Evaluation o f Xpert MTB/RIF for the Diagnosis of Extrapulmonary Tuberculosis in China*
YUAN Mei, LYU Yan, CHEN Su Ting, CAI Chao, LI Yuan, ZHANG Zhi Guo,
LI Yun Xu3, DONG Ling Ling3, FU Yu Hong3, HUANG Hai Rong3, GAO Ji M in1,#, and LI Wei M in3,#
Letter to the Editor
Evaluation o f Xpert MTB/RIF for the Diagnosis of Extrapulmonary Tuberculosis in China*
YUAN Mei1,3,¶, LYU Yan2,¶, CHEN Su Ting3,¶, CAI Chao3,¶, LI Yuan4, ZHANG Zhi Guo5,
LI Yun Xu3, DONG Ling Ling3, FU Yu Hong3, HUANG Hai Rong3, GAO Ji M in1,#, and LI Wei M in3,#
We evaluate the performance of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis (EPTB) in China. The performance of Xpert was evaluated compared to the composite reference standard (CRS), drug susceptibility testing(DST), and imaging examination. The overall sensitivity and specificity of Xpert were 64.1%(195/304) and 100% (24/24), respectively, using CRS as the gold standard. The sensitivity was significantly higher than that of culture for pus(P<0.05). The proportion of EPTB-positive cases diagnosed by imaging was two times more than that diagnosed using Xpert; however, 6 out of 19 cases may have been overdiagnosed by imaging. Compared to phenotypic DST, the sensitivity and specificity of Xpert were 80% (12/15) and 100%(75/75), respectively. Considering its high sensitivity and specificity, Xpert MTB/RIF may be used as a rapid initial test for EPTB diagnosis, and may also support a quicker decision on the treatment regimen. The combination of imaging and Xpert testing could provide high efficiency and accurate diagnosis of suspected EPTB.
Tuberculosis (TB), which is mainly caused by Mycobacterium tuberculosis, remains one of the most serious challenges for public health. China is one of the high-burden countries not only in TB but also in multidrug-resistant TB (MDR-TB)[1]. TB is clinically classified as pulmonary (PTB) and extrapulmonary TB (EPTB). Compared to PTB, the diagnosis of EPTB is more difficult and challenging. Existing tests, such as radiological methods, lacked specificity and objectivity, while traditional bacteriological tests such as smears and cultures were limited by their lack of sensitivity and were time-consuming (one to two months for diagnosis)[2]. Xpert MTB/RIF, a newly developed molecular test,specifically amplifies a TB-specific rpoB gene fragment of 81 bp and can simultaneously detect M. tuberculosis and rifampicin (RIF) resistance within 2 hours[3]. The Xpert assay has been used by the World Health Organization (WHO) for the diagnosis of PTB since 2010[4]and for the diagnosis of EPTB since 2013[5]. However, the performance of the Xpert assay in the diagnosis of EPTB was found to be variable among different regions[6]. The aim of this study was to evaluate the clinical application of the Xpert system in diagnosing EPTB and detecting RIF resistance in a national TB referral center in China(Beijing Chest Hospital).
We retrospectively collected the medical records of hospitalized patients whose EPTB specimens were submitted for Xpert MTB/RIF assay Between April 1, 2014 and March 31, 2015. Specimens without the results of acid-fast bacillus(AFB) smear microscopy and mycobacterial culture were excluded. For all the above methods, the processing of specimens was in accordance with our standard laboratory procedures[7]. The performance of the Xpert assay was compared to the compositereference standard (CRS)[7], drug susceptibility testing (DST)[7], and examination using high-resolution computed tomography/magnetic resonance imaging (HR-CT/MRI)[7]. The chi-square test (SPSS version 19.0; Chicago, IL, USA) was used for statistical analysis of categorical variables, and a P-value <0.05 was considered statistically significant. The CRS diagnosis of EPTB in this study included the following: (1) all culture-confirmed cases (in this group, microscopy may or may not be positive); (2)microscopy-positive cases with imaging suggestive of TB; (3) pathology confirmed for TB; and (4) all the above tests negative, with only clinical symptoms,imaging findings, and a response to empirical antituberculosis treatment.
A total of 328 hospitalized patients with suspected EPTB were recruited, from whom EPTB specimens were simultaneously submitted for Xpert testing, AFB staining, and culture. Pus accounted for 75.6% (248/328) of all specimens, of which 221(89.1%, 221/248) came from bone and joint lesions,w ith the rest from chest wall (11), psoas (6), lymph node (4), abdominal wall (1), and other lesions (5). Other than pus specimens, 19.2% (63/328) and 5.2%(17/328) of all specimens were pleural fluid and cerebrospinal fluid (CSF), respectively. According to CRS, 304 patients were diagnosed with EPTB and 24 patients were not considered TB.
