阿尔兹海默病睡眠障碍的机制与治疗进展
孙文静, 贺 斌, 尹 又
第二军医大学长征医院神经内科,上海 200003
·综述·
阿尔兹海默病睡眠障碍的机制与治疗进展
孙文静, 贺斌, 尹又*
第二军医大学长征医院神经内科,上海200003
阿尔兹海默病(Alzheimer's disease, AD)是一种与年龄高度相关的、以进行性认知功能障碍和记忆力损害为主的中枢神经系统变性疾病,又称为老年性痴呆。调查显示,40%以上的AD患者合并不同程度的睡眠问题(睡眠结构改变、昼夜节律紊乱或睡眠呼吸障碍),可导致患者认知与行为能力加速恶化、神经-内分泌系统功能失调、情绪易怒和低落,甚至导致死亡。由于目前AD的一线治疗药物疗效欠佳,且近10余年几乎所有的AD新药研发无明显阳性结果,故如何有效改善AD伴随症状亦成为临床延缓AD进展的治疗重点之一。近5年针对睡眠与AD的关系及相关药物、非药物治疗等研究日益受到重视。本文就目前对于AD患者睡眠障碍的发病机制、治疗现状作一综述。
睡眠障碍;阿尔兹海默病;机制;治疗
阿尔兹海默病(Alzheime's disease,AD)是最常见的痴呆类型。40%~80%的AD患者伴有不同程度的睡眠障碍,轻度AD即出现睡眠时间及结构紊乱[1]。目前,AD相关睡眠障碍的诊断主要依据Yesavage等[2]制定的标准。AD相关睡眠障碍中,尤以睡眠-觉醒节律紊乱、日落综合征等给患者及其家属的生活带来的身心及经济负担更为沉重[3]。
1 AD药物研发进展
近10余年针对不同靶点的AD药物的研发均进展缓慢,且疗效评估中未涉及AD睡眠障碍的改善报道(表1)[4-6]。
2 AD睡眠障碍的可能发病机制
AD睡眠障碍的可能发病机制包括生物节律中枢的紊乱、松果体区褪黑素(melatonine,MT)及其受体的改变、授时因子(zeitgeber)的变化以及基因因素等,其中睡眠/觉醒节律紊乱是其核心假说[7]。
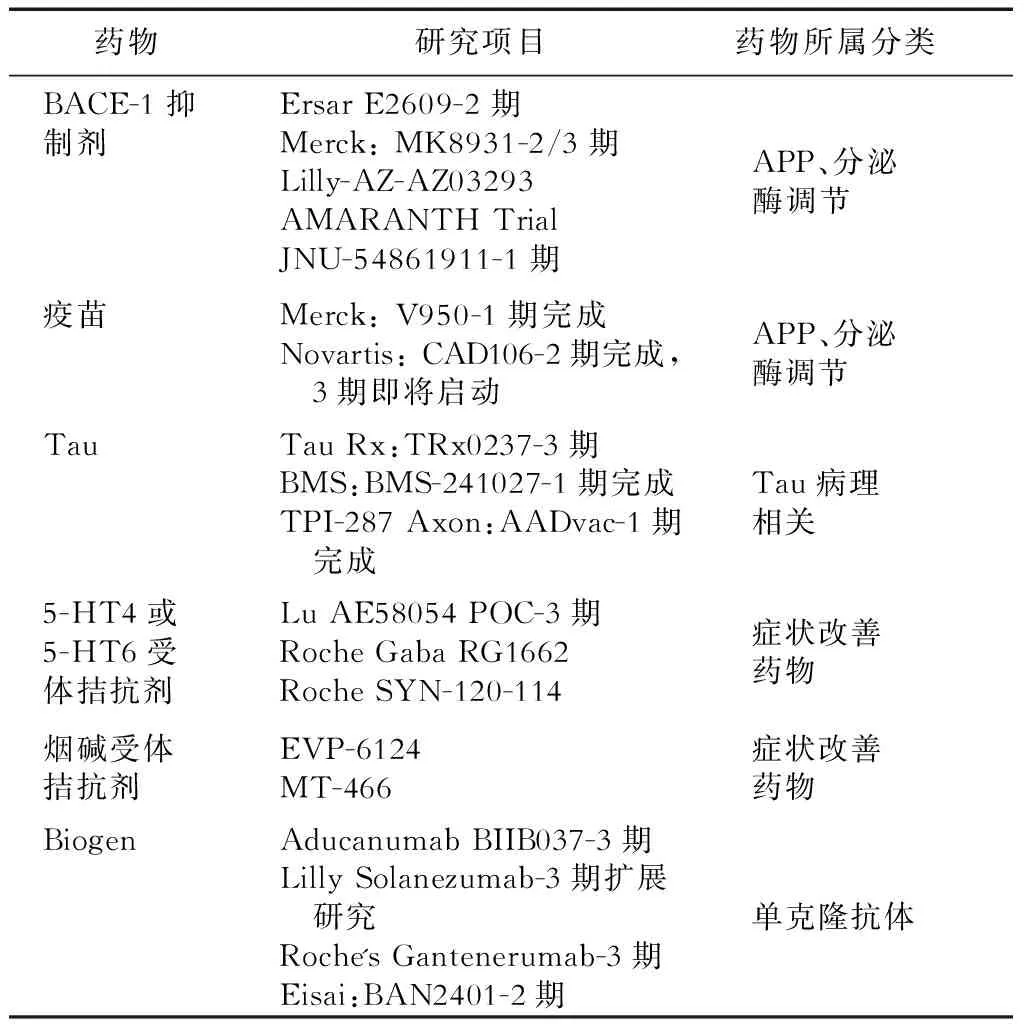
表1 近10年AD睡眠障碍新药研发报告(1期、2期)
2.1生物节律中枢紊乱作为生物节律的起始点,AD患者的视交叉上核神经元数目明显减少,并出现神经纤维缠结和β淀粉样蛋白沉积。
2.2褪黑素及其受体改变褪黑素由松果体分泌后通过其受体MT1(占主要部分)和MT2调控昼夜节律。AD患者脑脊液中褪黑素水平仅占同龄健康人的1/5,MT1的表达也明显降低,晚期神经元的数量明显减少。
