官能团化烯烃的氢甲酰化反应研究及应用进展
刘 旭,刘仲能,顾松园
(1.中国石化绿色化工与工业催化国家重点实验室,上海 201208;2.中国石化上海石油化工研究院,上海 201208)
综述与展望
官能团化烯烃的氢甲酰化反应研究及应用进展
刘旭1,2*,刘仲能1,2,顾松园1,2
(1.中国石化绿色化工与工业催化国家重点实验室,上海 201208;2.中国石化上海石油化工研究院,上海 201208)
氢甲酰化反应已发展成为重要的工业均相催化反应之一,通过官能团化烯烃氢甲酰化反应可以得到官能团化的醛类化合物,该类化合物大多是精细化学品或合成中间体,官能团化烯烃显示出很多不一样的特性。综述近年来官能团化烯烃氢甲酰化反应研究进展,介绍乙烯基芳烃、α-官能团化烯烃以及β-官能团化烯烃的氢甲酰化反应及应用,并对官能团化烯烃氢甲酰化反应进行展望。
精细化学工程;官能团化烯烃;氢甲酰化;醛
氢甲酰化反应是指烯烃在催化剂作用下与CO/H2反应生成醛的过程,已发展成为迄今最重要的工业均相催化反应之一。据统计,全球通过氢甲酰化生产醛和醇的能力已达千万吨规模[1]。通过官能团化烯烃氢甲酰化反应可以得到官能团化的醛类化合物,而官能团化的醛大多是精细化学品或药物、香精香料的合成中间体。与非官能团化烯烃相比,官能团的存在影响烯烃氢甲酰化反应,显示出很多不一样的特性[2],这些特性是由于环状金属中间体的稳定性不同所致,同时影响反应的区域选择性[3]。
本文综述近年来官能团化烯烃氢甲酰化反应研究进展,介绍乙烯基芳烃、α-官能团化烯烃以及β-官能团化烯烃的氢甲酰化反应及应用,并对官能团化烯烃氢甲酰化反应进行展望。
1 乙烯基芳烃
通常,烯基芳烃和烯基杂环类化合物[4-6]氢甲酰化反应生成支链醛,这是由于反应过程中形成了稳定的η3-丙烯基中间体[7]。在该类底物中,经常使用苯乙烯考察新的配体、催化剂和添加剂,并用于机理研究。使用四齿配体,有可能改变苯乙烯衍生物氢甲酰化反应的区域选择性,对位取代基的电子效应在该反应中显而易见(l/b:p-氟-苯乙烯,14.2;p-甲基-苯乙烯,19.4;苯乙烯,21.2;p-甲氧基-苯乙烯,26.0)[8]。当苯环上邻位取代基的位阻较大时,也会倾向于生成支链醛。
美国安进公司报道了一种全合成钙受体激动剂西那卡塞的方法[9],由间三氟甲基苯乙烯出发,经氢甲酰化反应和还原氨化反应得到药物活性成分,氢氨甲基化反应可以不经分离中间体一步完成[10]。

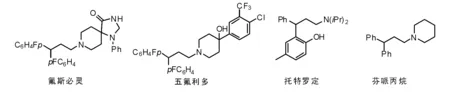
Botteghi C等[11]研究发现,在烯基-1,1-二芳基化合物的氢甲酰化反应中,Rh催化剂可以代替Co体系,与还原氨化反应结合可以合成抗精神病药物氟斯必灵和五氟利多以及泌尿系统药物托特罗定[12],也可用于合成芬哌丙烷[13],治疗肠胃功能紊乱。
2 α-官能团化烯烃
文献[14]报道了氯乙烯在Rh催化下的氢甲酰化反应可以得到2-氯丙醛,由于化合物热稳定性欠佳,采用反应条件比Co催化剂温和的Rh催化剂,得到更好的结果。为了避免催化剂被HCl分解,体系中加入缓冲液或胺。反应区域选择性较高,产物经氧化以及取代反应可以生成消旋的乳酸。

作为烯基酯类底物,使用P(OPh)3作为配体时,乙酸乙烯酯基本生成2-乙酰氧基丙醛[15];使用双膦配体时,主要得到支链产物[16]。体系内存在弱碱时,甲酰基醋酸酯可转化为β-乙酰氧基酮[17];温度较高且使用非改性Co催化剂时,可生成单乙酰基保护的丙二醇[18]。使用改性金属/三齿膦配体在相对较低温度下,区域选择性会发生翻转,并高选择性生成乙酸-3-羟基丙酯(>99.9%)[19],产物氢化后可生成1,3-丙二醇,单乙酰基保护的丙二醇可与对苯二甲酸聚合生成聚对苯二甲酸丙二醇酯。

在未改性Co催化剂的催化作用下,丙烯腈经氢甲酰化反应可得到β-甲酰基丙腈,选择性还原甲酰基可得到γ-羟基丁腈。使用Rh/P(OPh)3作为催化剂时,主要得到α-甲酰基丁腈,是合成聚甲基丙烯酸甲酯的起始原料[20]。作为共聚单体的2-三氟甲基丙烯酸,可以由3,3,3-三氟丙烯通过Rh催化的氢甲酰化反应再经氧化、卤化和消除反应得到[21]。
丙烯酰胺同样可以发生氢甲酰化反应,由于氨基的导向作用,反应主要发生在α-碳原子上,生成异构的醛[22],在Rh催化反应中,单膦配体的反应效果优于双膦配体[23],丙烯酸酯的氢甲酰化反应也发生在α-位[24]。当α位有取代基的丙烯酸衍生物发生氢甲酰化反应时,醛基依然在三级碳原子上生成[25],生成的醛可转化为α,α-取代的β氨基羧酸酯。

