Zika virus infection,transmission,associated neurological disorders and birth abnormalities∶A review of progress in research,priorities and knowledge gaps
Yitades Gebre,Nikkiah Forbes,Teshome Gebre
1Pan American Health Organization/World Health Organization,Country Office,Paramaribo,Suriname
2National HIV/AIDS and Infectious Disease Programme,Ministry of Health,Nassau,Bahamas
3International Trachoma Initiative,the Task Force for Global Health,Addis Ababa,Ethiopia
Zika virus infection,transmission,associated neurological disorders and birth abnormalities∶A review of progress in research,priorities and knowledge gaps
Yitades Gebre1,Nikkiah Forbes2,Teshome Gebre3*
1Pan American Health Organization/World Health Organization,Country Office,Paramaribo,Suriname
2National HIV/AIDS and Infectious Disease Programme,Ministry of Health,Nassau,Bahamas
3International Trachoma Initiative,the Task Force for Global Health,Addis Ababa,Ethiopia
ARTICLE INFO
Article history:
in revised form 15 Jul,2nd revised on 25 Jul 2016
Accepted 6 Aug 2016
Available online 26 Aug 2016
Zika virus
Zika virus transmission Vector
Microcephaly
Guillain-Barr´e syndrome Zika virus research
On February 1,2016,the World Health Organization declared that the cluster of microcephaly cases and other neurological disorders constitute public health emergency of international concern.Furthermore,few studies demonstrated that there was an increased evidence of causal relationship of Zika virus(ZIKAV)infection and microcephaly,birth abnormalities and neurological disorders such as Guillain-Barr´e syndrome. ZIKAV transmission occurs mainly by the bite of infected mosquitos(Aedes species),but there are also reports that infections could occur via the placenta,breast milk,saliva,blood transfusion and sex.This article reviews the global efforts,progress in scientific research to understand the pathogenesis of ZIKAV infection&disease,clinical presentations,congenital transmission and autoimmune neurological disorders.The paper further explores the knowledge gaps,future priority research agenda for strategic response including vector control and prevention.We conducted a systematic literature review to synthesise available evidence on ZIKAV infection and its vector and host interaction from electronic databases.
Review articlehttp://dx.doi.org/10.1016/j.apjtb.2016.08.008
1.Introduction
Zika virus(ZIKAV)is an emerging mosquito-borne flavivirus that was first identified in Uganda in 1947 and later found in humans with increasing outbreaks in Uganda,Yap Island,French Polynesia in 1952,2007,2013,respectively,and New Caledonia,Easter Islands and Cook Islands in 2014[1-4].
From 1 January 2007 to 4 May 2016,ZIKAV outbreaks were reported in a total of 57 countries and territories.In 44 countries these outbreaks were reported for the first time.Four of them(Cook Islands,French Polynesia,Isla de Pascua-Chile,and New Caledonia)reported a ZIKAV outbreak that is now over. Seven countries(Argentina,Chile,France,Italy,New Zealand, Peru and the US)have now documented locally acquired infections without any evidence indicating the presence of the presumptive vectors,probably through sexual transmission[5].
ZIKAV is primarily transmitted by the bite of infected mosquitoes,and the virus has been isolated from a number of Aedes mosquito species[6],notably Aedes aegypti(Ae.aegypti). The Ae.aegypti mosquito species are predominantly found in tropical and sub-tropical areas.Another potential transmitter,Aedes albopictus(Ae.albopictus),is entomologically well recognized in several parts of Europe,particularly in Mediterranean countries.Aedes polynesiensis is also incriminated as a possible contributor to ZIKAV transmission in the outbreak of French Polynesia.Other possible ways of ZIKAV transmission are detailed below.However there are several unknown facts and knowledge gaps about the pathogenesis,transmission,complications and treatment of ZIKAV infection.
This article discusses in detail the occurrence of ZIKAV as a public health threat,the progress and gaps in research related to the epidemiology of the virus,pathology,clinical presentations,knowledge,host and vector interaction,vector surveillance,transmission patterns,immunity and immunogenicity,genetic and molecular studies,the development of vaccines,newtherapeutics and new methods of vector control strategies.The paper reviews the current progress in research and the gaps by elucidating the association of complications of ZIKAV infections,clinical presentations,neurological disorders,and foetal/birth abnormalities such as microcephaly.
We conducted a systematic literature review to synthesise available evidence on ZIKAV infection and its vector and host interactions from electronic data repositories namely from PubMed,Medline,Scopus,JSTOR,of peer reviewed published articles from January 2000 to 2016 including situational reports of World Health Organization(WHO).Publication bias was minimized by searching reports from well recognized international public health agencies and the ZIKAV research database created by the Pan American Health Organization.The search words“Zika”,“ZIKAV”,and“Zika virus”were used.All authors independently screened and short listed abstracts using the search strategy.
2.ZIKAV epidemiology
2.1.Geographic distribution
ZIKAV was first isolated in 1947 from rhesus monkey in Uganda.The virus was later named after the geographic location,Zika Forest,where it was isolated[7].The virus was first confirmed as a cause of human disease when three persons became ill in Nigeria in 1953[8].Thereafter,ZIKAV was associated with sporadic infections and only 13 cases were documented over the next 57 years[9-11].It was thus astounding when the first major outbreak of ZIKAV was recognised in the Yap Islands of Micronesia in 2007 with approximately 5000 cases reported among a total population of 6700[2].French Polynesia also reported an outbreak of 32000 persons infected in 2013-2014[4,12-16].Subsequently,ZIKAV outbreaks occurred on other Pacific islands[4,15,16]. ZIKAV was first reported as causing an outbreak in the Western Hemisphere in 2014 in Chile's Easter Island[17].The virus was subsequently detected in Brazil in March 2015[6,18]. ZIKAV had spread to 14 Brazilian states by October 2015 and an estimated 1.3 million cases had occurred[19].Officials in the Pan American Health Organization on May 2016 estimated as many as 550 million people at risk of ZIKAV infection in the Americas.
Recent results of phylogenetic and molecular clock analyses point towards an introduction of ZIKAV in Brazil as early as May 2013[20].However,ZIKAV transmission was only confirmed in the north-eastern states of Brazil in May 2015[6].This event was followed by a rapid spread throughout the country,and subsequently to most of the countries in the Americas.In 2016,it was reported that an outbreak of ZIKAV was occurring in the Americas,the Pacific and the Caribbean[21-23].
In December 2014,an outbreak of ZIKAV infection was reported in Haiti rural community[24].In this outbreak,researchers were able to isolate the virus from three students at different locations in west of Port-au-Prince.The study with phylogenetic analysis suggested that the infection was wide spread in the communities.The viral sequencing showed genetic similarity and relations with ZIKAV from Brazil.Furthermore,the analysis showed the existence of at least three major African sub-lineages and established that the South American epidemic may have begun through the introduction of an initial ZIKAV outbreak in French Polynesia into Easter Island,then to the countries and territories in the Americas[24].
Similarly,in October 2015,the sera of four ZIKAV patients from Suriname were subjected for genomic sequencing and it was identified to be of the Asian genotype as opposed to the African lineage.The Suriname genotype shows over 99%protein and gene homology with the strain isolated from French Polynesia in 2013[25].
In 2016,numerous countries have reported autochthonous ZIKAV transmission including Aruba,Barbados,Belize,Bolivia,Bonaire,Brazil,Cape Verde,Colombia,Costa Rica,Cuba,Curacao,Dominica,Dominican Republic,Ecuador,El Salvador,Fiji,French Guiana,Guadeloupe,Guatemala,Guyana,Haiti,Honduras,Jamaica,Korea,Federated States of Micronesia,Marshall Islands,Martinique,Mexico,NewCaledonia,Nicaragua,Panama,Paraguay,Saint Lucia,Saint Martin,Saint Maarten,Saint Vincent and the Grenadines,Samoa,Suriname,Tonga,Trinidad and Tobago,and Venezuela[26-28].
The characterization of ZIKAV strains collected from six African and Asian countries,as published by researchers,showed that there are two geographically distinct lineages of the phylogenetic trees of the virus.The relationships and hence the epidemiological evidence of the Yap Island outbreak may confirm that it originated in South-East Asia[29].