2.3授时因子变化光照是最强的授时因子,而AD患者常不愿外出,且伴有视网膜和视神经病变、视野缺损等[8],导致光照作用减弱。2.4基因因素基因可能决定AD个体睡眠障碍的严重程度。转基因鼠睡眠调节紊乱较野生型鼠更早,进一步证实AD患者睡眠障碍与基因密切相关[5]。
3 AD睡眠障碍的药物治疗
目前,目前约有10余项较大规模的AD睡眠障碍相关临床药物研究,涉及胆碱酯酶抑制剂、褪黑素、抗精神病药及非药物疗法等,其中涉及681例患者的6项随机对照试验(RCT)研究中仅有一半阳性结果(表2)。
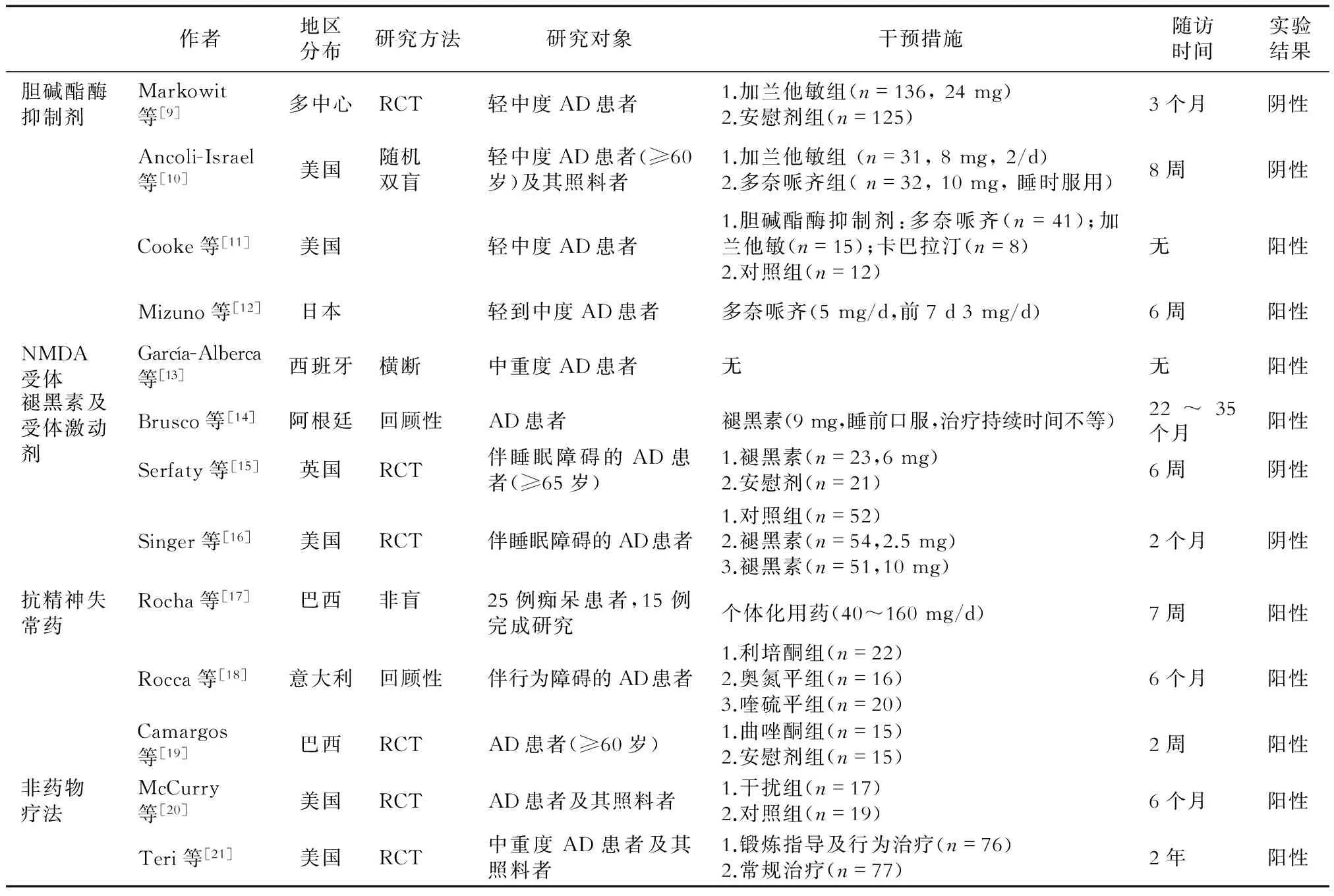
表2 AD睡眠障碍的大规模临床研究
3.1改善认知症状的药物
3.1.1胆碱酯酶抑制剂基底前脑作为胆碱能传导通路的发源地,可能存在胆碱能/腺苷/睡眠-觉醒的控通路。两项对加兰他敏的临床试验研究均为阴性结果[9-10]。Cooke等[11]对分别服用加兰他敏、重酒石酸卡巴拉汀和多奈哌齐的AD患者的睡眠结构进行观察,发现AD患者快速动眼睡眠时间均增加,而非快速动眼时间1期减少,2期增加;多奈哌齐组比加兰他敏组1期睡眠减少,较安慰剂组2期睡眠增加,表明多奈哌齐对患者睡眠有一定益处。 Mizuno等[12]也证实,5 mg多奈哌齐能增加患者的快速动眼睡眠时间百分比,提高睡眠效率,减少睡眠片段化。
3.1.2NMDA受体激动剂美金刚胺是第1个被证实对AD(尤其是中、重度AD)患者有显著疗效的药物。美金刚胺可以阻止过量谷氨酸等的传递而达到保护神经元的作用。García-Alberca等[13]研究表明,AD患者使用美金刚后睡眠紊乱量表(神经精神、神经心理及功能状态)评分均下降。
3.2褪黑素及其受体激动剂
3.2.1褪黑素AD睡眠障碍的主要因素是褪黑素合成和分泌减少,导致正常生物节律紊乱。一项包含14例参与者的回顾性研究[14]发现,每日口服9 mg褪黑素能明显改善睡眠质量。但另一项对比研究[15]中,AD睡眠障碍每日口服6 mg褪黑素7周后,睡眠参数无变化,分别服用2.5 mg、5 mg和10 mg褪黑素8周后亦睡眠无显著改善[16]。
3.2.2褪黑素受体激动剂AD患者体内不仅褪黑素水平下降[22],其受体表达也明显下降,导致患者生理节律紊乱加重。瑞美替昂是已经被美国食品和药品管理局(FDA)认证的治疗失眠的药物。瑞美替昂对MT1及MT2均有激动作用,但目前对其疗效的观察时间未超过35 d[23]。
3.3抗精神失常药


4 非药物治疗
