右美托咪定对单肺通气大鼠术后早期空间记忆功能的影响*
鲍 冲, 金 哲, 柯剑娟, 陈 凯, 王焱林
(武汉大学中南医院, 湖北 武汉 430071)
右美托咪定对单肺通气大鼠术后早期空间记忆功能的影响*
鲍冲, 金哲, 柯剑娟, 陈凯, 王焱林**
(武汉大学中南医院, 湖北 武汉430071)
目的: 探讨右美托咪定对单肺通气大鼠术后早期空间记忆能力的影响。方法: 选择雄性SD大鼠72只,月龄10~11月,随机分为双肺通气组(TLV组)、单肺通气组(OLV组)和右美托咪定组(DEX组);DEX组于诱导前30 min腹腔注射盐酸右美托咪定25 μg/kg,TLV组与OLV组给予等量生理盐水腹腔注射;各组大鼠采用异氟烷诱导并维持麻醉,经口气管插管,TLV组全程双肺通气,OLV组和DEX组双肺通气5 min后采用插入过深法行单肺通气,90 min后恢复双肺通气30 min;每组各取6只大鼠,行右颈内静脉、左侧颈总动脉逆血流置管,以开始单肺通气0、30、60、90和120 min时 (分别记作T0、T1、T2、T3、T4)采集右颈内静脉和左侧颈总动脉血行血气分析,计算脑氧摄取率(CERO2);各组剩余18只大鼠,于术前5 d开始每天行Morris水迷宫定向航行训练,并于术后第1 、3 、7 天时行空间探索实验,记录各组大鼠逃避潜伏期、目标象限停留时间和穿台次数。结果: 随着术后通气时间延长,各组CERO2逐渐上升(P<0.01),OLV组在T1至T4时点CERO2明显高于TLV组(P<0.01),恢复双肺通气后,仍高于TLV组;DEX组在T1至T4时点CERO2均明显低于OLV组(P<0.05或P<0.01);各组大鼠随着术前训练天数的增加,逃避潜伏期逐渐缩短(P<0.01);OLV组在术后第 1、3、7 天,逃避潜伏期均较TLV组明显延长(P<0.01),目标象限停留时间缩短(P<0.01),穿台次数减少(P<0.01);DEX组在术后第 1、3、7 天逃避潜伏期均短于OLV组,且目标象限停留时间增加,穿台次数增加(P<0.05)。结论: 右美托咪定可以改善单肺通气大鼠术后早期空间记忆功能,其机制可能与降低脑组织氧耗有关。
右美托咪定; 单肺通气; 脑氧摄取率; 认知功能障碍
[Abstract]Objective: To investigate the effect of Dexmedetomidine on the early spatial memory function in rats undergoing one-lung ventilation surgery. Methods: 72 male sprague-dawley rats were randomly divided into 3 groups with 24 of each: group of two-lung ventilation (group TLV),group of one-lung ventilation(group OLV) and group of one-lung ventilation accompanied with the injection of Dexmedetomidine(group DEX). Group DEX received Dexmedetomidine by intraperitoneal injection with a dose of 25 μg/kg in 30 minutes before induction, while an equal volume of saline was given to group TLV and group OLV at the same time. All three groups receive isoflurane anesthesia (with a flow of 1.5%), two-lung ventilation were given for 5min after intubation for each group followed by one-lung ventilation for 90min and lung inflate for 30min for group OLV and group DEX while only two-lung ventilation were given for group TLV. 6 rats of each group were chosen randomly to capture blood samples from left carotid artery and right internal jugular vein at the time of the beginning(T0), 30 min(T1), 60 min(T2),90 min (T3) of one-lung ventilation and 30 min after lung inflate (T4) for blood gas analysis to calculate CERO2. The remaining rats in each group were divided randomly into 3 subgroups with 6 of each. The spatial memory function was evaluated by Morris Water Maze(MWM). The place navigation test was taken for five consecutive day before ventilation. The 3 subgroups had the spatial probe test respectively on 1, 3 or 7 post-operative day (post-1d, post-3d, post-7d) for detecting escape latency(EL), percentage of time spend in target quadrant and times of cross over the platform. Results: CERO2in every groups increased gradually while ventilation. Compared with group TLV, CERO2increased significantly at T1、T2、T3、T4in group OLV(P<0.01) . Compared with group OLV, CERO2were lower in group DEX at T1、T2、T3、T4(P<0.05 orP<0.01). EL shortened gradually in each training day(P<0.01) and no difference among groups. Compared with group TLV, EL prolonged in group OLV at 1、3、7 post-operative days(P<0.01). Compared with group OLV, EL shortened, times of cross over the platform and percentage of time spend in target quadrant enhanced at 1、3、7 postoperative days in group DEX (P<0.05 orP<0.01). Conclusions: Dexmedetomidine improves early spatial memory function in rats undergoing one-lung ventilation, the mechanism may be related with reducing oxygen consumption in the brain.