According to the Pan American Health Organization surveillance data,the geographical distribution of ZIKAV has steadily widened since the virus was first detected in the Americas in 2015.Autochthonous ZIKAV transmission(mosquito borne infection)has been reported in 35 countries and territories of the region.In those countries and locations where the presence of competent mosquito vectors like Ae.aegypti have been identified,ZIKAV outbreaks and transmission are most likely to occur[1].
2.2.Zika case definitions
The WHO has issued interim case definitions of ZIKAV infections.These definitions identified three categories;i.e.,suspected,probable and confirmed ZIKAV cases[30].A suspected case of ZIKAV is characterized by the presence of rash and/or fever with arthralgia,arthritis,or non-purulent conjunctivitis.A probable case requires these symptoms in conjunction with the presence of anti-Zika immunoglobulin M(IgM)antibodies and an epidemiologic link within two weeks prior to symptom onset to a region with local autochthonous transmission.A confirmed case of ZIKAV disease requires laboratory confirmation of recent ZIKAV infection by either presence of ZIKAV RNA or antigen in serum or other samples(e.g.saliva,tissues,urine,whole blood),or IgM antibody against ZIKAV positive and PRNT90 for ZIKAV with titre 20 and ZIKAV PRNT90 titre ratio 4 compared to other flaviviruses,and exclusion of other flaviviruses.
2.3.Methods of ZIKAV transmission
Humantohuman transmissionofZIKAVpredominantly occur from the bite of an infected mosquito of the Aedes species[1,31]. ZIKAV RNA has been isolated in blood,semen,urine,saliva,amniotic fluid,breast milk and cerebrospinal fluid[15,27,32-37]. Transmission of ZIKAV has been suggested via other non-vector means such as sex,maternal-foetal transmission and blood transfusions[38-49].However,no study described transmission ofZIKAV person to person casually or by non-sexual close contact. There has been one reported case of laboratory-acquired ZIKAV disease in the US[32].
2.3.1.ZIKAV transmission by mosquito
The Aedes mosquito bite,which transmits numerous medically significant arboviruses such as Zika,dengue,chikungunya and yellow fever viruses,is in abundance in tropical countries[6,16,32,50-53].Ae.aegypti,which is widely spread in the tropics and subtropics,is the main mosquito vector associated with the transmission of ZIKAV[23].Other Aedes species are capable of transmitting ZIKAV such as Ae.albopictus,Aedes africanus,Aedes luteocephalus,Aedes vittatus,Aedes furcifer,Aedes hensilii and Aedes apicoargenteus[54].Many reports have indicated that Ae.albopictus is established in many parts of Europe,aswellasinMediterraneancountries.Aedes polynesiensisisalsosuspectedtocontributetoZIKAV transmission in French Polynesia.
Ae.aegypti lives in close proximity to people and their environs and may be found both indoors and outdoors.The mosquitoes typically breed in stagnant water which may be found in puddles,flower pots,buckets and other small containers.Aedes mosquitoes bite people mainly in the daytime but may also bite at twilight and at night.In the process,they may be infected by ZIKAV from humans.Infected mosquitoes feeding on un-infected non-immune persons may then transmit ZIKAV infection[32,55].
2.3.2.Sexual transmission
Isolations of ZIKAV in semen and few cases of transmission by sexual intercourse were reported in few settings[38-42,44,45,55-58].A man can sexually transmit ZIKAV to his partner and ZIKAV was first reported as being sexually transmittedin2008,Colorado,USA[39].ZIKAVwas transmitted from a male to a female after vaginal sexual intercourse that occurred prior to the onset of the man's clinical illness.Hematospermia was noted in this case report. In 2014,an autochthonous case of ZIKAV infection possibly due to sexual transmission was described in Florence,Italy[45].Several reports of confirmed cases of sexual transmission of ZIKAV have been documented in 2016 associated with the outbreakofZIKAVintheWesternHemisphere[44,56]. Replication-competent ZIKAV has been isolated in semen up to 62 days after the onset of fever while blood RT-PCR was negative at the time[40,41,53].The duration of persistence of ZIKAV in semen is unknown.
All reports of sexual transmission of ZIKAV involve transmission from a man to his partner following vaginal or anal intercourse before the development or after the resolution of clinical symptoms.Although presence of ZIKAV in female genital tract of ZIKAV infected woman is theoretically possible,no reports were made on the possibilities or potential sexual transmission of ZIKAV from an infected woman to her partner[43].
2.3.3.Transplacental and perinatal transmission
Pregnant women can become infected with ZIKAV and maternal-foetal ZIKAV transmission can occur throughout pregnancy[47,48].ZIKAV RNA has been detected in the amniotic fluid of the expectant mothers whose foetuses were found to have cerebral abnormalities on ultrasonography[48,57,59,60].Zika viral antigen and RNA has been isolated in the placenta and brain tissues of babies with microcephaly who died soon after birth and in the tissues after miscarriages[61].There have been two reports of perinatal ZIKAV transmission described in mother-infant pairs where the mothers developed clinical features of ZIKAV infection in the perinatal period.In both cases,the mothers and the babies had ZIKAV infection confirmed within 4 days of the delivery date.In the first case,the infant was asymptomatic while the other infant was described as having an isolated diffuse rash[47].Microcephaly can be classified as primary(congenital)or secondary(postnatal)[62].Primary microcephaly can be detected before 36 weeks of gestation[61].Breastfeeding has not yet been described as a mode of ZIKAV transmission,however,it has been reported that other flaviviruses could be transmitted through breast milk[32,63,64].
2.3.4.Transmission by blood transfusion
Transmission of ZIKAV by blood and blood related products has not been reported.However the possibilities and potential risk of transfusion-related ZIKAV transmission definitely exist. As theoretical possibilities,molecular screening was implemented in French Polynesia for blood donors during the territories'outbreak.It was noted that 2.8%of asymptomatic blood donors,at the time of donation,were found to be positive for acute ZIKAV infection after screening[33].
3.Clinical presentation of ZIKAV disease
Before 2013,ZIKAV infection had been described as a mild,self-limiting illness associated with fever,rash,joint pain and conjunctivitis[2].Other clinical presentations have been described in association with ZIKAV infection during the current outbreak.
The incubation period for ZIKAV is unknown but the symptoms may appear within 3-12 days after the infected mosquito bite and may resolve within 7 days[65].The illness is usually mild and 80%of cases of ZIKAV infection may be asymptomatic[1].Symptoms and signs of ZIKAV may include“low-grade fever,maculopapular rash,arthralgia,conjunctivitis,malaise,myalgia,retro-orbital pain and asthenia”[23,66,67]. Rarely,other features such as nausea,diarrhoea,abdominal pain,mucus membrane ulceration and pruritus may occur[68]. Thrombocytopenia has been noted in case reports[69,70].The symptoms of ZIKAV infection usually resolve within 7 days. Severe disease is uncommon,hospitalization is not usually required and the case fatality rate is low[71,72].
4.Zika-associated neurological syndromes
A rise in Guillain-Barr´e syndrome(GBS)has been reported in several countries in the Americas and the Pacific in association with the current ZIKAV outbreak[12,14,36,73,74].GBS is a serious,immune-mediated illness exhibiting as progressive paralysis over 1-3 weeks,with a 5%death rate and up to 20%of patients left with a significant disability.Based on the study methods and case ascertainment,the annual incidence of GBS could be estimated in the range of 0.4-4.0 cases per 100000 population per year.Furthermore,earlier studies have also found that GBS is more common in adults,with risk increasing with age and men more likely to be affected than women[75].During the outbreak of ZIKAV in French Polynesia,in the 4 months between November 2013 and February 2014,42 patients werediagnosed with GBS among 28000 persons presenting for medical care[12],representing a marked increase from the approximately 5 cases detected annually in the previous 4 years[29].A case-control study in French Polynesia also showed an odds ratio of>34 between GBS and a history of ZIKAV infection.Overall,7 countries in the Americas have reported an increase in cases of GBS with at least one case detected to have laboratory confirmed ZIKAV[1,4].
Other neurological complications have been reported in associationwithZIKAVincludingacutemyelitis[76],meningoencephalitis[77]and brain ischemia[78].