4.1光照光照主要通过视网膜下丘脑束引起视交叉上核神经细胞膜去极化,增强神经元发放率,并阻止年龄相关的精氨酸加压素(AVP)表达,从而改善老年人的睡眠、神经内分泌、体温及睡眠-觉醒节律。AD患者上述正常节律常较健康老年人破坏更为严重,对光照治疗反应良好。McCurry等[20]证实,AD睡眠障碍患者可以进行光照等治疗。
4.2其他方法研究[21]显示,增加体育锻炼不仅对老年人的睡眠-觉醒节律有明显的改善作用,而且可以提高老年人的身心健康及生活热情,但此研究没有进行睡眠相关参数的评估。此外,踱步似乎能改善AD患者睡眠-觉醒周期的同步化[29]。通过经皮电刺激激活AD患者背部胸1~胸5脊髓神经元也可以改善睡眠节律[30]。有研究[31]初步表明,针灸可以通过增加夜间褪黑素的分泌而减少失眠及焦虑。也有研究[32]通过直接干预体温(如热水澡)来提高老年人的睡眠质量。
AD患者睡眠障碍的发生率高、危害大,尽管不断对其病因、发病机制进行探索,但仍未找到较好的治疗方法。目前常用的药物各有利弊,应针对AD睡眠障碍表现的差异选择药物。胆碱酯酶抑制剂中的多奈哌齐在改善认知功能的同时有助于调节睡眠,有望成为该类AD患者的基础治疗药物;褪黑素可提高患者的睡眠及认知能力,但治疗周期较长;患者对小剂量(40 mg)齐拉西酮的耐受性良好,且照料者的负担较轻,但类帕金森表现等不良反应常见;小剂量非典型抗精神病药对伴有行为障碍或夜间睡眠-觉醒紊乱的AD患者更优;曲唑酮是目前最受肯定的AD睡眠障碍辅助药物,其能改善AD患者的睡眠质量和睡眠结构,且无认知损伤等不良反应。总之,AD睡眠障碍的治疗正逐渐受到关注,其作为AD辅助治疗的意义日益凸显,但治疗方案尚缺乏大样本、前瞻性以及对照研究的支持。
[1]Petit D, Gagnon JF, Fantini ML, et al. Sleep and quantitative EEG in neurodegenerative disorders[J].J Psychosom Res,2004,56(5):487-496.
[2]Yesavage JA, Friedman L, Ancoli-Israel S, et al. Development of diagnostic criteria for defining sleep disturbance in Alzheimer's disease[J].J Geriatr Psychiatry Neurol,2003,16(3):131-139.