3 β-官能团化烯烃
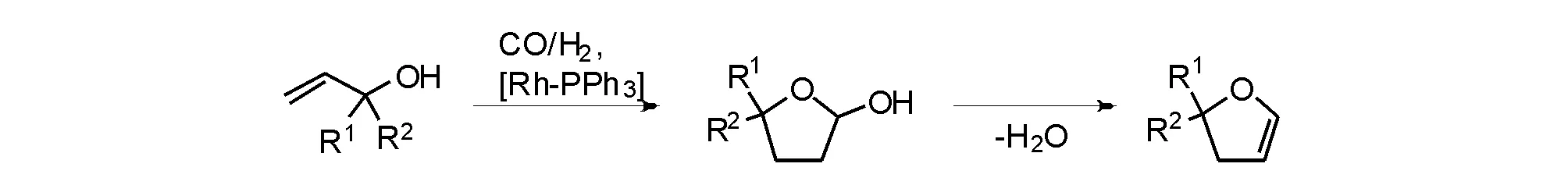
Zhang X等[26]使用膦-亚磷酰胺配体与Rh的催化体系,考察了该类底物反应的区域选择性。烯丙醇的氢甲酰化反应主要生成4-丁醇醛,该化合物可以合成1,4-丁二醇和四氢呋喃。除支链产物外,氢甲酰化反应还可生成一些C3副产物,如正丙醇和丙醛[27]。最初反应使用未经改性的Rh催化剂,随后单膦[28]或双膦配体[29]逐渐应用于该反应。1-丁烯-3-醇的反应主要发生在γ位,得到α-羟基-β-甲基四氢呋喃,经脱水可以制备取代的二氢呋喃[30]。
Ruiz N等[31]研究了单膦配体与Rh组成的催化剂对非环状烯丙基醚氢甲酰化反应。Polo A等[32]使用二氢呋喃和二氢吡喃异构体研究了杂环化合物中氧原子的导向作用,通常,使用二氢呋喃反应条件较温和,可先异构化为平面性更好的五元环,从而更好形成金属络合物。2H,5H-二氢呋喃易转化为相应的2H,3H-异构体,与合成气进行氢甲酰化反应。使用位阻较大的单膦配体时,主要生成2-甲酰基四氢呋喃;使用PPh3作为配体时,主要生成3-甲酰基四氢呋喃,二氢吡喃也有类似的特性。

Briggs J R等[33]研究了支链烯丙基硅和烯丙醇类化合物的氢甲酰化反应,使用Rh-BIPHEPHOS催化剂可以得到更高的正异比,也应用于抗心律失衡药物伊布利特和抗组胺剂非索非那定[34]的全合成。
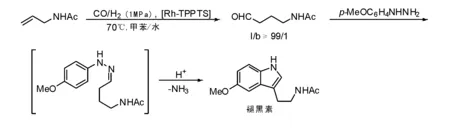
Verspui G等[35]研究了N-乙酰基烯丙胺在水油两相体系中氢甲酰化反应的区域选择性,结果表明,使用Rh-PPh3作为催化剂时,仅得到中等的区域选择性,但活性较高;使用Xantphos作为配体时,产物的正异比可达20∶1,但反应速率较低。使用水溶性Rh-TPPTS得到的效果最好,4-乙酰氨基丙烯可转化为N-乙酰基-5-甲氧基色胺(褪黑素),是一种可以调节睡眠周期的天然产物。
官能团化烯丙胺在Rh的催化作用下可发生氢甲酰化反应[36-37],区域选择性较高,产物可作为合成β内酰胺的中间体,用于合成抗生素。对于N-烯丙基-邻苯二甲酰亚胺,Rh-BIPHEPHOS作为催化剂时,目标产物收率可达95%,产物正异比可达18∶1[38]。

Lambers-Verstappen M M H等[39]研究了烯丙腈的氢甲酰化反应,正异比可达77∶23。使用手性双膦配体时,更易得到支链的醛,并且对映选择性非常好[40]。Lazzaroni R等[41]由光学纯的α-氨基酸高对映选择性合成手性吲哚里西啶类化合物,先合成手性N-乙烯基吡咯,然后在未经修饰的Rh催化剂作用下经氢甲酰化反应得到正构的醛,再经关环脱水反应得到稠杂环化合物。

烯丙基芳基的高区域选择性氢甲酰化反应引起关注,因为单萜类丁香酚、黄樟素、甲基胡椒酚和其异构体可得到相应的醛,在香精香料和药物领域应用广泛,如黄樟素在2位选择性氢甲酰化反应可以得到香料新洋茉莉醛[42]。Da Silva等[43]研究发现,NAPHOS作为配体可得到较高的区域选择性,dppp作为配体时主要得到支链醛。单膦配体与Rh组成的催化剂在丁香酚的氢甲酰化反应中,区域选择性最高可达84%[44]。
4 结语与展望
经过多年发展,官能团化烯烃的氢甲酰化反应取得了显著进步,开发了多种配体和金属络合物,表现出较高的活性和区域选择性,成功应用于天然产物类药物的合成。但存在烯基砜和亚砜类化合物的氢甲酰化反应研究不够深入、反应区域选择性不易控制、配体及催化剂制备限制了在大规模合成中的应用、底物类型不够丰富和催化剂通用性欠佳等缺点。在官能团化烯烃的氢甲酰化反应中,新配体和催化剂开发仍具有挑战性,具有广阔的应用前景。
[1]Naqvi S.Oxo Alcohols[C]//Process Economics Program Report 21E,Menlo Park,CA:SRI Consulting,2010.
