The anti-tubercular activity of Melia azedarach L.and Lobelia chinensis Lour.and their potential as effective anti-Mycobacterium tuberculosis candidate agents
Won Hyung Choi,In Ah Lee
1Department of Biomedical Science,School of Medicine,Kyung Hee University,26 Kyunghee-daero,Dongdaemun-gu,Seoul 130-701,Republic of Korea
2Department of Medical Zoology,School of Medicine,Kyung Hee University,26 Kyunghee-daero,Dongdaemun-gu,Seoul 130-701,Republic of Korea
3Department of Chemistry,College of Natural Science,Kunsan National University,558 Daehak-ro,Gunsan-si,Jeonbuk
573-701,Republic of Korea
The anti-tubercular activity of Melia azedarach L.and Lobelia chinensis Lour.and their potential as effective anti-Mycobacterium tuberculosis candidate agents
Won Hyung Choi1,2*,In Ah Lee3
1Department of Biomedical Science,School of Medicine,Kyung Hee University,26 Kyunghee-daero,Dongdaemun-gu,Seoul 130-701,Republic of Korea
2Department of Medical Zoology,School of Medicine,Kyung Hee University,26 Kyunghee-daero,Dongdaemun-gu,Seoul 130-701,Republic of Korea
3Department of Chemistry,College of Natural Science,Kunsan National University,558 Daehak-ro,Gunsan-si,Jeonbuk
573-701,Republic of Korea
ARTICLE INFO
Article history:
Lobelia chinensis Lour. Melia azedarach L.
MGIT 960 system
Drug susceptibility
Tuberculosis
Objective:Toevaluatetheanti-mycobacterialactivityofMeliaazedarachL.(M.azedarach)and Lobelia chinensis Lour.(L.chinensis)extracts against the growth of Mycobacterium tuberculosis(M.tuberculosis).
Methods:The anti-M.tuberculosis activity of M.azedarach and L.chinensis extracts were evaluated using different indicator methods such as resazurin microtiter assay(REMA)and mycobacteria growth indicator tube(MGIT)960 system assay.The M.tuberculosis was incubated with various concentrations(50-800μg/mL)of the extracts for 5 days in the REMA,and for 4 weeks in MGIT 960 system assay.
Results:M.azedarach and L.chinensis extracts showed their anti-M.tuberculosis activity by strongly inhibiting the growth of M.tuberculosis in a concentration-dependent manner in the REMA and the MGIT 960 system assay.Particularly,the methanol extract of M.azedarach and n-hexane extract of L.chinensis consistently exhibited their effects by effectively inhibiting the growth of M.tuberculosis in MGIT 960 system for 4 weeks with a single-treatment,indicating higher anti-M.tuberculosis activity than other extracts,and their minimum inhibitory concentrations were measured as 400μg/mL and 800μg/ mL,respectively.
Conclusions:These results demonstrate that M.azedarach and L.chinensis extracts not only have unique anti-M.tuberculosis activity,but also induce the selective anti-M.tuberculosiseffectsbyconsistentlyinhibitingorblockingthegrowthof M.tuberculosis through a new pharmacological action.Therefore,this study suggests the potential of them as effective candidate agents of next-generation for developing a new anti-tuberculosis drug,as well as the advantage for utilizing traditional medicinal plants as one of effective strategies against tuberculosis.
Original articlehttp://dx.doi.org/10.1016/j.apjtb.2016.08.007
1.Introduction
Recently chronic infectious diseases including tuberculosis,hepatitis,filariasis,leishmaniasis,amoebiasiscausedby Entamoeba histolytica,as well as acute infectious zoonosis such as malaria,Middle East respiratory syndrome,influenza and Ebola,have been occurred or persisted in various countries,particularly,in less-developed countries globally.Furthermore,the prevalence of tuberculosis has still been causing a serious globalchallengeofdrug-resistanttuberculosissuchasextensively drug-resistant tuberculosis(XDR-TB)and multi-drug resistant tuberculosis(MDR-TB)despite various efforts and studies worldwide.Mycobacterium tuberculosis(M.tuberculosis)is one of the major infectious factors causing the highest mortality through the co-infection with HIV/AIDS as well as the most dangerous infectious bacteria causing resistant strain through fastinfectivity and the adaptability against environmental variation. Recently,it was estimated that 9.0 million people in the world wereconfirmedasnewtuberculosiscasesin2013,and1.1million of them had HIV-positive response.Particularly,tuberculosis cases co-infected with HIV/AIDS showed high-infection rates in the African region compared with other countries,and most of tuberculosis cases in 2013 and 2014 occurred in Asia(56%)and theAfrican region(29%)[1,2].Intheseaspects,variousstudiesfor the inhibition and/or the treatment of tuberculosis have been carried out in anti-tuberculosis drug discovery/development field.However,the current anti-tuberculosis drugs not only showed the limit that can't inhibit the increase of the tuberculosis patients,but also caused various side effects.Recently,the global pharmaceuticalcompanies forthe developmentof novel/effective anti-tuberculosis drugs are testing the repurposed compounds such as PA-824,SQ109 and linezolid through chemical remodeling of the existing drugs,as well as newly developed compounds such as Q203,TMC207,pyridomycin,and thiophenes in clinical trials[1,3-7].Nevertheless,the rapid emergence of tuberculosis including MDR-TB and XDR-TB is still inducing serious concerns and problems in the public health field worldwide.Recently,various extracts and/or natural products derived from medicinal plants or traditional oriental medicine were studied or reported for discovering novel anti-M.tuberculosis candidate drugs,which are being progressed for developing antituberculosis drugs of more effective/safe next-generation[8-13]. Furthermore,various medicinal plants of oriental medicine have been utilized as traditional resources for treating diseases and/or symptoms such as diabetes,arteriosclerosis,hepatitis,parasite,cancer both“in vivo”and“in vitro”[14-18].