5.Congenital microcephaly
Babies born to women infected with ZIKAV have severe neurological sequelae.An unusual cluster of cases of congenital microcephaly and other neurological disorders have been reported.Congenital microcephaly(Figure 1)and other foetal malformations potentially associated with ZIKAV infection or suggestive of congenital infection have been reported in Brazil(1271 cases),Cabo Verde(3 cases),Colombia(7 cases),French Polynesia(8 cases),Martinique(3 cases)and Panama(4 cases). TwoweredetectedintheUSandSloveniawithlinksoftemporary stay in Brazil[5].Available preliminary data suggest that severe congenital abnormalities are linked to ZIKAV infection[79].The cases described share many characteristics with congenital abnormalities associated with other viral infections,although the abnormalitiespresumablylinkedtoZIKAVmayhave distinguishing characteristics.In some cases,ZIKAV foetal syndrome resembles other viral infection related congenital disorders,but it is more severe than that observed with many other intrauterine viral infections[80].
Few studies have reported that the presence of ZIKAV infection as evident through laboratory confirmation.ZIKAV RNA either in the amniotic fluid,or brain tissues of infants born with microcephaly as well as the high rates of microcephaly among infants born to mothers with proven antecedent acute ZIKAV infection,provides strong evidence linking microcephaly to maternal ZIKAV infection[48,57,59,61,81-83].ZIKAV infection in utero may contribute to the development of microcephaly.The outbreaks in Brazil,Polynesia and Micronesia suggest that there is a high probability of microcephaly in the newborns if the infection has taken place in the first trimester[84-88],however microcephaly may occur at up to 18 weeks gestational age[57,59,82,85,89].According to the Brazilian Ministry of Health,more than 5000 reported cases of microcephaly with suspected or associated ZIKAV infection have been investigated and classified[27,88,90-92].
Ophthalmological findings have also been reported in infants with microcephaly and presumable intra-uterine ZIKAV infection[49,86-88].These findings include severeocular abnormalities including important macular and optic nerve abnormalities.Focal pigment mottling,chorioretinal atrophy andopticdiscabnormalitieswerereportedamongthe ophthalmologic manifestations described[92-94].The range of congenital abnormalities seen with infants born to ZIKAV infected mothers indicates the relationship of ZIKAV and microcephaly,neurological disorders and ophthalmological disorders and a potential of ZIKAV congenital syndromes.
6.Zika diagnosis and treatment
In many advanced laboratories,diagnostic testing for ZIKAV is performed primarily on serum.Other specimen types such as urine,saliva,amniotic fluid,and tissue has also been evaluated. However,ZIKAV is routinely diagnosed using RT-PCR for Zika viral RNA or ZIKAV serology[66,95,96].A definitive diagnosis of ZIKAV infection is made by the recognition of viral nucleic acid in serum while viremia is often brief and diagnosis by RT-PCR will be productive if undertaken in the first week after the commencement of clinical symptoms[97,98]. RT-PCR is positive for a short window period after clinical infection and during viremia[97].Additionally,it may be difficult to isolate nucleic acid from clinical samples in the low level viremia generally observed in ZIKAV infection[98]. For these reasons,negative results of RT-PCR testing cannot be used to exclude ZIKAV infection.Experience from the study of similar arbo-flaviviruses indicates that as the presence of active virus in the blood diminishes the ZIKAV,IgM antibodies will occur and will remain detectable for several months[99]. ZIKAV IgM and neutralizing antibodies classically develop towards the end of the first week of clinical presentation[100]. ZIKAV-specificIgMantibodiesdetectedbyIgM-capture enzyme-linked immunosorbent assay or immunofluorescence assays in serum may be used for the diagnosis of ZIKAV infection six or more days after the onset of clinical symptoms[95].A major challenge in the interpretation of serological test results is cross-reacting antibodies against related arbo-viruses(e.g.dengue,chikungunya and yellow fever viruses).The plaque reduction neutralization test can be used to differentiate antibodies of closely related antibodies and aid in the verification of results[101].There is limited data that ZIKAV RNA can be detected by RT-PCR in urine[34-36,102]and saliva[35].
Antenatal diagnostic procedures and techniques for the diagnosis of ZIKAV infection have not yet been reliably established. ZIKAV has been identified by RT-PCR in the amniotic fluid ofcongenital ZIKAV infected cases[48,57,82,103,104].Immunohistochemistry and RT-PCR have also identified ZIKAV infection in the tissues of foetal losses and infant demise shortly after birth[79].Ultrasonographymaydetectmicrocephalyand congenital malformations associated with ZIKAV infection including brain atrophy,hydranencephaly,abnormal gyration,cerebralcalcifications,absentcorpuscallosum,ventricular dilatation,hydrops fetalis,anhydramnios and intrauterine growth retardation[57,82,89,105].These findings were noted as early as 18-20 weeks of gestation although they are often detected later[57,89,106].Challenges exist with respect to clinical and technical factors associated with the use of ultrasonography to detect microcephaly[107]and the sensitivity of detecting microcephaly using ultrasonography[108].A more detailed evaluation of the foetalintracranialanatomybymeansofserialfoetal ultrasonography or foetal brain MRI might be recommended.
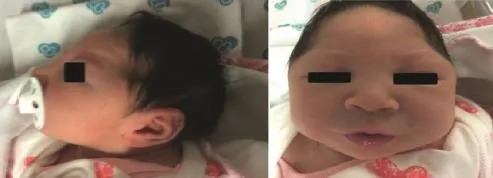
Figure 1.Newborn with microcephaly.
Treatment of ZIKAV infection is non-specific.Treatment is generally supportive and is based on relieving symptoms mainly based on pain relief,fever reduction,and anti-histamines for pruritic rash including preventing dehydration with oral fluids. Treatment with acetylsalicylic acid and non-steroidal anti-inflammatory drugs is discouraged because of a potential increased risk of haemorrhagic syndrome.Pregnant women should be treated with acetaminophen for alleviation of fever[105].
The immunological response of the human host following ZIKAV infection is subject for future studies.The experience and lessons learnt from other human infection and immunological reaction from dengue infection in humans may be used as a referencepointuntilfurtherimmunologicalobservationisknown. The potential role of antibody dependent enhancement of ZIKAV infection anddiseasehas notbeenexamined[109].Immunological analysis as illustrated by two case reports,demonstrated that recovery from ZIKAV infection is associated with restoration of normal numbers of immune cells in the periphery as well as with normal function of antigen-presenting cells[69].
7.Vector control interventions and personal protection
Several epidemiological studies examined risk factors for ZIKAV transmission and human exposure to mosquitos and conditions for ZIKAV circulation.Historically ZIKAV transmission is known to take two different pathways;an enzootic sylvan cycle,where the virus circulates between arboreal Aedes spp.mosquitoes and non-human primates;and a human cycle,betweenhumansandperidomestic/domesticAedesspp. mosquitoes[110].
ZIKAV is mosquito transmitted flaviviridae.Among the various mosquito species,Aedes(Stegomyia)mosquitoes appear to be the most important vector for ZIKAV transmission,although some Anopheles,Culex,Eretmapodites,and Mansonia species are known to contribute for the transmission of ZIKAV. The relative importance of non-human primates or other potential hosts compared to humans in the transmission cycle of ZIKAV is not well known.
As introduced earlier,ZIKAV was isolated first from mosquito vectors in 1948 and in 1956 from Aedes africanus specimens.In addition to this,other researchers have also surveyed many other Aedes species including human and non-human primates to isolate ZIKAV using RT-PCR methods[109].It is alsointerestingtonotethatsomeresearchersfailedto critically consider looking into the possibilities of other vector species such as Culex.Faye et al.reported a long list of mosquito species from which ZIKAV strains were isolated,including several species of Aedes and Anopheles coustani[111].The presence of ZIKAV in any mosquito species does not necessarily mean that the vector is an efficient vehicle for transmission.
Vectorcontrolactivitiesshouldaim at reducingAedesmosquito densities to the lowest possible levels to ensure that ZIKAV transmission is not taking place or is fullyinterrupted.A comprehensive vectorcontrolactivityshouldincludewell-structuredentomological surveillance focusing on local geospatial distribution of the vectors andmeasurementsofthevectorpopulationovertimetofacilitatethe right control interventions.WHO promotes integrated vector management as a strategic approach to vector control[110,112]. Vector control approaches need to implement studies and methods that focus on the local ecology,species,susceptibility and resistance patterns to insecticides used,as well as the programmatic operations at different stages of the life cycle of the mosquitoes[113].Countries need to strengthen vector surveillance and collaborate to close the research gaps through data sharing in order to monitor the spread of arboviruses,the dynamics and insecticide susceptibility status of vector populations and the risk of disease outbreaks.