Using CRS as the reference, our studies showed that the pooled sensitivity of Xpert assay (64.1%,195/304) was higher than that of culture (31.3%,95/304) and smear (19.7%, 60/304), with a specificity of 100% (Table 1). These results were consistent with the reports of previous studies and supported the WHO recommendation of this assay for the diagnosis of EPTB[5]. However, compared to the pooled sensitivity of the Xpert assay reported by a previous study (81.3%)[8], the overall sensitivity of the Xpert assay seemed to be lower in our study. Other than the different sources of specimens, the HIV-positive patients (0%) and pediatric patients(0-18 years, 8.8%) included in our study might be a major reason, as these represented 10% and 33.5%,respectively, in a previous study[8]. Consistent with previous research, the sensitivity of the Xpert assay in patients with HIV co-infection was higher than in non-HIV-infected TB patients[9], and the sensitivity in pediatric patients was higher than in adults[8].
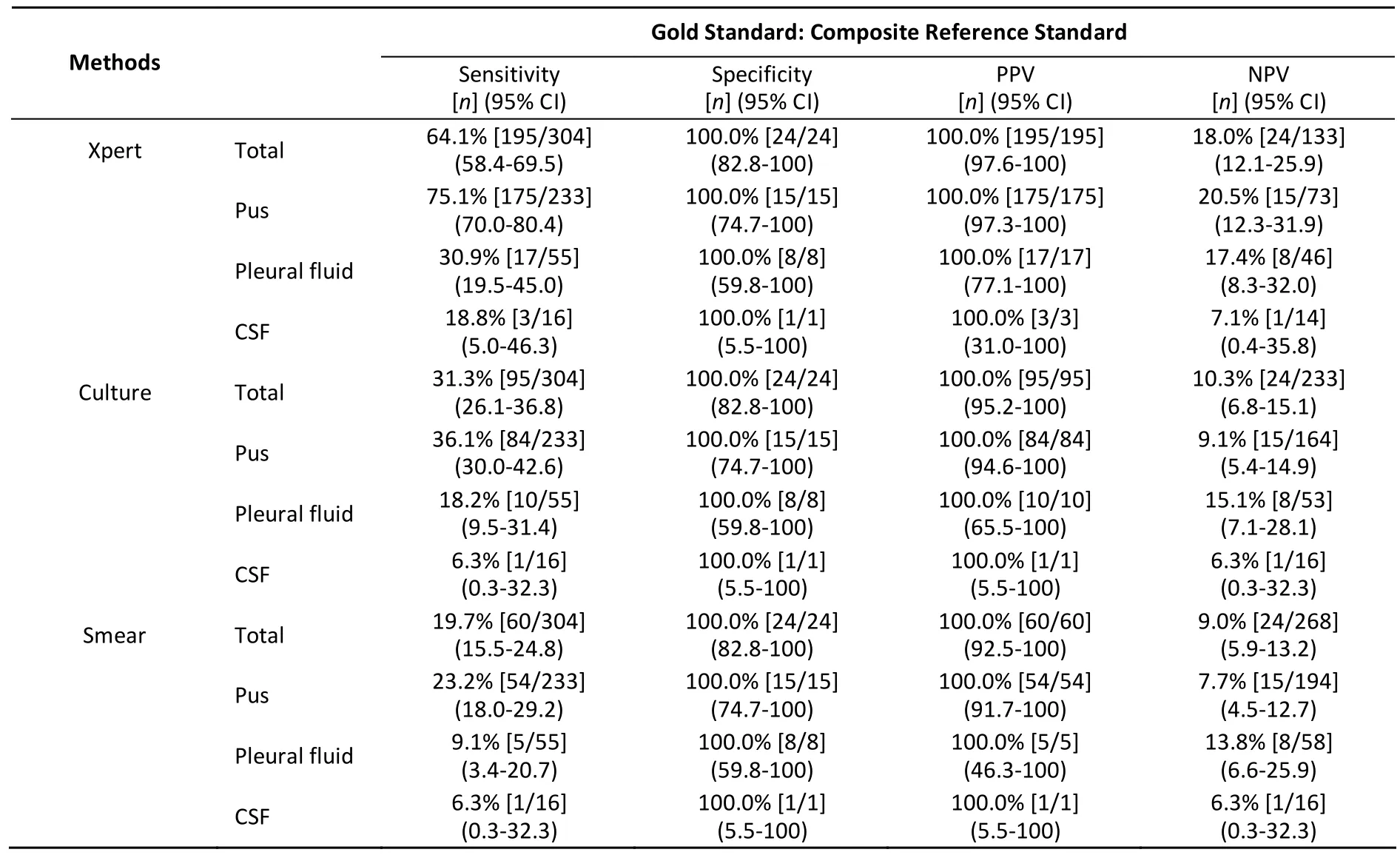
Table 1. Performance of Xpert MTB/RIF Assay for Different EPTB Specimens Compared to CRS, Culture, and Smear
The Xpert assay had the highest sensitivity for pus (75.1%, 175/233), followed by pleural fluid(30.9%, 17/55) and CSF (18.8%, 3/16) (Table 2). Some reports[8]showed that Xpert MTB/RIF had better sensitivity than smears, but was still not optimal in comparison with culture. However, we demonstrated in the current study that the Xpert assay achieved higher sensitivities for all specimens,especially compared to pus culture (75.1% versus 36.1%, P<0.05). The low sensitivity of culture might be due to the fact that 82% (269/328) of patients in this study received antituberculosis treatment for various periods of time before sample collection,and there might be a loss of viable bacilli during the pretreatment of specimens. As for pus from bone and joints of tuberculosis patients, the bacteria tend to remain dormant in an anaerobic or low oxygen joint cavity and are difficult to detect by traditional culture methods.