For these reasons,various studies for developing the effective/safe anti-tuberculosis drugs with novel mechanisms of action,low cytotoxicity,and the least side-effects are urgently needed to block the tuberculosis.In this aspect,this study was carried out to evaluate anti-M.tuberculosis effect of Melia azedarach L.(M.azedarach)and Lobelia chinensis Lour.(L.chinensis)that are effectively utilized as a medicinal plant in traditional oriental medicine,and to identify the potential of them as candidates for developing novel antitubercular drugs.
2.Materials and methods
2.1.Materials
Various drugs used in this study including rifampicin,isoniazid,resazurin powder and dimethylsulfoxide,were purchased from Sigma-Aldrich Chemical Co.Ltd.(St.Louis,MO,USA),and MGIT™960 system indicator 7 mL growth tubes with BACTEC™MGIT™960 supplement kit were purchased from Becton-Dickinson and Company(Sparks,MD,USA).All other chemicals and reagents were purchased from Merck Chemical Co.,Ltd.(Darmstadt,Germany)and Sigma-Aldrich Chemical Co.,Ltd.(St.Louis,MO,USA).
2.2.Preparation and extracts of medicinal plants
The dried roots and barks of M.azedarach and dried roots,stem,leaves and flowers of L.chinensis were provided by the Oriental Medical Center,Kyung Hee University(Seoul,Republic of Korea)and Bundang Oriental Hospital,Dongguk University(Seoul,Republic of Korea)for this study.The M.azedarach and L.chinensis were extracted according to solvent extraction system as follows:Five hundred grams of each powdered M.azedarach and L.chinensis were independently extracted with 4 L of n-hexane,chloroform,ethyl acetate,methanol,and distilled water at room temperature for 24 h,respectively.These extracts were filtered using filter paper and a vacuum pump,and then were evaporated under reduced pressure using a rotary evaporator in a vacuum at 45°C.The extracts were lyophilized after evaporation.All extracts were filtered using a 0.45μm syringe filter(Roshi Kaisha,Ltd.,Tokyo,Japan)and stored at-80°C until use.
2.3.Preparation of anti-M.tuberculosis drugs
The anti-M.tuberculosis first-line drugs,isoniazid,was dissolved in sterile distilled water,and rifampicin was dissolved in dimethylsulfoxide,toaconcentrationof50mg/mLaccordingtothe manufacturer'sinstruction.Theanti-M.tuberculosisfirst-linedrugs wereusedasreferencestandarddrugs.Allcompoundswerefiltered using0.45μmmembranesyringefilter(RoshiKaisha,Ltd.,Tokyo,Japan)before use and stored at-80°C deep-freezer until use.
2.4.PreparationandgrowthconditionsofM.tuberculosis
M.tuberculosis H37Rv(ATCC 27294)and H37Ra(ATCC 25177)used in this study were purchased from American Type CultureCollection(Manassas,VA,USA).M.tuberculosisH37Rv and H37Ra were grown in Middlebrook 7H9 broth(Difco Laboratories,Detroit,MI,USA)supplemented with 10%(v/v)oleic acid/albumin/dextrose/catalase enrichment(Becton-Dickinson and Company,Sparks,MD,USA)and 0.05%(v/v)Tween 80(Sigma-Aldrich Chemical,St.Louis,MO,USA)to the log phase at 37°C for 4-5 weeks on shaking incubation of 120 r/min.