[Key words]dexmedetomidine; one-lung ventilation; cerebral oxygen uptake rates; cognitive dysfunction
术后认知功能障碍(postoperative cognitive dysfunction,POCD)表现为麻醉和手术后出现的学习、空间记忆等功能的减退,早期术后认知功能障碍通常指术后1周内出现的认知功能减退,可延长住院日,增加医疗负担,近年来在临床受到重视。POCD的发生机制复杂,可能涉及到神经炎性反应及脑氧供需平衡失调[1-2]。单肺通气(one-lung ventilation, OLV)广泛用于各类心胸外科手术,可有效改善手术视野,但这种非生理性通气方式,不可避免的导致了通气/血流比失调,并对组织氧供产生影响,从而增加POCD的发生率[3-4]。盐酸右美托咪定(dexmedetomidine,DEX)是高选择性的α2受体激动剂,具有稳定的镇静效益[5]。研究证明,DEX在器官缺血、缺氧性损伤中有器官保护作用[6-7]。本研究拟通过Morris水迷宫观察盐酸右美托咪定对单肺通气大鼠术后脑氧摄取率(CERO2)及空间记忆功能的影响,探讨其是否能够调节单肺通气时的脑氧供需平衡,为临床麻醉提供参考。
1 材料与方法
1.1材料
健康雄性SD大鼠72只,月龄10~11月,体重(260±20)g,由湖北省预防科学院疾病预防控制中心动物实验中心提供,饲养于武汉大学中南医院动物实验中心SPF级动物房。大鼠呼吸机为美国KENT公司TOPO动物呼吸机。大鼠Morris水迷宫:直径160 cm、深50 cm的圆形水池,内壁黑色,池内水深30 cm,自动控温在(26±1)℃,房间内光照恒定,无光线直射在水池内,将水池均分为4个象限,第3象限(目标象限)中央放置一直径为12 cm,高28 cm的圆形黑色高台,台面位于水下2 cm,池壁内等距贴有4个不同形状的标记。采用西班牙Panlab公司的SMART 3.0软件进行行为学分析。
1.2方法
1.2.1动物分组适应性饲养7 d后,大鼠随机分为双肺通气组(TLV组),单肺通气组(OLV组)及右美托咪定组(DEX组),每组24只。温度24 ℃,湿度60%,通风良好,自由进食及饮水,光周期为12 h(7∶00开灯)条件下饲养,开始行为学实验前3 d分笼饲养。
1.2.2适应性训练每组各选18只,于术前5 d,每天14~16时行Morris水迷宫定向航行训练。训练时,分别于各象限标记点将大鼠面向池壁放入水中,记录大鼠自入水点到找到并爬上固定平台所需时间(潜伏期);如果大鼠在60 s内未找到平台,则人为引导大鼠到达平台且停留30 s,此时潜伏期记为60 s。每日14∶00~18∶00进行测试,4次/d,4次潜伏期成绩的均值记为当日成绩。由SMART3.0系统采集和分析大鼠的逃避潜伏期。
1.2.3动物模型制备各组24只大鼠置于透明诱导箱中经异氟烷诱导麻醉后,经口明视下气管插管,置管深度距门齿5 cm,吸入氧浓度 100%,呼吸频率 60次/min,压力控制模式下行双肺通气5 min,1.5%异氟烷维持麻醉,5 min双肺通气完成时记作0 min[8]。TLV组: 诱导前30 min腹腔注射生理盐水6.25 mL/kg,0 min后呼吸频率 60次/min,压力控制模式下行双肺通气120 min。OLV组:诱导前30 min腹腔注射生理盐水6.25 mL/kg,0 min后将气管导管插入过深,距门齿10 cm,气囊充气0.3 mL,通过胸廓起伏及听诊器听诊确认右肺单肺通气,在呼吸频率80次/min,压力控制模式下行单肺通气90 min后气管导管退至距门齿5 cm,恢复双肺通气30 min[9]。DEX组:诱导前30 min腹腔注射25 μg/kg(DEX用0.9% NaCl溶液稀释成4 mg/L,即DEX用量为 6.25 mL/kg)DEX,其余操作同OLV组。