[2]Reinius H K,Krause A O I.Hydroformylation of functional alkenes with heterodonor phosphine rhodium catalysts:substrate or ligand directed regioselectivity?[J].Catalysis Letters,2000,70(3):149-154.
[3]Fremy G,Monflier E,Carpentier J F,et al.An unusual enhancement of catalytic activity in biphasic catalysis:the rhodium catalyzed hydroformylation of acrylic esters[J].Journal of Molecular Catalysis A:Chemical,1998,129(1):35-40.
[4]Settambolo R,Pucci S,Bertozzi S,et al.Remarkable α-regioselectivity in the rhodium-catalyzed hydroformylation of 2-vinylpyridine[J].Journal of Organometallic Chemistry,1995,489(1/2):50-51.
[5]Botteghi C,Marchetti M,Paganelli S,et al.Study on the regioselectivity in the rhodium-catalyzed hydroformylation of vinyl-pyridine derivatives[J].Journal of Molecular Catalysis A:Chemical,1997,118:173-179.
[6]Lazzaroni R,Settambolo R,Caiazzo A,et al.Rhodium-catalyzed hydroformylation of 4-vinylpyridine:4-ethylpyridine formation via an unusual cleavage of the Rh-C bond by the enolic form of the oxo product[J].Organometallics,2002,21:2454-2459.
[7]Del Rio I,Pamies O,Van Leeuwen P W N M,et al.Mechanistic study of the hydroformylation of styrene catalyzed by the rhodium/BDPP system[J].Journal of Organometallic Chemistry,2000,608:115-121.
[8]Yu S,Chie Y M,Guan Z H,et al.Highly regioselective hydroformylation of styrene and its derivatives catalyzed by Rh complex with tetraphosphorus ligands[J].Organic Letters,2009,11(1):241-244.
[9]Thiel O,Bernard C,Larsen R,et al.Methods of synthesizing cinacalcet and salts thereof:WO,2009002427[P].2008-12-31.
[10]Fogg D E,Dos Santos E N.Tandem catalysis:a taxonomy and illustrative review[J].Coordination Chemistry Reviews,2004,248(21/24):2365-2379.
[11]Botteghi C,Marchetti M,Paganelli S,et al.Rhodium catalyzed hydroformylation of 1,1-bis(p-fluorophenyl)allyl or propargyl alcohol:a key step in the synthesis of fluspirilen and Penfluridol[J].Tetrahedron,2001,57:1631-1637.