Of 304 cases diagnosed with EPTB based on CRS,the Xpert showed excellent sensitivity (100%) in smear-positive/culture-positive (S+/Culture+), and smear-positive/culture-negative (S+/Culture-) cases,and was 91.7% in smear-negative/culture-positive(S-/Culture+) cases. In smear-negative/culturenegative (S-/Culture-) cases, the sensitivity of the Xpert assay was 43.5% (80/184) (Table 2). This suggested that the rapid detection of MTB by Xpert has great clinical application value when considering the long culture time.
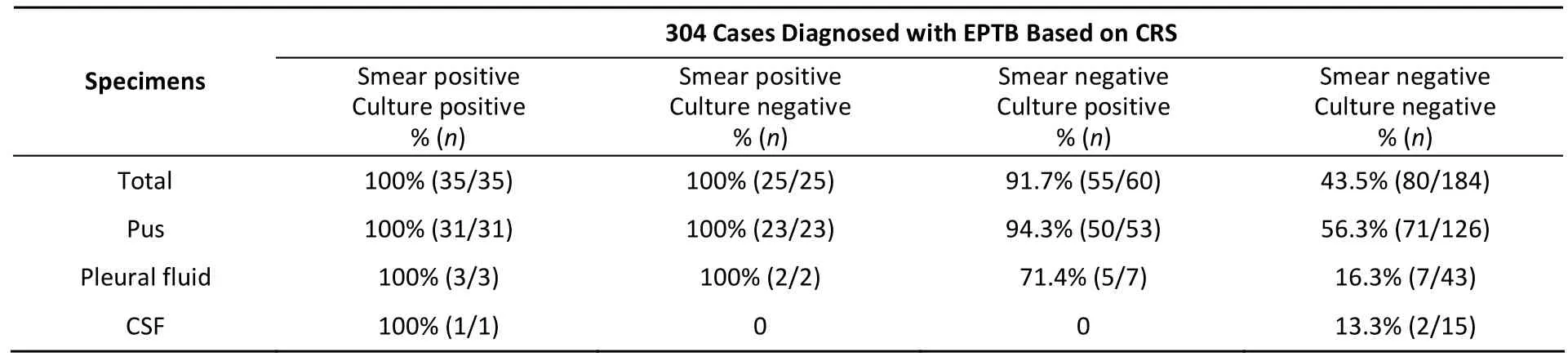
Table 2. The Sensitivity of Xpert Assay in Different Smear and Culture Groups
Other than bacteriological exam ination, the HR-CT/MRI is an important imaging diagnostic method for EPTB in China. In our study, a total of 175 cases (84.1%, 175/208) had radiological (HR-CT/MRI)examination results among all smear and culture negative cases. On the basis of CRS, 156 cases were diagnosed as EPTB, while 19 were not EPTB (Figure 1). The number of EPTB-positive cases diagnosed with CT/MRI was statistically significantly greater(P<0.05) (140/156) than those diagnosed with Xpert(74/156). However, imaging might lead to misdiagnosis and overdiagnosis due to subjectivity. In the present study, 6 (31.6%, 6/19) cases were considered EPTB by HR-CT/MRI, but were not considered TB by CRS. Considering its reliability and excellent specificity, the Xpert assay could providemore accurate information for EPTB cases diagnosed by HR CT/MRI, and could be complementary to the imaging methods.
In this study, 95 M. tuberculosis-positive isolates underwent conventional phenotypic drug susceptibility testing (DST). Among these samples, 90 were M. tuberculosis-positive by Xpert assay. By using phenotypic DST as the gold standard, the sensitivity of the Xpert assay for detecting RIF resistance was 80% (12/15), while the specificity was 100% (75/75) for detecting RIF susceptibility, which is consistent w ith previous studies[9]. By sequencing three RIF-inconsistent strains (RIF sensitive by Xpert but RIF resistant by DST), all were found to have a w ild-type sequence on the RIF resistancedeterm ining region. The RIF resistance of the three isolates detected by conventional DST m ight be due to other mechanisms or to off-site mutations from the RIF resistance-determ ining region in the rpoB gene[10]. Considering the long time required for results by conventional DST, the Xpert assay could support quicker decisions on a treatment regimen.