1.2.4CERO2每组取6只未行适应性训练的大鼠,于诱导后插管前,沿颈部正中切开皮肤,游离右颈内静脉、左侧颈总动脉,分别逆血流方向置管。于0、30、60、90和120 min(分别记作T0、T1、T2、T3、T4)时,采集右颈内静脉、左侧颈总动脉血各0.2 mL即刻送血气分析。根据Fick公式计算CERO2[10]。
1.2.5认知功能检测每组分别于术后第1、3、7天各取6只大鼠行Morris水迷宫空间探索试验,记录大鼠的逃避潜伏期、目标象限停留时间及穿台次数。
1.3统计学分析
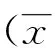
2 结果
2.1CERO2
与T0时点比较,随着通气时间延长,TLV组CERO2逐渐上升;OLV组、DEX组在单肺通气期间CERO2渐上升,在T3达到峰值(P<0.01),恢复双肺通气后,CERO2下降。组间比较,各组在T0时点,CERO2差异无统计学意义;随着单肺通气时间延长,OLV组在T1至T4时点的CERO2明显高于TLV组(P<0.01)。DEX组在单肺通气期间CERO2明显低于OLV组,恢复双肺通气后仍低。见表1。

表1 3组大鼠各时点
与T0比较,(1)P<0.01,(2)P<0.05;与TLV组比较,(3)P<0.01,(4)P<0.05;与OLV组比较,(5)P<0.01,(6)P<0.05
2.2Morris水迷宫行为学检测
各组大鼠随着术前训练天数的增加,逃避潜伏期逐渐缩短(P<0.01),各组术前组间比较差异无统计学意义(P>0.05)。术后各组大鼠逃避潜伏期都较术前延长。同TLV组比较,术后第1、3及7天,OLV组逃避潜伏期均明显延长(P<0.01),目标象限停留时间缩短(P<0.01),穿台次数减少(P<0.01)。同OLV组比较,术后第1、3及7 天,DEX组逃避潜伏期明显缩短,目标象限停留时间延长,穿台次数增加(P<0.01或P<0.05)。见表2、表3。
3 讨论
POCD最早于1955年提出,其发生受到年龄、基础疾病、手术及麻醉方式、持续时间等多种因素的影响[11-12]。其发病机制涉及神经、内分泌等多系统,尚待深入研究。目前,对其机制的研究主要集中在炎性应激与脑组织氧供不足两方面。脑组织具有高灌注高代谢低储备的特点,对缺血缺氧的耐受性较差。随着胸科手术量的增加,经单肺通气手术后的POCD受到越来越多的关注。行单肺通气时,肺循环血流重新分布,流经非通气侧肺的血液未经氧合,导致脑氧饱和度下降。研究证实单肺通气期间脑氧饱和度下降,其会导致术后认知功能的改变[13-14]。CERO2为脑组织在单位时间内消耗的氧量,排除了血红蛋白浓度的影响[15]。CERO2增加,说明脑氧摄取增加,脑血流相对于脑氧耗不足;CERO2降低,提示脑氧摄取减少,脑血流相对于脑氧耗过剩。本研究通过检测CERO2反应脑氧供需平衡,其结果显示随着通气时间延长,各组CERO2均升高,脑氧耗逐渐增加。同TLV组比较, OLV组CERO2值于在单肺通气后各个时点升高更显著,脑组织需氧量明显增加,氧供相对不足。从Morris水迷宫的结果分析,OLV组大鼠术后第1 、3 和7天时逃避潜伏期均较TLV组延长,目标象限停留时间缩短,穿台次数减少,说明单肺通气后,大鼠术后早期空间记忆能力出现明显减退。

表2 3组大鼠术前Morris水迷宫透应性训练逃避潜伏期
(1)与术前5 d比较,P<0.01
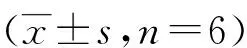
表3 3组大鼠术后Morris水迷宫空间探索实验结果
与TLV组同指标比较,(1)P<0.01;与OLV组同指标比较,(2)P<0.01,(3)P<0.05