2.5.Drug susceptibility testing of M.tuberculosis
The in vitro anti-M.tuberculosis activity of M.azedarach and L.chinensis extracts was confirmed by resazurin microtiter assay(REMA)usinga96-wellmicro-plate.Briefly,M.tuberculosis was grown in fresh Middlebrook 7H9 broth supplemented with 10%(v/v)oleic acid/albumin/dextrose/ catalase enrichment and 0.05%(v/v)Tween 80 until the culture reached a turbidity equal to that of 1.0 McFarland standard(3.0×108CFU/mL)at 37°C.The bacteria were adjusted to a density of 2×106CFU/mL in fresh culture broth.Finally,the bacterial suspensions were inoculated into all wells of a 96-well microtiter plate containing final concentrations(50-200μg/mL)of M.azedarach and L.chinensis extracts and anti-tuberculosis first-line drugs(1.25-5.00μg/mL).M.tuberculosis growth controls containing no anti-M.tuberculosis first-line drugs and blank controls without inoculation were also included.The 96-well plates,covered with lids,placed in a plastic bag,were incubated at 37°C for 5 days.After incubation,20μL of freshly prepared 0.05%(w/v)resazurin solution was added to all wells,and the plates were re-incubated at 37°C for 36 h.A change in color from blue to pink indicating bacterial growth was observed after 36 h of incubation.The minimum inhibitory concentration(MIC)was expressed as the lowest concentration of the drug that inhibited M.tuberculosis growth or prevented change in color of the resazurin solution from blue to pink based on a REMA.
2.6.Evaluation of drug susceptibility of M.tuberculosis by MGIT 960 system assay
For evaluate anti-M.tuberculosis effects of M.azedarach and L.chinensis extracts against the growth of M.tuberculosis,the drug susceptibility testing of the strain was further performed using the BACTEC™MGIT 960 system(Becton,Dickinson and Company,Sparks,MD,USA).In brief,100μL of a suspension of M.tuberculosis culture,adjusted to 9.6×106CFU/ mL,was inoculated in an MGIT growth media tube with BACTEC™MGIT 960 growth supplement(Becton,Dickinson and Company),which were incubated with different concentrations(25-800μg/mL)of the extracts and anti-M.tuberculosis first-line drugs(10μg/mL),and M.tuberculosis growth controls containing no anti-M.tuberculosis first-line drugs were also included.They were incubated into the BACTEC™MGIT 960 system device for 4 weeks for the accurate determination of M.tuberculosis drug-susceptibility.
2.7.Statistical analysis
All results were expressed as mean±SD of three independent experiments.Statistical analysis of the data was performed using the Student's t-test and One-way ANOVA.P<0.05 was considered to be statistically significant.
3.Results
3.1.Evaluation of anti-M.tuberculosis activity of the extracts using REMA
The anti-M.tuberculosis activity of M.azedarach and L.chinensis extracts were evaluated using the REMA.The M.azedarach and L.chinensis were independently extracted using different solvents at room temperature for 24 h,respectively(Figure 1).After the bacteria were incubated with various concentrations(50-200μg/mL)of the extracts and anti-M.tuberculosis first-line drugs(rifampicin and isoniazid,1.25-5.00μg/mL)for 5 days,the growth of M.tuberculosis were markedly inhibited in a concentration-dependent manner,and then their viabilities were 0%at a concentration of 100μg/mL of all extracts(Table 1).The minimal inhibitory concentration(MIC)valuesofallextractsagainsttheviabilityof M.tuberculosis were measured as 100μg/mL(Table 2). Furthermore,theextractsobviouslydemonstratedtheir M.tuberculosis-inhibitory effect through the REMA.Although the superiority and specificity between the extracts regarding anti-M.tuberculosis effect were not confirmed in the REMA,the extracts showed similar anti-M.tuberculosis activity in both M.tuberculosis H37Ra and M.tuberculosis H37Rv.These results indicate that M.azedarach and L.chinensis extracts have not only anti-M.tuberculosis effects by strongly inhibiting the growth of M.tuberculosis but also the potential that can beutilized or developed as promising anti-M.tuberculosis candidate agents through their new pharmacological activity.
