,Miranda ET,Mendes-Giannini MJ.Cryptococcosis outbreak in psittacine birds in Brazil.Med Mycol 2004;42(4):355-62.
[35]Clinical and Laboratory Standards Institute.Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. 2nded.Wayne,PA:ClinicalandLaboratoryStandardsInstitute;2008.
[36]de Paula E Silva AC,Costa-Orlandi CB,Gullo FP,Sangalli-Leite F,de Oliveira HC,da Silva Jde F,et al.Antifungal activity of decyl gallate against several species of pathogenic fungi.Evid Based Complement Altern Med 2014;2014:506273.
[37]de Aguiar Cordeiro R,Mourão CI,Rocha MF,de Farias Marques FJ,Teixeira CE,de Oliveira Miranda DF,et al.Antifolates inhibit Cryptococcus biofilms and enhance susceptibility of planktonic cells to amphotericin B.Eur J Clin Microbiol Infect Dis 2013;32(4):557-64.
[38]Hamada Y,Takano S,Ayano Y,Tokunaga M,Koashi T,Okamoto S,et al.Structure-activity relationship of oligomeric flavan-3-ols:importance of the upper-unit b-ring hydroxyl groups in the dimeric structure for strong activities.Molecules 2015;20(10):18870-85.
[39]Cao H,Chen X,Jassbi AR,Xiao J.Microbial biotransformation of bioactive flavonoids.Biotechnol Adv 2015;33(1):214-23.
[40]Singh G,Kumar P,Joshi SC.Treatment of dermatophytosis by a new antifungal agent‘apigenin'.Mycoses 2014;57(8):497-506.
[41]Taleb-Contini SH,Schorr K,Da Costa FB,de Oliveira DCR. Detection of flavonoids in glandular trichomes of Chromolaena species(Eupatorieae,Asteraceae)by reversed-phase high-performance liquid chromatography.Rev Bras Ciˆenc Farm 2007;43(2): 315-21.
[42]Ozçelik B,Kartal M,Orhan I.Cytotoxicity,antiviral and antimicrobial activities of alkaloids,flavonoids,and phenolic acids. Pharm Biol 2011;49(4):396-402.
[43]Pendota SC,Aderogba MA,Ndhlala AR,Van Staden J.Antimicrobial and acetylcholinesterase inhibitory activities of Buddleja salviifolia(L.)Lam.leaf extracts and isolated compounds. J Ehnopharmacol 2013;148(2):515-20.
[44]Wang J,Lou J,Luo C,Zhou L,Wang M,Wang L.Phenolic compounds from Halimodendron halodendron(Pall.)voss and their antimicrobial and antioxidant activities.Int J Mol Sci 2012;13(9):11349-64.
[45]Manayi A,Saeidnia S,Faramarzi MA,Samadi N,Jafari S,Vazirian M,et al.A comparative study of anti-Candida activity and phenolic contents of the calluses from Lythrum salicaria L.in different treatments.Appl Biochem Biotechnol 2013;170(1):176-84.
[46]PicernoP,MencheriniT,SansoneF,DelGaudioP,GranataI,PortaA,et al.Screening of a polar extract of Paeonia rockii:composition and antioxidant and antifungal activities.J Ethnopharmacol 2011;138(3):705-12.
[47]Lattˊe KP,Kolodziej H.Antifungal effects of hydrolysable tannins and related compounds on dermatophytes,mould fungi and yeasts. Z Naturforsch C 2000;55(5-6):467-72.
[48]Siler B,Zivkoviˊc S,Banjanac T,Cvetkoviˊc J,Nestoroviˊc ˆZivkoviˊc J,Ciriˊc A,et al.Centauries as underestimated food additives:antioxidant and antimicrobial potential.Food Chem 2014;147:367-76.
[49]Martins N,Barros L,Henriques M,Silva S,Ferreira ICFR.Activity of phenolic compounds from plant origin against Candida species. Ind Crop Prod 2015;74:648-70.
19 Nov 2015
Luis Octavio Regasini,Laboratory of Green and Medicinal Chemistry,Department of Chemistry and Environmental Sciences,Institute of Biosciences,Letters and Exact Sciences,São Paulo State University(UNESP),São Josˊe do Rio Preto,Sao Paulo,Brazil.
Tel:+55 17 3221 2362
E-mail:regasini@ibilce.unesp.br
Foundation Project:Supported by Support Foundation of São Paulo Research(FAPESP,Grant No.2014/05445-3),National Council of Technological and Scientific Development(CNPq),and Office of Research of the São Paulo State University.
Peer review under responsibility of Hainan Medical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Hainan Medical University.This is an open access article under the CC BYNC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Pacific Journal of Tropical Biomedicine2016年10期
Asian Pacific Journal of Tropical Biomedicine2016年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A review on promising phytochemical,nutritional and glycemic control studies on Moringa oleifera Lam.in tropical and sub-tropical regions
- A rare cause of acute abdomen-Spontaneous rectus sheath hematoma
- Evaluation of proline,chlorophyll,soluble sugar content and uptake of nutrients in the German chamomile(Matricaria chamomilla L.)under drought stress and organic fertilizer treatments
- ProductionofsecondarymetaboliteE2.2fromPhaleriamacrocarpaendophyticfungus
- Cytotoxic,genotoxic and apoptotic effects of naringenin-oxime relative to naringenin on normal and cancer cell lines
- Pandanusamaryllifoliusleafextractincreasesinsulinsensitivityinhigh-fatdiet-induced obese mice