选择性α2受体激动剂右美托咪定,具有抑制交感神经、镇静、遗忘等作用[16]。大量研究证明DEX在肝脏、肾脏、肠等组织的缺血、缺氧及再灌注损伤中具有器官保护作用。在大脑缺氧缺血的实验模型中,DEX显示出了降低CERO2,减轻缺氧缺血性脑损伤,并改善脑损伤后神经功能的预后的作用[17-19]。Sanders等研究证明了DEX对成年大鼠的神经保护呈现出剂量依赖效应;进一步研究显示,同更小的剂量相比,25~50 μg/kg的剂量可以产生良好的神经保护作用[20]。因此本研究在诱导前30 min腹腔注射25 μg/kg剂量的DEX,来观察其是否能降低单肺通气期间的CERO2,以及能否改善单肺通气大鼠术后的空间记忆能力。
本研究显示,腹腔注射DEX后,单肺通气期间,CERO2仍上升。相对于未给予DEX的OLV组,DEX可以有效降低单肺通气期间及恢复双肺通气后的各个时点的CERO2,即右美托咪定可以降低单肺通气期间脑组织氧耗。从Morris水迷宫的结果分析,DEX组大鼠在术后第1、3、7天逃避潜伏期均较OLV组缩短,目标象限停留时间延长,穿台次数增加,即DEX可以改善单肺通气后的空间记忆能力。
综上所述,DEX可改善单肺通气大鼠术后空间记忆能力,其机制可能与改善脑氧供需平衡相关。
[1]Hovens IB, Schoemaker RG, van der Zee EA, et al. Postoperative cognitive dysfunction: Involvement of neuroinflammation and neuronal functioning[J].Brain Behav Immun, 2014(38):202-210.
[2]Ni C, Xu T, Li N, et al. Cerebral oxygen saturation after multiple perioperative influential factors predicts the occurrence of postoperative cognitive dysfunction[J].BMC Anesthesiol, 2015(15):156.
[3]Yu YT, Zhu CC, Qian XZ,et al. Adult patient with pulmonary agenesis: focusing on one-lung ventilation during general anesthesia[J]. J Thorac Dis, 2016(1):124-129.
[4]Seo JH, Cho CW,Hong, et al. The effects of thermal softening of double-lumen endobronchial tubes on postoperative sore throat, hoarseness and vocal cord injuries: a prospective double-blind randomized trial[J]. Br J Anaesth, 2016(2): 282-288.
[5]Zhang WH, Wang ZX, Song XR, et al. Comparison of rescue techniques for failed chloral hydrate sedation for magnetic resonance imaging scans-additional chloral hydrate vs intranasal dexmedetomidine[J]. Paediatr Anaesth, 2015(3):273-279.