Countries and territories affected by ZIKAV need to target efforts by prioritizing vector control interventions based on surveillance information and research findings.It is most appropriate that vector control strategies to be more proactive and be able to promptly provide special care for pregnant women in order to prevent the occurrence of ZIKAV congenital syndromes by instituting preventive measures like adequate repellent lotion and insecticide treated nets.Furthermore,this kind of interventions targeting pregnant women need to be carefully monitored for its efficacy most particularly during ZIKAV outbreaks.Personal protection measures should be considered for women of childbearing age and at household level in general[113].
Intensified vector control activities have to be prioritized in areas with large populations and determining specific areas of focus for interventions[110].In order to curb the current global ZIKAV outbreak and tackle the existing burden of dengue viral infections,WHO has evaluated some newer tools[114]. This innovation includes a genetically modified mosquito prototype known as OX513A,which is a transgenic strain of Ae.aegypti engineered to carry a dominant,repressible,nonsex-specific,late-acting,lethal genetic system,together with a fluorescent marker.Larvae carrying the OX513A gene develop normally,but die before becoming an adult.This technology has been demonstrated to reduce Ae.aegypti populations in smallscale field trials in several countries,but there is a lack of data on epidemiological impact.Implementing this tool requires the sustained release of transgenic male mosquitoes to maintain suppression of wild Ae.aegypti populations.Another technique being developed involves a mass release of male insects that have been sterilized by low doses of radiation.The method actually decreases the chance of the female's eggs from developing to full maturity[114].
A promising biological control method is one that uses male mosquitoes carrying the naturally occurring Wolbachia bacteria,which are found in 60%of common insects,including butterflies and fruit flies.Laboratory results show that Wolbachia infection reduces the replication of dengue,chikungunya and ZIKAV within Aedes mosquitoes,and eliminates or substantially delaysthe appearance of virus in mosquito saliva,thus reducing the competence of the mosquito to transmit viruses.The mosquitoes are not genetically modified.Mosquitoes carrying Wolbachia bacteriahavebeenreleasedinseveralplaces,including Australia,Brazil,Indonesia and Vietnam as part of control strategies for dengue.Some countries affected by Zika are using biological methods as part of an integrated approach to mosquito control[112].
As ZIKAV exerts its presence in the Region of the Americas,researchers need to elucidate the interaction and adaptation of the new virus in these susceptible hosts and other intermediary organisms and vector species[109].Entomological surveillance is vital for any good vector control initiative.For areas with establishedpopulations,monitoringAedesmosquitoesat immature(mainly larvae and pupae)and adult life stages should be undertaken.
8.Promoting research and development
As of March 2nd,2016,67 companies and research institutions were already working on a number of products(31 on diagnostics,18 on vaccines,8 on therapeutics,10 on vector control),which are at various stages of early development.No vaccine or therapeutic modality has yet been tested on humans[112].However,the use of different animal models to study viral pathogenesisanddevelopcandidatevaccinesisurgently required.
According to the WHO emergency research and development plan and Zika product landscape,a number of in-vitro diagnostic(IVD)manufacturers have already expressed interest in developing assays to support the control effort.More than 30 IVDs have been developed or are at various stages of development.Of the few IVDs commercially available,even fewer have undergone regulatory premarket assessment.Several vaccine developing institutions and commercial interests are actively pursuing vaccine development.Vaccine approaches include purified inactivated virus,nucleic acid based vaccines(DNA,RNA),live vectored vaccines,subunit vaccines,virus-like particles technologies and live recombinant approach[115].
The rapid spread and occurrence of ZIKAV outbreak in the region of the Americas in the last two years and past outbreaks since 2007 in other regions begged for firm scientific knowledge and research about ZIKAV.Some of the research questions are directed in the areas of modes of transmission;i.e.if Zika is transmitted by other vectors,which mosquito species are involved?What is the efficiency of person-to-person transmission via bodily fluids such as saliva?What is the perinatal transmission rate and timing?How often does transmission occur sexually?What is the blood transfusion transmission rate?Is it possible for ZIKAV to result in a chronic infection?Is a long lasting protective immune response produced after ZIKAV infection?Is re-infection with ZIKAV possible[109]?
It is unclear what factors gave rise to the emergence of ZIKAV in the current outbreak in the Western Hemisphere. Several explanations have been proposed including common underlying mechanisms related to the emergence of ZIKAV,dengue and chikungunya and the Aedes mosquito vectors such as globalization,urbanization and environmental factor[52,116]. Another hypothesis is that ZIKAV recombination in nature as a possible adaptive response to the mosquito vectors as a proposed influence on ZIKAV spreading patterns[117].It has alsobeensuggestedthatmutationsinZIKAVaffecting virulence and viral introduction or transmission to vulnerable,non-immune populations may contribute to the spread of the epidemic[84].The incidence of ZIKAV infection in the current outbreak in the Americas is difficult to determine because ZIKAV infection may be asymptomatic or mild[118].
Research gaps exist with respect to Zika transmission. Although there is accumulating evidence to suggest that Zika can be transmitted sexually through semen,gaps in data exist on the risk of sexual transmission and the duration of ZIKAV detection in semen[42].It is unknown if a man can transmit ZIKAV to his partner if he has ZIKAV infection and is asymptomatic[42,43].There is also a paucity of available data on whether females can transmit ZIKAV sexually or if ZIKAV can be transmitted via oral sex[42].Zika may also be present in other body fluids such as saliva in the acute phase,but there are gaps in data on ZIKAV viability,viral load,duration and risk of transmission in saliva[37].ZIKAV has been isolated in stored blood samples by RT-PCR,however data are unavailable regarding the survival of ZIKAV in processed and stored substances of human origin[94].It is unknown if ZIKAV can be transmitted via breast milk or the frequency and persistence of the ZIKAV in breast milk.It is also unknown if breast milk contains protective antibodies in women previously infected with ZIKAV[114].
Prior to the outbreak of ZIKAV in the Americas,the causal and temporal link between ZIKAV infection,maternal-foetal transmissionandcongenitalabnormalitieshadnotbeen described.
Research gaps also exist on the frequency and risk factors for maternal-foetal ZIKAV transmission.It is unknown if the risk of a foetusdevelopingcongenitalanomaliesisdifferentbasedonsexual transmission versus mosquito-borne transmission[44].The role of viral load in pathogenesis and in utero transmission are also unknown.Information on the complications resulting from ZIKAV infection is still limited.Researches that define the clinical spectrum and characterization of the congenital abnormalities are still needed.Studies relating to the association between ZIKAV and other viral outbreaks such as chikungunya and dengue and the potential for immunological interaction or co-infection contributing to viral transmission and virulence are needed.It is recommendedthat furtherstudies are conducted on ZIKAV basic science and epidemiology to guide interventions[88].
Questionsexist on how Zika affects the nervoussystem and on the evidence and causality to guide interventions[77,116].The risk of a baby developing microcephaly after ZIKAV infection in pregnancyisalsounclear[65].Thefullspectrumof manifestations of congenital ZIKAV infection has also yet to be described[84].It has been proposed that the recently observed manifestations of neurologic and congenital malformations in association with ZIKAV infection may be a reflection of increased incidence or viral mutation[84].Challenges also exist pertaining to the availability of reliable antenatal diagnostic regimens to establish antenatal ZIKAV infection as well as the role of RT-PCR on amniotic fluid and cord blood samples[65].
Commercially available diagnostics for ZIKAV are under development,but currently there are few available[108].The Zika RT-PCR test is the first commercially available assay in the US.The test was developed from a commercial laboratory provider which was granted an Emergency Use Authorization for testing for ZIKAV RNA.Prior to this,the only ZIKAV tests approved by the Food and Drug Administration under Emergency Use Authorization were accessible from the Centers forDisease Control and were to be used in Centers for Disease Control designated laboratories.There is a need for the development of rapid ZIKAV serologic tests.Further research is recommended on the use and validation of non-blood specimens such as urine and saliva for diagnostic purposes[34,37,40,109]. Challenges also exist with respect to cross reactivity between other flavivirus antibodies and diagnosing Zika by serological methods[90].