[12]Botteghi C,Corrias T,Marchetti M,et al.A new efficient route to tolterodine[J].Organic Process Research & Development,2002,6(4):379-383.
[13]Botteghi C,Cazzaloto L,Marchetti M,et al.New synthetic route to pharmacologically active 1-(N,N-dialkylamino)-3,3-diarylpropanes via rhodium-catalyzed hydroformylation of 1,1-diarylethenes[J].Journal of Organic Chemistry,1995,60:6612-6615.
[14]Ono H,Kasuga T,Kiyono S,et al.Production process of 2-chloropropionaldehyde:EP,0260944[P].1988-03-23.
[15]Drent E.Process for preparation of branched aldehydes:GB,2217318[P].1989-10-25.
[16]Köckritz A,Bischoff S,Kant M,et al.Asymmetric hydroformylation and hydrogenation catalyzed by chiral rhodium and ruthenium complexes of phosphorylated 2,2’-bis(diphenyl-phosphino)-1,1’-binaphthyls[J].Journal of Molecular Catalysis A:Chemical,2001,174(1/2):119-126.
[17]Siegel H,Himmele W.Synthesis of intermediates by rhodium-catalyzed hydroformylation[J].Angewandte Chemie International Edition,1980,19(3):178-183.
[18]Fell B,Barl M.Hydroformylierung von vinylacetat zu propandiolderivaten[J].Journal of Molecular Catalysis,1977,2:301-306.
[19]Chen X.Methods for preparing ester of 1,3-propylene glycol and 1,3-propylene glycol:WO,2011075905[P].2011-06-30.
[20]Kurkov V P.Preparation of 2-cyanoaldehydes by rhodium-catalyzed hydroformylation:US,4344896[P].1982-08-17.
[21]Botteghi C,Lando C,Matteoli U,et al.Studies on the preparation of 2-(trifluoromethyl)acrylic acid and its esters from 3,3,3-trifluoropropene via hydrocarbonylation reactions[J].Journal of Fluorine Chemistry,1997,83(1):67-71.
[22]Consiglio G,Kollar L,Kolliker R.Cyclometallated compounds Ⅵ.Cyclopalladation of 2-phenylpyrimidines with pendant pyrazole donors[J].Journal of Organometallic Chemistry,1990,395(3):375-381.
[23]Garcia L,Claver C,Dieguez M,et al.A highly selective synthesis of 3-hydroxy-2-methylpropionamide involving a one-pot tandem hydroformylation-hydrogenation sequence[J].Chemical Communications,2006,2:191-193.
[24]Saidi O,Liu S,Xiao J.Effects of ligands on the rhodium-catalyzed hydroformylation of acrylate[J].Journal of Molecular Catalysis A:Chemical,2009,305(1/2):130-134.
[25]Reetz M T,Li X.The influence of mixtures of monodentate achiral ligands on the regioselectivity of transition-metal-catalyzed hydroformylation[J].Angewandte Chemie International Edition,2005,44(19):2962-2964.
[26]Zhang X,Cao B,Yu S,et al.Rhodium-catalyzed asymmetric hydroformylation of N-allylamides:highly enantioselective approach to β2-amino aldehydes[J].Angewandte Chemie International Edition,2010,49:4047-4050.
[27]White D F,Boogaerts I,Cole-Hamilton D J.Hydroformylation process:WO,2011084268[P].2011-07-14.
[28]Dureanleau R G,Knifton J F.Process for separation of product resulting from hydroformylation:US,4678857[P].1987-07-07.
[29]White D F,Boogaerts I,Cole-Hamilton J.Hydroformylation process:US,7790932[P].2010-09-07.
[30]Kilroy T G,O Sullivan T P,Guiry P J.Synthesis of dihydrofurans substituted in the 2-position[J].European Journal of Organic Chemistry,2005,2005(23):4929-4949.
[31]Ruiz N,Polo A,Castillon S,et al.Hydroformylation of allyl ethers.A study of the regioselectivity using rhodium catalysts[J].Journal of Molecular Catalysis A:Chemical,1999,137(1/3):93-100.
[32]Polo A,Claver C,Castillon S,et al.Regioselective hydroformylation of cyclic vinyl and allyl ethers with rhodium catalysts.Crucial influence of the size of the phosphorus cocatalyst[J].Organometallics,1992,11(11):3525-3533.
[33]Briggs J R,Klosin J,Whiteker G T.Synthesis of biologically active amines via rhodium-bisphosphite-catalyzed hydroaminomethylation[J].Organic Letters,2005,7(22):4795-4798.