[6]Sun Y, Gao Q, Wu N, et al. Protective effects of dexmedetomidine on intestinal ischemia-reperfusion injury[J]. Exp Ther Med, 2015(2):647-652.
[7]Pasechnik IN, Maklai AV, Tepliakova AN, et al. Cerebral dysfunction as a component of multiple organ failure in surgical patients(lecture) [J]. Khirurgiia (Mosk), 2015(6):4-16.
[8]Li ZQ, Li LX, Mo N. Duration-dependent regulation of autophagy by isoflurane exposure in aged rats[J].Neurosci Bull, 2015(4):505-513.
[9]林飞,潘灵辉,杨建,等. 不同单肺通气时间对大鼠肺组织中水通道蛋白1、4表达的影响[J].临床麻醉学杂志, 2013(1):69-71.
[10]Chen S, Zhu X, Wang Q, et al. The early effect of Voluven, a novel hydroxyethyl starch (130/0.4), on cerebral oxygen supply and consumption in resuscitation of rabbit with acute hemorrhagic shock[J]. J Trauma, 2009(3):676-682.
[11]Bedford PD.Adverse cerebral effects of anaesthesia on old people[J]. Lancet, 1955(6884): 259-263.
[12]莫桂熙,刘奕君,莫坚,等.全身麻醉和硬膜外麻醉对老年骨科手术患者术后短期认知功能的影响[J].现代生物医学进展, 2014(9):1704-1708.
[13]Karzai W, Schwarzkopf K. Hypoxemia during one-lung ventilation: prediction, prevention, and treatment[J]. Anesthesiology, 2009(6):1402-1411.
[14]Tang L, Kazan R, Taddei R, et al. Reduced cerebral oxygen saturation during thoracic surgery predicts early postoperative cognitive dysfunction[J]. Br J Anaesth, 2012(4):623-629.
[15]Mahmoud M, Mason KP. Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations[J]. Br J Anaesth, 2015(2):171-182.
[16]Reynolds J, Rogers A, Capehart S. Retrospective comparison of intranasal dexmedetomidine and oral chloral hydrate for sedated auditory brainstem response exams[J]. Hosp Pediatr, 2016(3):166-171.
[17]Tufek A, Tokgoz O, Aliosmanoglu I, et al. The protective effects of dexmedetomidine on the liver and remote organs against hepatic ischemia reperfusion injury in rats[J].Int J Surg, 2013(1):96-100
[18]Cakir M, Polat A, Tekin S, et al. The effect of dexmedetomidine against oxidative and tubular damage induced by renal ischemia reperfusion in rats[J]. Ren Fail, 2015(4):704-708.
[19]Rodriguez-Gonzalez R, Sobrino T, Veiga S, et al. Neuroprotective effects of dexmedetomidine conditioning strategies: Evidences from an in vitro model of cerebral ischemia[J]. Life Sci, 2016(1):162-169.
[20]Sanders RD, Giombini M, Ma D, et al. Dexmedetomidine exerts dose-dependent age-independent antinociception but age-dependent hypnosis in Fischer rats[J]. Anesth Analg, 2005(5):1295-1302.
(2016-02-12收稿,2016-07-11修回)
中文编辑: 文箐颍; 英文编辑: 刘华
Dexmedetomidine Improves the Early Spatial Memory Function in Rats Undergoing One-lung Ventilation
BAO Chong, JIN Zhe, KE Jianjuan, CHEN Kai, WANG Yanlin
(DepartmentofAnesthesiology,ZhongnanHospitalofWuhanUniversity,Wuhan430071,Hubei,China)
国家自然科学基金项目(81471858)
Email:wyl0342@sina.com
R453.9; R35.33
A
1000-2707(2016)08-0891-05
10.19367/j.cnki.1000-2707.2016.08.006
**
网络出版时间:2016-08-23网络出版地址:http://www.cnki.net/kcms/detail/52.5012.R.20160823.1343.046.html