In conclusion, the Xpert MTB/RIF system appears prom ising for EPTB diagnosis in China, given the good sensitivity compared to traditional tests. Its specificity indicated that it has high value in confirm ing a diagnosis of EPTB. The Xpert MTB/RIF may be used as a rapid initial test for EPTB diagnosis and can support quicker decisions on a treatment regimen, by simultaneously detecting M. tuberculosis and RIF-resistance in two hours.
Acknowledgement We thank the staff working at the National Clinical Laboratory on Tuberculosis,Beijing Chest Hospital, Capital Medical University.
¶These authors contributed equally to this work.
#Correspondence should be addressed to: LI Wei Min, E-mail: lwm_18@aliyun.com, Tel: 86-10-89509359,Fax: 86-10-89509359; GAO Ji Min, E-mail: jimingao64@ 163.com
Biographical notes of the first authors: YUAN Mei,female, master degree, majoring in bacteriology diagnosis of tuberculosis; LYU Yan, female, doctoral degree, a clinical doctor; CHEN Su Ting, female, doctoral degree, majoring in Molecular biology research; CAI Chao, male, doctoral degree, a clinical doctor.
Accepted: August 1, 2016
REFERENCES
1. World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB): global report on surveillance and response. Geneva, Switzerland, WHO, 2010.
2. Chegou NN, Hoek KG, Kriel M, et al. Tuberculosis assays: past,present and future. Expert Rev Anti Infect Ther, 2011; 9,457-69.
3. Lawn SD, Nicol MP. Xpert® MTB/RIF assay: development,evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol, 2011; 6, 1067-82.
4. World Health Organization. Report of the Tenth Meeting WHO Strategic and Technical Advisory Group for Tuberculosis(STAGTB). Geneva, World Health Organization, 2010.
5. World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system for the diagnosis of pulmonary and extra-pulmonary TB in adults and children. Geneva, Switzerland: WHO, 2013.
6. Jia LQ, Long HY, Hu QJ, et al. Diagnosis value with Xpert MTB/RIF assay for extrapulmonary tuberculosis: A meta-analysis. Chin J Clinicians, 2013; 7, 2587-91.
7. Chinese Ministry of Health. The diagnosis of Tuberculosis. The implement guide of Tuberculosis Prevent and Treatment in China. Beijing, China. Chinese Ministry of Health. 2008. (In Chinese)
8. Tortoli E, Russo C, Piersimoni C, et al. Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur Respir J, 2012; 40, 442-7.
9. Patel VB, Theron G, Lenders L, et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous meningitis in a high burden setting: a prospective study. PLoS Med, 2013; 10,e1001536.
10.Jenkins C. Rifampicin resistance in tuberculosis outbreak,London, England. Emerg Infect Dis, 2005, 11, 931-4.
10.3967/bes2016.080
March 4, 2016;
*This study was supported by research funding from National Natural Science Foundation of China (81273144); Beijing Natural Science Foundation Program and Scientific Research Key Program of Beijing Municipal Commission of Education(KZ201510025024); and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201304).
1. Zhejiang Provincial Key Laboratory for Technology & Application of Model Organisms, School of Laboratory Medicine and Life Science, Wenzhou Medical University, Wenzhou 325035, Zhejiang, China; 2. Department of Radiology, Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing Chest Hospital, Capital Medical University, Beijing 101149,China; 3. National Clinical Laboratory on Tuberculosis, Beijing Key Laboratory on Drug-resistant Tuberculosis Research,Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing Chest Hospital, Capital Medical University, Beijing 101149, China; 4. Department of Orthopaedics, Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing Chest Hospital, Capital Medical University, Beijing 101149, China; 5. Institute of Tuberculosis Prevention and Control of District Changping, Beijing 102200, China
 Biomedical and Environmental Sciences2016年8期
Biomedical and Environmental Sciences2016年8期
- Biomedical and Environmental Sciences的其它文章
- Risk of Treatment Failure in Patients with Drug-susceptible Pulmonary Tuberculosis in China*
- Viral Contamination Source in Clinical Microbiology Laboratory*
- Effect of Smo SiRNA-mediated Hedgehog Signaling Pathway Inhibition on Palatal Fusion*
- 8-isop rostane as Oxidative Stress Marker in Coal Mine Wo rkers
- Bio logical Effec ts o f Clo th Con taining Specific Ore Pow der in Patien ts w ith Po llen Allergy
- Alcohol Drinking, Dyslipidemia, and Diabetes: A Population-based Prospective Cohort Study among lnner Mongolians in China*