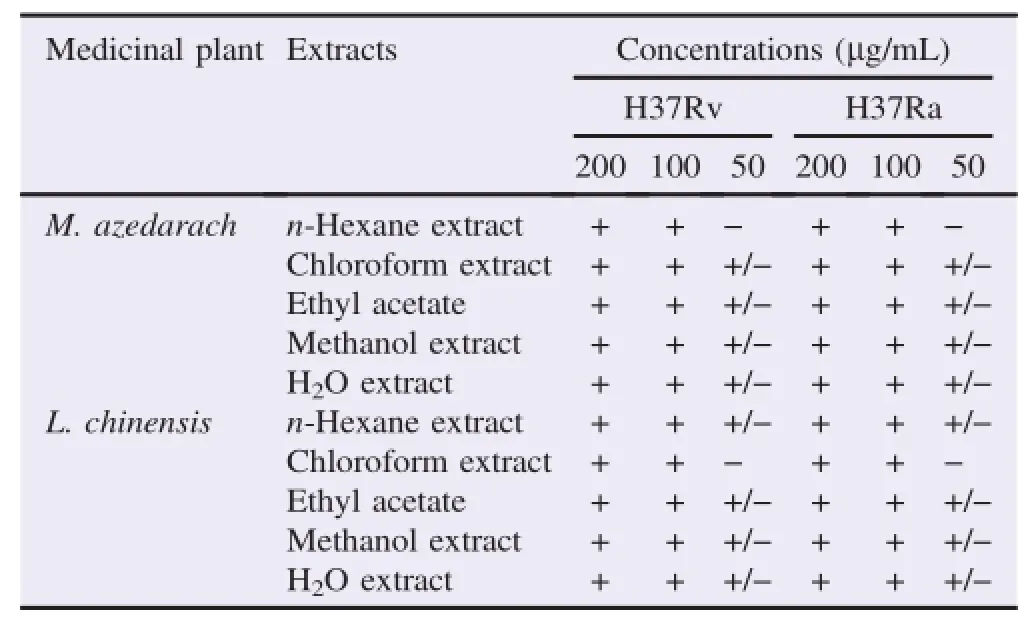
Table 1 The inhibitory effects of M.azedarach and L.chinensis extracts against the growth of M.tuberculosis(H37Rv and H37Ra)determined by the REMA.
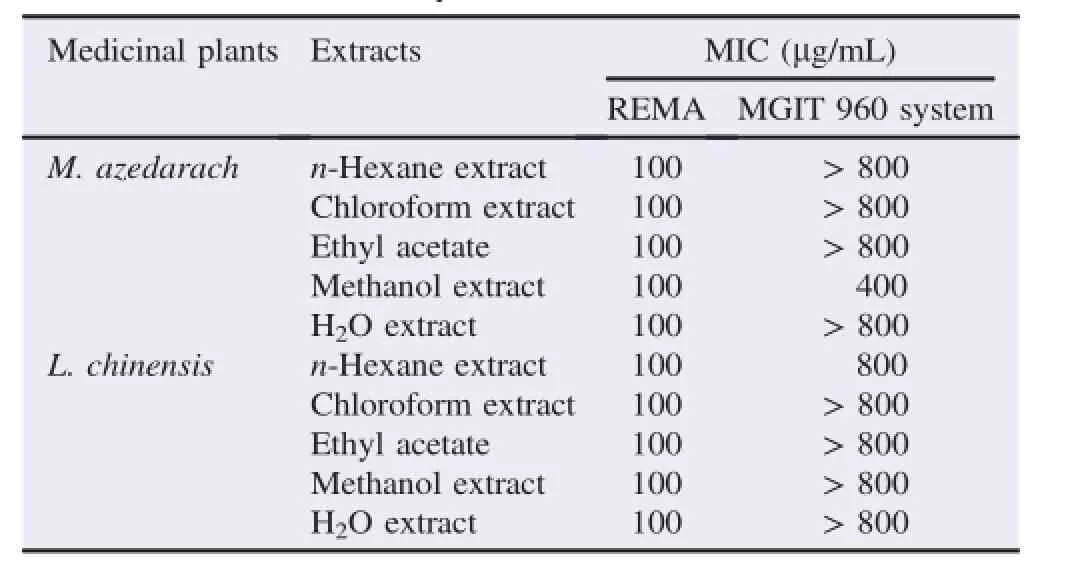
Table 2MICs of M.azedarach and L.chinensis extracts against the growth of M.tuberculosis(H37Rv and H37Ra)determined by different anti-M.tuberculosis indicator assays.
Figure 1.Extraction process of M.azedarach(A)and L.chinensis(B).