Extensiveantibodycross-reactivitybetweenZIKAVandother flaviviruses complicates the use of serology,which is often fundamentaltoviraldiagnosisandsurveillance[84].Pastinfection with other flaviviruses or previous flavivirus vaccination may resultinimmunoglobulinGantibodiesthatcouldbindtoZIKAV.
A priority research agenda is the development of convenient,easy to use serological assays for monitoring the transmission of ZIKAV and studying the associations between ZIKAV infection and severe clinical manifestations[109].
There is currently a paucity of evidence on the role of antivirals for the treatment of ZIKAV infection and studies in affected regions are needed to guide local risk assessment,therapeutics development and a vaccine[84].
9.Conclusion
The rapid distribution and outbreak of ZIKAV remains a global concern and threat.The spread and transmission of the virus will likely continue to be detected in new geographic locations and populations.Many countries harbour the mosquito vectors responsible for the transmission of ZIKAV.Other potential transmission patterns and ways may also contribute towards a long term circulation of the virus.
Ongoing ZIKAV emergency research and development plans need to be expanded based on the current evidence of the associations between ZIKAV infections,congenital Zika syndromes,foetal malformations;post-infectious GBS or any other autoimmune neurological disorders may have significant implications to public health.
Many uncertainties about ZIKAV transmission exist.The degree to which viremic persons or other species can transmit ZIKAV to insect vectors is not well understood.The previous transmission cycle and range of insect vectors associated with ZIKAV transmission in the past may differ from the transmission cycle and strain of ZIKAV,now circulating in the Americas.It is still unclear how infectious the circulating strain of ZIKAV is as well as how long viremia lasts,the degree of viremia and the risk of chronic viral persistence after initial infection[111].What is the relationship between pregnant women weakened immunity and foetal infections?The timing and prolonged infection in women and foetuses need further understanding.Overall there is a knowledge gap about the way zika infection progress in humans.
Opportunities exist for furthering ongoing research on novel vector interventions for the control of ZIKAV transmission,other flaviviruses as well as other potential emerging arthopod borne infections.What is the role(if any)of previous infections by(or immune responses to)other flaviviruses,in ZIKAV pathogenesis?What about co-infections with different arboviruses[118]?No vaccine or antivirals medications currently approved for ZIKAV infection.The only good reason for optimism is that vaccine trials have commenced and many researchers are working hard to close the knowledge gaps.
Conflict of interest statement
We declare that we have no conflict of interest.
[1]World Health Organization.Zika virus.Geneva:World Health Organization;2016.[Online]Available from:http://www.who. int/mediacentre/factsheets/zika/en/[Accessed on 5th May,2016]
[2]Duffy MR,Chen TH,Hancock T,Powers AM,Kool JL,Lanciotti RS,et al.Zika virus outbreak on Yap Island,Federated States of Micronesia.N Engl J Med 2009;360:2536-43.
[3]Tognarelli J,Ulloa S,Villagra E,Lagos J,Aguayo C,Fasce R,et al.A report on the outbreak of Zika virus on Easter Island,South Pacific,2014.Arch Virol 2016;161(3):665-8.
[4]Cao-Lormeau VM,Roche C,Teissier A,Robin E,Berry AL,Mallet HP,et al.Zika virus,French Polynesia,South Pacific,2013.Emerg Infect Dis 2014;20(6):1085-6.
[5]World Health Organization.Zika situation report.World Health Organization;2016.[Online]Available from:http://www.who. int/emergencies/zika-virus/situation-report/5-may-2016/en/[Accessed on 6th May,2016]
[6]Zanluca C,Melo VC,Mosimann AL,Santos GI,Santos CN,Luz K.First report of autochthonous transmission of Zika virus in Brazil.Mem Inst Oswaldo Cruz 2015;110(4):569-72.
[7]World Health Organization.Emergencies:the history of Zika virus.Geneva:World Health Organization;2016.[Online]Availablefrom:http://www.who.int/emergencies/zika-virus/ timeline/en/[Accessed on 6th May,2016]
[8]Macnamara FN.Zika virus:a report on three cases of human infection during an epidemic of jaundice in Nigeria.Trans R Soc Trop Med Hyg 1954;48:139-45.
[9]Fagbami AH.Zika virus infections in Nigeria:virological and seroepidemiological investigations in Oyo State.J Hyg(Lond)1979;83:213-9.
[10]Moore DL,Causey OR,Carey DE,Reddy S,Cooke AR,Akinkugbe FM,et al.Arthropod-borne viral infections of man in Nigeria,1964-1970.Ann Trop Med Parasitol 1975;69:49-64.
[11]Olson JG,Ksiazek TG,Suhandiman,Triwibowo.Zika virus,a cause of fever in Central Java,Indonesia.Trans R Soc Trop Med Hyg 1981;75:389-93.
[12]European Centre for Disease Prevention and Control.Rapid risk assessment:Zika virus infection outbreak.French Polynesia. Stockholm:European Centre for Disease Prevention and Control;2014.[Online]Availablefrom:http://ecdc.europa.eu/en/ publications/Publications/Zika-virus-French-Polynesia-rapid-riskassessment.pdf[Accessed on 7th May,2016]
[13]European Centre for Disease Prevention and Control.Rapid risk assessment.Zika virus disease epidemic:potential association with microcephaly and Guillain-Barr´e syndrome.Second update,8 February 2016.Stockholm:European Centre for Disease Prevention and Control;2016.[Online]Available from:http://ecdc. europa.eu/en/publications/Publications/zika-virus-rapid-riskassessment-8-february-2016.pdf[Accessed on 5th May,2016]
[14]Oehler E,Watrin L,Larre P,Leparc-Goffart I,Lastere S,Valour F,et al.Zika virus infection complicated by Guillain-Barr´e syndrome-case report,French Polynesia,December 2013.EuroSurveill2014;http://dx.doi.org/10.2807/1560-7917.ES2014.19.9.20720.
[15]Dupont-Rouzeyrol M,BironA,O'Connor O,Huguon E,Descloux E.Infectious Zika viral particles in breastmilk.Lancet 2016;387(10023):1051.
[16]Roth A,Mercier A,Lepers C,Hoy D,Duituturaga S,Benyon E,et al.Concurrent outbreaks of dengue,chikungunya and Zika virus infections an un-precedented epidemic wave of mosquitoborne viruses in the Pacific,2012-2014.Euro Surveill 2014;http://dx.doi.org/10.2807/1560-7917.ES2014.19.41.20929.
[17]Gatherer D,Kohl A.Zika virus:a previously slow pandemic spreads rapidly through the Americas.J Gen Virol 2016;97: 269-73.
[18]Campos GS,Bandeira AC,Sardi SI.Zika virus outbreak,Bahia,Brazil.Emerg Infect Dis 2015;21(10):1885-6.
[19]World Health Organization.Zika virus outbreaks in the Americas. Wkly Epidemiol Rec 2015;90:609-10.
[20]Faria NR,Azevedo RD,Kraemer MU,Souza R,Cunha MS,Hill SC,et al.Zika virus in the Americas:early epidemiological and genetic findings.Science 2016;352(6283):345-9.
[21]Fauci AS,Morens DM.Zika virus in the Americas-yet another arbovirus threat.N Engl J Med 2016;374(7):601-4.
[22]Hennessey M,Fischer M,Staples JE.Zika virus spreads to new areas-region of the Americas,May 2015-January 2016.MMWR Morb Mortal Wkly Rep 2016;65(3):55-8.
[23]Chen LH,Hamer DH.Zika virus:rapid spread in the Western Hemisphere.Ann Intern Med 2016;164(9):613-5.
[24]Lednicky J,Beau De Rochars VM,El Badry M,Loeb J,Telisma T,Chavannes S,et al.Zika virus outbreak in Haiti in 2014:molecular and clinical data.PLoS Negl Trop Dis 2016;10(4):e0004687.
[25]Hajra A,Bandyopadhyay D,Hajra SK.Zika virus:a global threat to humanity:a comprehensive review and current developments. N Am J Med Sci 2016;8(3):123-8.