[34]Whiteker G.Synthesis of fexofenadine via rhodium-catalyzed hydroaminomethylation[J].Topics in Catalysis,2010,53(15):1025-1030.
[35]Verspui G,Elbertse G,Sheldon F A,et al.Selective hydroformylation of N-allylacetamidein an inverted aqueous two-phase catalytic system,enabling a shortsynthesis of melatonin[J].Chemical Communications,2000,28(10):1363-1364.
[36]Dekeukeleire S,Dhooghe M,Muller C,et al.Rhodium-catalysed hydroformylation of N-(2-propenyl)-β-lactams as a key step in the synthesis of functionalised N-[4-(2-oxoazetidin-1-yl)but-1-enyl]acetamides[J].New Journal of Chemistry,2010,34:1079-1083.
[37]Cesarotti E,Rimoldi I.Stereoselective synthesis of 1-methylcarbapenem precursors:studies on the diastereoselective hydroformylation of 4-vinyl β-lactam with aminophosphonite-phosphinite and aminophosphine-phosphite rhodium(Ⅰ) complexes[J].Tetrahedron:Asymmetry,2004,15:3841-3845.
[38]Cuny G D,Buchwald S L.Practical,high-yield,regioselective,rhodium-catalyzed hydroformylation of functionalized alpha-olefins[J].Journal of the American Chemical Society,1993,115:2066-2068.
[39]Lambers-Verstappen M M H,De Vries J G.Rhodium-catalysed asymmetric hydroformylation of unsaturated nitriles[J].Advanced Synthesis & Catalysis,2003,345:478-482.
[40]Cobley C C,Gardner K,Klosin J,et al.Synthesis and application of a new bisphosphite ligand collection for asymmetric hydroformylation of allyl cyanide[J].Journal of Organic Chemistry,2004,69:4031-4040.
[41]Lazzaroni R,Settambolo R.Synthesis of indolizidines from optically pure α-amino acids via stereocontrolled rhodium-catalyzed hydroformylation of N-allylpyrroles[J].Chirality,2011,23(9):730-735.
[42]Alhaffar M,Suleiman R,El Ali B.Rhodium-catalyzed one-pot hydroformylation-cyclization of allylbenzene derivatives:simple and efficient route to 5,6-dihydronaphthalenes[J].Catalysis Communications,2010,11(8):778-782.
[43]Da Silva A C,De Oliveira K C B,Gusevskaya E V,et al.Rhodium-catalyzed hydroformylation of allylbenzenes and propenylbenzenes:effect of phosphine and diphosphine ligands on chemo-and regioselectivity[J].Journal of Molecular Catalysis A:Chemical,2002,179:133-141.
[44]Alhaffar M,Suleiman R,Hussain S M S,et al.Ultranox626 as a selective ligand in rhodium-catalyzed hydroformylation-acetalization of allylbenzene derivatives[J].Reaction Kinetics,Mechanisms and Catalysis,2011,104(2):323-336.
Research and application advance in hydroformylation of functionalized olefins
LiuXu1,2*,LiuZhongneng1,2,GuSongyuan1,2
(1.Sinopec State Key Laboratory of Green Chemical Engineering and Industrial Catalysis, Shanghai 201208, China; 2.Sinopec Shanghai Research Institute of Petrochemical Technology, Shanghai 201208, China)
Hydroformylation is one of the most important homogeneous catalytic processes in industry.Hydroformylation of functionalized olefins provides the routes to obtaining aldehydes with one or more additional functional groups.Such functionalized aldehydes can be sold as final products or used as intermediates in the synthesis of fine chemicals,pharmaceuticals and fragrances.The hydroformylation showed distinct differences compared to the reaction with unfunctionalized alkenes.In this paper, the progress in hydroformylation of functionalized olefins and its application were introduced,including vinyl arenes,α-functionalized olefins and β-functionalized olefins.The development prospects of hydroformylation of functionalized olefins was put forward.
fine chemical engineering; functionalized olefins; hydroformylation; aldehyde
TQ426.94;O643.36Document code: AArticle ID: 1008-1143(2016)08-0001-06
2016-04-14基金项目:中国石化基金(414088)资助项目
刘旭,1983年生,男,河南省郑州市人,博士,工程师,研究方向为均相催化。
刘旭。
10.3969/j.issn.1008-1143.2016.08.001
TQ426.94;O643.36
A
1008-1143(2016)08-0001-06
doi:10.3969/j.issn.1008-1143.2016.08.001