3.2.Anti-M.tuberculosis effects of the extracts using the MGIT 960 system assay
The anti-M.tuberculosis effects of the extracts were further evaluated using the MGIT 960 system assay.The bacteria were incubated with various concentrations(25-800μg/mL)of the extracts and anti-M.tuberculosis first-line drugs(10μg/mL)in an MGIT growth media tube of the BACTEC™MGIT 960 system device for drug susceptibility testing for 4 weeks,and their growth units were markedly inhibited in a concentrationdependentmanner(Figure2).Particularly,themethanol extract of M.azedarach and n-hexane extract of L.chinensis strongly inhibited the growth of M.tuberculosis compared with other extracts.When M.tuberculosis was incubated with 200μg/mL of the methanol extract of M.azedarach,their growth units were detected at about 8.7 days,whereas 400μg/ mL of the methanol extract strongly inhibited the growth of M.tuberculosis compared with other extracts,and the growth unit of M.tuberculosis was not detected until 28th day. Furthermore,when M.tuberculosis was incubated with 400μg/ mL of the n-hexane extract of L.chinensis,their growth units were detected at 20.6 days,whereas 800μg/mL of the n-hexane extract strongly inhibited the growth of M.tuberculosis,and the growth unit of M.tuberculosis was not detected until 28th day. In this MGIT 960 system assay,the methanol extract of M.azedarach and the n-hexane extract of L.chinensis consistently inhibited the growth of M.tuberculosis for 4 weeks with a single treatment(400μg/mL and 800μg/mL)respectively,and they showed more effectively the selective anti-M.tuberculosis activity as well as anti-M.tuberculosis action compared with other extracts.In addition,the extracts indicated higher activity against M.tuberculosis H37Ra than M.tuberculosis H37Rv in the MGIT 960 system assay(Figure 2).Particularly,the differences in anti-M.tuberculosis activity of them were obviously confirmed in the MGIT 960 system assay compared with the REMA assay(Table 2).These results demonstrate that the extracts not only induce anti-M.tuberculosis activity causing the inactivation of M.tuberculosis by effectively or consistently inhibiting the growth of M.tuberculosis,but also have unique anti-M.tuberculosis properties that inhibit or block the growth of M.tuberculosis in a concentration-dependent manner.
4.Discussion
The recent zoonotic diseases such as Middle East respiratory syndrome,avianinfluenzaandEbolahavebeenoccurredinvarious countries worldwide through diverse infectious pathways,particularly,in Asia,Middle East and Africa.Furthermore,the serious infectious pathogens such as influenza,M.tuberculosis,and methicillin-resistantStaphylococcusaureushavestrongly enhanced their infectivity and viability through resistance or adaptabilitytodrugsaswellasenvironmentalvariationforthepast decades,which induces serious difficulty to treatment and prevention of the disease as well as the development of the effective drug.In particular,these diseases have caused high risk symptoms to young children whose immune function is low and the elderly who have other underlying diseases or complications compared with normal adults.In this aspect,tuberculosis is a disease to cause infection through droplets,which has been causing high mortality and prevalence rate compared with other contagious diseases worldwide.Tuberculosis was one of the leading causes of death among various infectious diseases in 2013 and 2014 globally,particularly,in Africa[1,2].It is the most dangerous infectious disease causing complications such as bronchiectasis,emphysema,and pneumothorax through chronic symptom.In addition,the rapid increase and appearance of XDR-TB/MDR-TB imply an urgent need to develop anti-tuberculosis drugs of effective/safe next-generation for treating tuberculosis.Until recently,despite various efforts to discover and to develop new anti-tuberculosis drugs,the development of effective/safe anti-tuberculosis drugs is still facing with direct or indirect-difficulty including the cost or time as well as the economic aspect and the profitability of pharmaceuticals.Furthermore,the overuse of antibiotics and antibacterial drugs has rapidly increased the prevalence and incidencerate ofresistant-tuberculosissuchasXDR-TBandMDR-TB,whichhas causedmore difficulty to treat tuberculosis-patients comparedwith the past.In addition,the current anti-tuberculosis drugs induce various/serious side effects including hepatotoxicity,ototoxicity,andnephrotoxicity.Inthisaspect,asoneoffeasiblealternativesfor overcoming limitations of the drug's side effects,particularly,including rifampicin,isoniazid,ethambutol and moxifloxacin,various studies for developing anti-tuberculosis drugs were reported from active substances derived from traditional diverse medicinal plants and biological resources[19-23].However,antituberculosis agents of effective/safe next-generation that can be utilized or usedas potential/novel first-line anti-tuberculosis drugs,particularly,the extracts,natural products,and/or semi-synthetic compounds,have not yet been reported in the global pharmaceutical market.
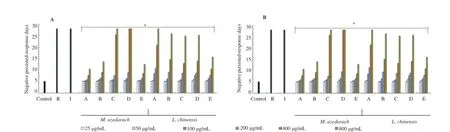
Figure 2.The anti-M.tuberculosis activity of M.azedarach and L.chinensis extracts against the growth of M.tuberculosis H37Rv(A)and H37Ra(B).