[26]Centers for Disease Control and Prevention.CDC adds countries to interim travel guidance related to Zika virus.DeKalb County: Centers for Disease Control and Prevention;2012.[Online]Available from:http://www.cdc.gov/media/releases/2016/s0122-zika-travel-guidance.html[Accessed on 25th April,2016]
[27]Pan American Health Organization.Zika virus infection and Zika fever:frequently asked questions.Washington D.C.:Pan American HealthOrganization;2016.[Online]Availablefrom:http://www.paho. org/hq/index.php?option=com_content&view=article&id=9183 &Itemid=41463&lang=en[Accessed on 31st March,2016]
[28]Centers for Disease Control and Prevention.Travelers'health: travel health notices.DeKalb County:Centers for Disease Control and Prevention;2016.[Online]Available from:http://wwwnc. cdc.gov/travel/notices[Accessed on 25th April,2016]
[29]Kindhauser MK,Allen T,Frank V,Santhana RS,Dye C.Zika: the origin and spread of a mosquito-borne virus.Bull World Health Organ 2016;http://dx.doi.org/10.2471/BLT.16.171082.
[30]World Health Organization.Zika virus disease.Geneva:World Health Organization;2016.[Online]Available from:http:// www.who.int/csr/disease/zika/case-definition/en[Accessed on 25th April,2016]
[31]World Health Organization.WHO statement on the first meeting of the International Health Regulations(2005)(IHR 2005)Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations.Geneva: World Health Organization;2016.[Online]Available from:http:// www.who.int/mediacentre/news/statements/2016/1st-emergencycommittee-zika/en/[Accessed on 3rd March,2016]
[32]Centers for Disease Control and Prevention.Transmission& risks.DeKalb County:Centers for Disease Control and Prevention;2016.[Online]Available from:http://www.cdc.gov/zika/ transmission/index.html[Accessed on 20th April,2016]
[33]Musso D,Nhan T,Robin E,Roche C,Bierlaire D,Zisou K,et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia,November 2013 to February 2014.Euro Surveill 2014;http://dx.doi.org/ 10.2807/1560-7917.ES2014.19.14.20761.
[34]Gourinat AC,O'Connor O,Calvez E,Goarant C,Dupont-Rouzeyrol M.Detection of Zika virus in urine.Emerg Infect Dis 2015;21(1):84-6.
[35]Musso D,Roche C,Nhan TX,Robin E,Teissier A,Cao-Lormeau VM.Detection of Zika virus in saliva.J Clin Virol 2015;68:53-5.
[36]Roz´e B,Najioullah F,Ferg´e J,Apetse K,Brouste Y,Cesaire R,et al.Zika virus detection in urine from patients with Guillain-Barr´e syndrome on Martinique,January 2016.Euro Surveill 2016;http://dx.doi.org/10.2807/1560-7917.ES.2016.21.9.30154.
[37]Barzon L,Pacenti M,Berto A,Sinigaglia A,Franchin E,Lavezzo E,et al.Isolation of infectious Zika virus from saliva and prolonged viral RNA shedding in a traveller returning from the Dominican Republic to Italy,January 2016.Euro Surveill 2016;http://dx.doi.org/10.2807/1560-7917.ES.2016.21.10.30159.
[38]Dallas County Health and Human Services.DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission.Dallas:Dallas County Health and Human Services;2016.[Online]Available from:http://www.dallascounty.org/ department/hhs/press/documents/PR2-216DCHHSReportsFirst CaseofZikaVirusThroughSexualTransmission.pdf[Accessed on 7th April,2016]
[39]Foy BD,Kobylinski KC,Chilson Foy JL,Blitvich BJ,Travassos da Rosa A,Haddow AD,et al.Probable non-vector-borne transmission of Zika virus,Colorado,USA.Emerg Infect Dis 2011;17(5):880-2.
[40]Musso D,Roche C,Robin E,Nhan T,Teissier A,Cao-Lormeau VM.Potential sexual transmission of Zika virus.Emerg Infect Dis 2015;21(2):359-61.
[41]Atkinson B,Hearn P,Afrough B,Lumley S,Carter D,Aarons EJ,et al.Detection of Zika virus in semen.Emerg Infect Dis 2016;22(5):940.
[42]Centers for Disease Control and Prevention.CDC encourages following guidance to prevent sexual transmission of Zika virus. DeKalb County:Centers for Disease Control and Prevention;2016.[Online]Availablefrom:http://www.cdc.gov/media/ releases/2016/s0223-zika-guidance.html[Accessedon28th April,2016]
[43]Oster AM,Brooks JT,Stryker JE,Kachur RE,Mead P,Pesik NT,et al.Interim guidelines for prevention of sexual transmission of Zika virus-United States,2016.MMWR Morb Mortal Wkly Rep 2016;65:120-1.
[44]Hills SL,Russell K,Hennessey M,Williams C,Oster AM,Fischer M,et al.Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission-continental United States,2016.MMWR Morb Mortal Wkly Rep 2016;65(8):215-6.
[45]Venturi G,Zammarchi L,Fortuna C,Remoli ME,Benedetti E,Fiorentini C,et al.An autochthonous case of Zika due to possible sexual transmission,Florence,Italy,2014.Euro Surveill 2016;http://dx.doi.org/10.2807/1560-7917.ES.2016.21.8.30148.
[46]Marano G,Pupella S,Vaglio S,Liumbruno GM,Grazzini G.Zika virus and the never-ending story of emerging pathogens and transfusion medicine.Blood Transfus 2016;14(2):95-100.
[47]Besnard M,Last`ere S,Tessier A,Cao-Lormeau VM,Musso D. Evidence of perinatal transmission of Zika virus,French Polynesia,Decemebr 2013 and February 2014.Euro Surveill 2014;http://dx.doi.org/10.2807/1560-7917.ES2014.19.13.20751.
[48]Oliveira Melo AS,Malinger G,Ximenes R,Szejnfeld PO,Alves Sampaio S,Bispo de Filippis AM.Zika virus intrauterine infection causes fetal brain abnormality and microcephaly:tip of the iceberg?Ultrasound Obstet Gynecol 2016;47:6-7.
[49]Schuler-Faccini L,Ribeiro EM,Feitosa IM,Horovitz DD,Cavalcanti DP,Pessoa A,et al.Possible association between Zika virus infection and microcephaly-Brazil,2015.MMWR Morb Mortal Wkly Rep 2016;65:59-62.
[50]Terzian AC,Auguste AJ,Vedovello D,Ferreira MU,da Silva-Nunes M,Speranca MA,et al.Isolation and characterization of Mayaro virus from a human in Acre,Brazil.Am J Trop Med Hyg 2015;92:401-4.
[51]Gautret P,Simon F.Dengue,chikungunya and Zika and mass gatherings:what happened in Brazil,2014.Travel Med Infect Dis 2016;14(1):7-8.
[52]Musso D,Cao-Lormeau VM,Gubler DJ.Zika virus:following the path of dengue and chikungunya?Lancet 2015;386:243-4.
[53]Marcondes CB,Ximenes Mde F.Zika virus in Brazil and the danger of infestation by Aedes(Stegomyia)mosquitoes.Rev Soc Bras Med Trop 2016;49(1):4-10.
[54]Hayes EB.Zika virus outside Africa.Emerg Infect Dis 2009;15(9):1347-50.
[55]Calvez E,Guillaumot L,Millet L,Marie J,Bossin H,Rama V,et al.Genetic diversity and phylogeny of Aedes aegypti,the main arbovirus vector in the Pacific.PLoS Negl Trop Dis 2016;10(1): e0004374.
[56]Pan American Health Organization.PAHO statement on Zika virus transmissionandprevention.WashingtonD.C.:PanAmericanHealth Organization;2016.[Online]Available from:http://www.paho.org/ hq/index.php?option=com_content&view=article&id=11605% 3A2016-paho-statement-on-zika-transmission-prevention-&catid=8424%3Acontent&lang=en[Accessedon22ndMarch,2016]
[57]Oster AM,Russell K,Stryker JE,Friedman A,Kachur RE,Petersen EE,et al.Update:interim guidance for prevention of sexual transmission of Zika virus-United States,2016.MMWR Morb Mortal Wkly Rep 2016;65:323-5.