In this perspective,the new pharmacological activity of medicinal plants used in oriental medicine or traditional medicine can provide the advantages of both the safety and efficacy compared with newly developed drugs such as semi-synthetic compounds or biomedicine,which may be used or utilized as oneofeffective/feasiblestrategiesfordevelopingantituberculosis drugs of effective/safe next-generation.Moreover,the plants selected from the existing medicinal plants through new pharmacological action may increase the potential which can be utilized as a useful/feasible resource for developing new substances in a medical field.For these reasons,this study has been focused on major key points for the discovery of new candidate substances as one of different strategies for developing anti-tuberculosis drugs,which has included crucial factors such as the minimization of side effects and the safety of drugs,as well as the finding of novel pharmacological activity and function of the medicinal plants used in Korean traditional medicine. In these aspects,M.azedarach and L.chinensis are medicinal plants that are used as biological resources of traditional oriental medicine in Korea,Japan and China as well as various countries and regions globally,which include various bioactive substances.Until recently,pharmacological activity and function of them have been variously reported as follows:(1)the aqueous extracts from M.azedarach induce the effect of wound healing byactivatingthegrowthofkeratinocyte[24];(2)the pediculicidal activity of ethanol extracts of M.azedarach[25];(3)the leaves and bark extracts of M.azedarach through cyclooxygenase-2 and the inducible NO synthase inhibition cause anticancer activity and anti-inflammatory effects[26-28];(4)the anti-diabetic activity of ethanol extract of M.azedarach[29];(5)theanti-parasiticeffectofhexaneextractof M.azedarach against gastrointestinal nematodes[30];(6)the antibacterial effect and antifungal activity of the extracts of M.azedarach against pathogenic bacterial and fungus strains[31,32];(7)the methanol extracts and the compounds isolated fromL.chinensisinduceanti-oxidantactivityandantiinflammatory effects through the nuclear factor-kB pathways[33,34];(8)the anti-viral effects of L.chinensis extracts in mouse model infected by herpes simplex virus type 1[35];(9)the anticancer effects of the compounds isolated from L.chinensis against human lung cancer cells[36,37].
Taken together,these studies show substantial evidence that M.azedarach and L.chinensis can be used or utilized as therapeutic agents through their unique pharmacological activity and function in both“in vitro”and“in vivo”.However,despite the pharmacological activity or actions of them identified through these studies,their anti-tubercular activity have not yet been reported in both“in vitro”and“in vivo”.For this reason,this study has been begun from hypothesis that M.azedarach and L.chinensis may strongly inhibit or block and effectively modulate the growth of M.tuberculosis.As mentioned above,the results of this study showed novel anti-tubercular activity of M.azedarach and L.chinensis extracts by effectively inhibiting the growth of M.tuberculosis through their novel pharmacological activity and properties.Particularly,anti-M.tuberculosis activity and the ability of M.azedarach and L.chinensis extracts were obviously demonstrated through different M.tuberculosis susceptibility-indicator assays such as the REMA and MGIT 960 system assay,and they consistently showed their anti-M.tuberculosis activity by strongly inhibiting the growth of M.tuberculosis for 4 weeks with a single treatment(400μg/mL and 800μg/mL)in MGIT 960 system assay,respectively.In addition,the specificity and differences in anti-M.tuberculosis activity of them were obviously confirmed in the MGIT 960 system assay compared with the REMA assay,and the methanol extract of M.azedarach more effectively inhibited thegrowthofM.tuberculosiscomparedwiththoseof L.chinensis extracts.These results imply that the intracellular signaling-pathways for replication as well as key-proteins for regulating cell cycle in cytoplasm that accelerate the growth of M.tuberculosisand/orfunctionsofthecellwallof M.tuberculosis are strongly inhibited or deactivated by the binding of the extracts.Furthermore,the extracts showed higher anti-M.tuberculosis activity in M.tuberculosis H37Ra than M.tuberculosis H37Rv in the MGIT 960 system assay,and it suggests that these results can be associated with differences in pathogenesis and virulence of the two strains or different pathogenic phenotypes between H37Rv and H37Ra.
In conclusion,M.azedarach and L.chinensis extracts effectivelyinhibitedthegrowthofM.tuberculosisthatcancauseactive tuberculosis in those with weakened immune systems through their novel pharmacological activity or function,and their anti-M.tuberculosis activity were obviously demonstrated through different anti-M.tuberculosis indicator assays such as the MGIT 960 system and the REMA.These results showed that the effective use of M.azedarach and L.chinensis extracts can be utilized as potential anti-M.tuberculosis agents for consistently inhibiting or blocking tuberculosis causing various complications in clinical fields.Therefore,this study provides significant evidence and the potential that M.azedarach and L.chinensis extracts can be used or utilized as promising candidate substances for developing novel anti-tubercular drugs of the effective nextgeneration in the near future through their new pharmacological activity concerning anti-M.tuberculosis effect.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors would like to express sincere gratitude to the staffs of Kyung Hee University School of Medicine for providing research equipment and facilities during the course of the experiment.This study was supported by C&K pharmaceutical company in Korea.
[1]World Health Organization.Global tuberculosis report 2013. Geneva:World Health Organization;2013.
[2]World Health Organization.Global tuberculosis report 2014. Geneva:World Health Organization;2014.
[3]Koul A,Arnoult E,Lounis N,Guillemont J,Andries K.The challenge of new drug discovery for tuberculosis.Nature 2011;469(7331):483-90.