[58]Mansuy JM,Dutertre M,Mengelle C,Fourcade C,Marchou B,Delobel P,et al.Zika virus:high infectious viral load in semen,a new sexually transmitted pathogen?Lancet Infect Dis 2016;16(4):405.
[59]Jouannic JM,Friszer S,Leparc-Goffart I,Garel C,Eyrolle-Guignot D.Zika virus infection in French Polynesia.Lancet 2016;387(10023):1051-2.
[60]Calvet G,Aguiar RS,Melo AS,Sampaio SA,de Filippis I,Fabri A,et al.Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil:a case study. Lancet Infect Dis 2016;16:653-60.
[61]Martines RB,Bhatnagar J,Keating MK,Silva-Flannery L,Muehlenbachs A,Gary J,et al.Notes from the field:evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses-Brazil,2015.MMWR Morb Mortal Wkly Rep 2016;65:159-60.
[62]De Carvalho NS,De Carvalho BF,Fugaça CA,D´oris B,Biscaia ES.Zika virus infection during pregnancy and microcephaly occurrence:a review of literature and Brazilian data.Braz J Infect Dis 2016;20(3):282-9.
[63]BarthelA,GourinatAC,Cazorla C,JoubertC,Dupont-RouzeyrolM,DesclouxE.Breastmilkasapossiblerouteofvertical transmission of dengue virus?Clin Infect Dis 2013;57(3):415-7.
[64]Hinckley AF,O'Leary DR,Hayes EB.Transmission of West Nile virus through human breast milk seems to be rare.Pediatrics 2007;119(3):e666-71.
[65]Petersen E,Wilson M,Touch S,McCloskey B,Mwaba P,Bates M,et al.Rapid spread of Zika in the Americas-implications for public health preparedness for mass gatherings at the 2016 Brazil Olympic Games.Int J Infect Dis 2016;44:11-5.
[66]Centers for Disease Control and Prevention.Emergency preparednessandresponse:recognizing,managing,andreportingZika virus infections in travelers returning from Central America,South America,the Caribbean,and Mexico.DeKalb County:Centers for Disease Control and Prevention;2016.[Online]Available from: http://emergency.cdc.gov/han/han00385.asp[Accessed on 20th April,2016]
[67]PetersenEE,StaplesJE,Meaney-DelmanD,FischerM,Ellington SR,Callaghan WM,et al.Interim guidelines for pregnant women during a Zika virus outbreak-United States,2016. MMWR Morb Mortal Wkly Rep 2016;65:30-3.
[68]Ministry of Health-Manat¯u Hauora.Zika virus.Wellington: Ministry of Health;2016.[Online]Available from:http://www. health.govt.nz/our-work/diseases-and-conditions/zika-virus[Accessed on 29th March,2016]
[69]Zammarchi L,Stella G,Mantella A,Bartolozzi D,Tappe D,G¨unther S,et al.Zika virus infections imported to Italy:clinical,immunological and virological findings,and public health implications.J Clin Virol 2015;63:32-5.
[70]Karimi O,Goorhuis A,Schinkel J,Codrington J,Vreden SG,VermaatJS,etal.Thrombocytopeniaandsubcutaneousbleedingsina patient with Zika virus infection.Lancet 2016;387(10022):939-40.
[71]Centers for Disease Control and Prevention.About Zika.DeKalb County:Centers for Disease Control and Prevention;2016.[Online]Available from:http://www.cdc.gov/zika/about/index. html[Accessed on 20th March,2016]
[72]Centers for Disease Control and Prevention.For health care providers:clinical evaluation&disease.DeKalb County:Centers for Disease Control and Prevention;2016.[Online]Available from: http://www.cdc.gov/zika/hc-providers/preparing-for-zika/ clinicalevaluationdisease.html[Accessed on 20th March,2016]
[73]Thomas DL,Sharp TM,Torres J,Armstrong PA,Munoz-Jordan J,Ryff KR,et al.Local transmission of Zika virus-Puerto Rico,November 23,2015-January 28,2016.MMWR Morb Mortal Wkly Rep 2016;65:154-8.
[74]Broutet N,Krauer F,Riesen M,Khalakdina A,Almiron M,Aldighieri S,et al.Zika virus as a cause of neurological disorders. N Engl J Med 2016;374(16):1506-9.
[75]Musso D,Nilles EJ,Cao-Lormeau VM.Rapid spread of emerging Zika virus in the Pacific area.Clin Microbiol Infect 2014;20: O595-6.
[76]M´echarles S,Herrmann C,Poullain P,Tran TH,Deschamps N,Mathon G,et al.Acute myelitis due to Zika virus infection. Lancet 2016;387(10026):1481.
[77]Cartreaux G,Maquart M,Bedet A,Contou D,Brugi`eres P,Fourati S,et al.Zika virus associated with meningoencephalitis. N Engl J Med 2016;374(16):1595-6.
[78]Baud D,Van Mieghem T,Musso D,Truttmann AC,Panchaud A,Vouga M.Clinical management of pregnant women exposed to Zika virus.Lancet Infect Dis 2016;16(5):523.
[79]Miranda-Filho Dde B,Martelli CM,Ximenes RA,Araújo TV,Rocha MA,Ramos RC,et al.Initial description of the presumed congenitalzikasyndrome.AmJPublicHealth2016;106(4):598-600.
[80]Malone RW,Klase Z,Khakhina S,Callahan MV,Glasspool-Malone J,De Bernardi Schneider A.Zika fetal neuropathogenesis:etiology of a viral syndrome.bioRxiv 2016;http:// dx.doi.org/10.1101/050674.
[81]Mlakar J,Korva M,Tul N,Popoviˊc M,Poljˆsak-Prijatelj M,Mraz J,et al.Zika virus associated with microcephaly.N Eng J Med 2016;374:951-8.
[82]Brasil P,Pereira JP Jr,Raja Gabaglia C,Damasceno L,Wakimoto M,Ribeiro Nogueira RM,et al.Zika virus infection in pregnant women in Rio de Janeiro-preliminary report.N Eng J Med 2016;http://dx.doi.org/10.1056/NEJMoa1602412.
[83]DriggersRW,HoCY,KorhonenEM,KuvinanenS,JˆaˆaskelˆainenAJ,Smura T,et al.Zika virus infection with prolonged maternal viremia and fetal brain abnormalities.N Engl J Med 2016;374:2142-51.
[84]Cauchemez S,Bernard M,Bompard P,Dub T,Guillemette-Artur P,Eyrolle-Guignot D,et al.Zika virus infection in French Polynesia.Lancet 2016;387(10033):2125-32.
[85]Meaney-Delman D,Hills SL,Williams C,Galang RR,Iyengar P,Hennenfent AK,et al.Zika virus infection among U.S.pregnant travelers-August 2015-February 2016.MMWR Morb Mortal Wkly Rep 2016;65:211-4.
[86]Kleber de Oliveira W,Cortez-Escalante J,De Oliveira WT,do Carmo GM,Henriques CM,Coelho GE,et al.Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy-Brazil,2015.MMWR Morb Mortal Wkly Rep 2016;65:242-7.
[87]Reefhuis J,Gilboa SM,Johansson MA,Valencia D,Simeone RM,HillsSL,etal.Projectingmonthofbirthforat-riskinfantsafterZika virus disease outbreaks.Emerg Infect Dis 2016;22(5):828-32.
[88]European Centre for Disease Prevention and Control.Rapid risk assessment:Zika virus epidemic in the Americas:potential associations with microcephaly and Guillain-Barr´e syndrome.Stockholm:European Centre for Disease Prevention and Control;2015.[Online]Available from:http://ecdc.europa.eu/en/publications/ Publications/zika-virus-americas-association-with-microcephalyrapid-risk-assessment.pdf[Accessed on 27th April,2016]
[89]Petersen LR,Jamieson DJ,Powers AM,Honein MA.Zika virus. N Engl J Med 2016;374:1552-63.