[4]Hartkoorn RC,Sala C,Neres J,Pojer F,Magnet S,Mukherjee R,et al.Towards a new tuberculosis drug:pyridomycin-nature's isoniazid.EMBO Mol Med 2012;4(10):1032-42.
[5]Lee M,Lee J,Carroll MW,Choi H,Min S,Song T,et al.Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med 2012;367(16):1508-18.
[6]Pethe K,Bifani P,Jang J,Kang S,Park S,Ahn S,et al.Discovery of Q203,a potent clinical candidate for the treatment of tuberculosis.Nat Med 2013;19(9):1157-60.
[7]Wilson R,Kumar P,Parashar V,Vilch`eze C,Veyron-Churlet R,Freundlich JS,et al.Antituberculosis thiophenes define a requirement for Pks13 in mycolic acid biosynthesis.Nat Chem Biol 2013;9(8):499-506.
[8]Luo X,Pires D,Aínsa JA,Gracia B,Duarte N,Mulhovo S,et al. Zanthoxylum capense constituents with antimycobacterial activity against Mycobacterium tuberculosis in vitro and ex vivo within human macrophages.J Ethnopharmacol 2013;146(1):417-22.
[9]Lu CH,Ye FW,Shen YM.Siderochelins with anti-mycobacterial activity from Amycolatopsis sp.LZ149.Chin J Nat Med 2015;13(1):69-72.
[10]Singh R,Hussain S,Verma R,Sharma P.Anti-mycobacterial screening of five Indian medicinal plants and partial purification of active extracts of Cassia sophera and Urtica dioica.Asian Pac J Trop Med 2013;6(5):366-71.
[11]Madikizela B,Ndhlala AR,Finnie JF,Staden JV.In vitro antimicrobial activity of extracts from plants used traditionally in South Africa to treat tuberculosis and related symptoms.Evid Based Complement Altern Med 2013;2013:840719.
[12]Luo X,Pires D,Aínsa JA,Gracia B,Mulhovo S,Duarte A,et al. Antimycobacterialevaluationandpreliminaryphytochemical investigation of selected medicinal plants traditionally used in Mozambique.J Ethnopharmacol 2011;137(1):114-20.
[13]Green E,Samie A,Obi CL,Bessong PO,Ndip RN.Inhibitory properties of selected South African medicinal plants against Mycobacterium tuberculosis.J Ethnopharmacol 2010;130(1):151-7.
[14]Ding PL,Liao ZX,Huang H,Zhou P,Chen DF.(+)-12alpha-Hydroxysophocarpine,a new quinolizidine alkaloid and related anti-HBV alkaloids from Sophora flavescens.Bioorg Med Chem Lett 2006;16(5):1231-5.
[15]Choi WH,Chu JP,Jiang MH,Baek SH,Park HD.Effects of fraction obtained from Korean Corni fructus extracts causing antiproliferation and p53-dependent apoptosis in A549 lung cancer cells.Nutr Cancer 2011;63(1):121-9.
[16]Choi WH,Jiang MH,Chu JP.Antiparasitic effects of Zingiber officinale(Ginger)extract against Toxoplasma gondii.J Appl Biomed 2013;11(1):15-26.
[17]Qiu LP,Chen KP.Anti-HBV agents derived from botanical origin. Fitoterapia 2013;84:140-57.
[18]Li T,Peng T.Traditional Chinese herbal medicine as a source of molecules with antiviral activity.Antivir Res 2013;97(1):1-9.
[19]Copp BR,Pearce AN.Natural product growth inhibitors of Mycobacterium tuberculosis.Nat Prod Rep 2007;24(2):278-97.
[20]Tandon R,Ponnan P,Aggarwal N,Pathak R,Baghel AS,Gupta G,et al.Characterization of 7-amino-4-methylcoumarin as an effectiveantitubercularagent:structure-activityrelationships. J Antimicrob Chemother 2011;66(11):2543-55.
[21]Saikia D,Parveen S,Gupta VK,Luqman S.Anti-tuberculosis activity of Indian grass KHUS(Vetiveria zizanioides L.Nash). Complement Ther Med 2012;20(6):434-6.
[22]Gemechu A,Giday M,Worku A,Ameni G.In vitro antimycobacterialactivityofselectedmedicinalplantsagainst Mycobacterium tuberculosis and Mycobacterium bovis strains. BMC Complement Altern Med 2013;13:291.
[23]Leitão F,Leitão SG,de Almeida MZ,Cantos J,Coelho T,da Silva PE.Medicinal plants from open-air markets in the State of Rio de Janeiro,Brazil as a potential source of new antimycobacterial agents.J Ethnopharmacol 2013;149(2):513-21.
[24]Alerico GC,Beckenkamp A,Vignoli-Silva M,Buffon A,von Poser GL.Proliferative effect of plants used for wound healing in Rio Grande do Sul state,Brazil.J Ethnopharmacol 2015;176:305-10.