[90]PanAmericanHealthOrganization.Questionandanswers:Zikaand pregnancy.Washington D.C.:Pan American Health Organization;2016.[Online]Available from:http://www.paho.org/hq/index.php?option=com_content&view=article&id=11552&Itemid=41672& lang=en[Accessed on 12th April,2016]
[91]PanAmericanHealthOrganization.Epidemiologicalalert: neurological syndrome,congenital malformations,and Zika virus infection.Implications for public health in the Americas.Washington D.C.:Pan American Health Organization;2016.[Online]Available from:http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32405&lang=en[Accessed on 14th May,2016]
[92]Ventura CV,Maia M,Ventura BV,Linden VV,Araújo EB,Ramos RC,et al.Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq Bras Oftalmol 2016;79(1):1-3.
[93]Jampol LM,Goldstein DA.Zika virus infection and the eye.JAMA Opthalmol2016;http://dx.doi.org/10.1001/jamaophthal.2016.0284.
[94]dePaulaFreitasB,deOliveiraDiasJR,PrazeresJ,Sacramento GA,Ko AI,Maia M,et al.Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador,Brazil.JAMA Ophthalmol 2016;http:// dx.doi.org/10.1001/jamaopthal mol.2016.0267.
[95]Pan American Health Organization.Zika virus infection.Washington D.C.:Pan American Health Organization;2016.[Online]Available from:http://www.paho.org/hq/index.php?option=com_ topics&view=article&id=427&Itemid=41484&lang=en[Accessed on 2nd May,2016]
[96]Oduyebo T,Petersen EE,Rasmussen SA,Mead PS,Meaney-Delman D,Renquist CM,et al.Update:interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure-United States,2016.MMWR Morb Mortal Wkly Rep 2016;65:122.
[97]Bearcroft WG.Zika virus infection experimentally induced in a human volunteer.Trans R Soc Trop Med Hyg 1956;50:442-8.
[98]Lanciotti RS,Kosoy OL,Laven JJ,Velez JO,Lambert AJ,Johnson AJ,et al.Genetic and serologic properties of Zika virus associated with an epidemic,Yap State,Micronesia,2007.Emerg Infect Dis 2008;14(8):1232-9.
[99]Busch MP,Kleinman SH,Tobler LH,Kamel HT,Norris PJ,Walsh I,et al.Virus and antibody dynamics in acute West Nile virus infection.J Infect Dis 2008;198:984-93.
[100]Centers for Disease Control and Prevention.Zika virus:symptoms,testing&treatment.DeKalb County:Centers for Disease Control and Prevention;2016.[Online]Available from:http://www.cdc. gov/zika/symptoms/index.html[Accessed on 12th May,2016]
[101]Roehrig JT,Hombach J,Barrett AD.Guidelines for plaquereduction neutralization testing of human antibodies to dengue viruses.Viral Immunol 2008;21:123-32.
[102]Kutsuna S,Kato Y,Takasaki T,Moi M,Kotaki A,Uemura H,et al.Two cases of Zika fever imported from French Polynesia to Japan,December 2013 to January 2014[corrected].Euro Surveill 2014;http://dx.doi.org/10.2807/1560-7917.ES2014.19.4.20683.
[103]Reddy UM,Abuhamad AZ,Levine D,Saade GR;Fetal imaging Workshop Invited Participants.Fetal imaging:executive summary ofajointEuniceKennedyShriverNationalInstituteofChildHealth and Human Development,Society for Maternal-Fetal Medicine,AmericanInstituteofUltrasoundinMedicine,AmericanCollegeof Obstetricians and Gynecologists,American College of Radiology,Society for Paediatric Radiology,and Society of Radiologists in Ultrasound Fetal Imaging Workshop.J Ultrasound Med 2014;33(5):745-57.
[104]Leibovitz Z,Daniel-Spiegel E,Malinger G,Haratz K,Tamarkin M,Gindes L,et al.Prediction of microcephaly at birth using three reference ranges for fetal head circumference:can we improve prenatal diagnosis?Ultrasound Obstet Gynecol 2015;47(5):586-92.
[105]Sarno M,Sacramento GA,Khouri R,do Ros´ario MS,Costa F,Archanjo G,et al.Zika virus infection and stillbirths:a case of hydrops fetalis,hydranencephaly and fetal demise.PLoS Negl Trop Dis 2016;10(2):e0004517.
[106]Malone RW,Homan J,Callahan MV,Glasspool-Malone J,Damodaran L,Schneider Ade B,et al.Zika virus:medical countermeasure development challenges.PLoS Negl Trop Dis 2016;10(3):e0004530.
[107]denHollanderNS,WesselsMW,LosFJ,UrsemNT,NiermeijerMF,Wladimiroff JW.Congenital microcephaly detected by prenatal ultrasound:genetic aspects and clinical significance.Ultrasound Obstet Gynecol 2000;15:282-7.
[108]Weaver SC,Costa F,Garcia-Blanco MA,Ko AI,Ribeiro GS,Saade G,et al.Zika virus:history,emergence,biology,and prospects for control.Antivir Res 2016;130:69-80.
[109]Ayres CF.Identification of Zika virus vectors and implications for control.Lancet Infect Dis 2016;16:278-9.
[110]World Health Organization.Mosquito control:can it stop Zika at source?Geneva:WorldHealthOrganization;2016.[Online]Available from:http://www.who.int/emergencies/zika-virus/articles/mosquitocontrol/en/[Accessed on 4th May,2016]
[111]Faye O,Freire C,Iamarino A,Faye O,de Oliveira JV,Diallo M,et al.Molecular evolution of Zika virus during its emergence in the 20(th)century.PLoS Negl Trop Dis 2014;8(1):e2636.
[112]World Health Organization.Zika virus outbreak global response. Geneva:WorldHealthOrganization;2016.[Online]Availablefrom: http://www.who.int/emergencies/zika-virus/response/en/[Accessed 3rd May,2016]
[113]Wilder-Smith A,Gubler DJ.Geographic expansion of dengue:the impact of international travel.Med Clin North Am 2008;92(6): 1377-90.
[114]World Health Organization.Breastfeeding in the context of Zika virus:interim guidance.Geneva:World Health Organization;2016.[Online]Available from:http://www.who.int/csr/resources/ publications/zika/breastfeeding/en/[Accessed on 4th May,2016][115]World Health Organization.Current Zika product pipeline. Geneva:World Health Organization;2016.[Online]Available from:http://www.who.int/csr/research-and-development/zika-rdpipeline.pdf[Accessed on 4th May,2016]
[116]Pan American Health Organization/World Health Organization. Epidemiologic update:neurological syndrome,congenital anomalies,and Zika virus infection.Washington D.C.:Pan American Health Organization/WorldHealthOrganization;2016.[Online]Availablefrom: http://www.paho.org/hq/index.php?option=com_docman&task=doc_ view&Itemid=270&gid=32879&lang=en[Accessed on 21st March,2016]
[117]Lazear HM,Stringer EM,de Silva AM.The emerging zika virus epidemic in the Americas:research priorities.JAMA 2016;315(18):1945-6.
[118]Duarte Dos Santos CN,Goldenberg S.Zika virus and microcephaly:challenges for a long-term agenda.Trends Parasitol 2016;32(7):508-11.
18 May 2016
Teshome Gebre,Regional Director for Africa,International Trachoma Initiative,the Task Force for Global Health,P.O.Box 10001,Addis Ababa,Ethiopia.
Tel:+251 91 120 3524
Fax:+251 11 467 4174
E-mails:tgebre@taskforce.org,teshumanga2002@yahoo.com
Peer review under responsibility of Hainan Medical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Pacific Journal of Tropical Biomedicine2016年10期
Asian Pacific Journal of Tropical Biomedicine2016年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A review on promising phytochemical,nutritional and glycemic control studies on Moringa oleifera Lam.in tropical and sub-tropical regions
- A rare cause of acute abdomen-Spontaneous rectus sheath hematoma
- Evaluation of proline,chlorophyll,soluble sugar content and uptake of nutrients in the German chamomile(Matricaria chamomilla L.)under drought stress and organic fertilizer treatments
- ProductionofsecondarymetaboliteE2.2fromPhaleriamacrocarpaendophyticfungus
- Cytotoxic,genotoxic and apoptotic effects of naringenin-oxime relative to naringenin on normal and cancer cell lines
- Pandanusamaryllifoliusleafextractincreasesinsulinsensitivityinhigh-fatdiet-induced obese mice