[25]Rutkauskis JR,Jacomini D,Temponi LG,Sarragiotto MH,da Silva EA,Jorge TC.Pediculicidal treatment using ethanol and M.azedarach L.Parasitol Res 2015;114(6):2085-91.
[26]Pan X,Matsumoto M,Nishimoto Y,Ogihara E,Zhang J,Ukiya M,et al.Cytotoxic and nitric oxide production-inhibitory activities of limonoids and other compounds from the leaves and bark of Melia azedarach.Chem Biodivers 2014;11(8):1121-39.
[27]JafariS,SaeidniaS,HajimehdipoorH,ArdekaniMR,Faramarzi MA,Hadjiakhoondi A,et al.Cytotoxic evaluation of Melia azedarach in comparison with,Azadirachta indica and its phytochemical investigation.Daru 2013;21(1):37.
[28]Kim HW,Kang SC.The toxicity and anti-cancer activity of the hexane layer of Melia azedarach L.var.japonica Makino's bark extract.Toxicol Res 2012;28(1):57-65.
[29]Khan MF,Rawat AK,Pawar B,Gautam S,Srivastava AK,Negi DS.Bioactivity-guided chemical analysis of M.azedarach(Meliaceae),displaying antidiabetic activity.Fitoterapia 2014;98: 98-103.
[30]Cala AC,Chagas AC,Oliveira MC,Matos AP,Borges LM,Sousa LA,et al.In vitro anthelmintic effect of Melia azedarach and Trichilia claussenii C.against sheep gastrointestinal nematodes. Exp Parasitol 2012;130(2):98-102.
[31]Orhan IE,Guner E,Ozcelik B,Senol FS,Caglar SS,Emecen G,et al.Assessment of antimicrobial,insecticidal and genotoxic effects of Melia azedarach(chinaberry)naturalized in Anatolia.Int J Food Sci Nutr 2012;63(5):560-5.
[32]Khan AV,Ahmed QU,Mir MR,Shukla I,Khan AA.Antibacterial efficacy of the seed extracts of Melia azedarach against some hospital isolated human pathogenic bacterial strains.Asian Pac J Trop Biomed 2011;1(6):452-5.
[33]Li KC,Ho YL,Huang GJ,Chang YS.Anti-oxidant and antiinflammatory effects of Lobelia chinensis in vitro and in vivo. Am J Chin Med 2015;43(2):269-87.
[34]Kuo PC,Hwang TL,Lin YT,Kuo YC,Leu YL.Chemical constituents from Lobelia chinensis and their anti-virus and anti-inflammatory bioactivities.Arch Pharm Res 2011;34(5): 715-22.
[35]Kuo YC,Lee YC,Leu YL,Tsai WJ,Chang SC.Efficacy of orally administered Lobelia chinensis extracts on herpes simplex virus type 1 infection in BALB/c mice.Antivir Res 2008;80(2):206-12.
[36]Yang S,Shen T,Zhao L,Li C,Zhang Y,Lou H,et al.Chemical constituents of Lobelia chinensis.Fitoterapia 2014;93:168-74.
[37]Chen MW,Chen WR,Zhang JM,Long XY,Wang YT.Lobelia chinensis:chemical constituents and anticancer activity perspective.Chin J Nat Med 2014;12(2):103-7.
24 Feb 2016
Won Hyung Choi,Department of Biomedical Science,Department of Medical Zoology,Kyung Hee University School of Medicine,26 Kyunghee-daero,Dongdaemun-gu,Seoul 130-701,Republic of Korea.
Tel:+82 10 20715679
Fax:+82 70 82695679
E-mail:whchoi@khu.ac.kr
Peer review under responsibility of Hainan Medical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
Received in revised form 9 Apr 2016 Accepted 18 Jun 2016
Available online 26 Aug 2016
 Asian Pacific Journal of Tropical Biomedicine2016年10期
Asian Pacific Journal of Tropical Biomedicine2016年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A review on promising phytochemical,nutritional and glycemic control studies on Moringa oleifera Lam.in tropical and sub-tropical regions
- A rare cause of acute abdomen-Spontaneous rectus sheath hematoma
- Evaluation of proline,chlorophyll,soluble sugar content and uptake of nutrients in the German chamomile(Matricaria chamomilla L.)under drought stress and organic fertilizer treatments
- ProductionofsecondarymetaboliteE2.2fromPhaleriamacrocarpaendophyticfungus
- Cytotoxic,genotoxic and apoptotic effects of naringenin-oxime relative to naringenin on normal and cancer cell lines
- Pandanusamaryllifoliusleafextractincreasesinsulinsensitivityinhigh-fatdiet-induced obese mice