[3]Ostrowski M, Mietkiewicz MC. Night of the Alzheimer's patient: the nightmare of the caregiver via the caregivers'guides[J].Geriatr Psychol Neuropsychiatr Vieil,2015,13(2):179-186.
[4]Castello MA, Jeppson JD, Soriano S. Moving beyond anti-amyloid therapy for the prevention and treatment of Alzheimer's disease[J].BMC Neurol,2014,14:169.
[5]De Strooper B. Lessons from a failed γ-secretase Alzheimer trial[J].Cell, 2014,159(4):721-726.
[6]尹又, 黄流清, 李澎, 等. 帕金森病患者睡眠障碍的研究进展[J].中华行为医学与脑科学杂志,2014,23(1):91-93.
[7]Wu YH, Swaab DF. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer's disease[J].Sleep Med,2007,8(6):623-636.
[8]Valenti DA. Alzheimer's disease: visual system review[J].Optometry,2010,81(1):12-21.
[9]Markowitz JS, Gutterman EM, Lilienfeld S, et al. Sleep-related outcomes in persons with mild to moderate Alzheimer disease in a placebo-controlled trial of galantamine[J]. Sleep,2003,26(5):602-606.
[10]Ancoli-Israel S, Amatniek J, Ascher S, et al. Effects of galantamine versus donepezil on sleep in patients with mild to moderate Alzheimer disease and their caregivers: a double-blind, head-to-head, randomized pilot study[J].Alzheimer Dis Assoc Disord,2005,19(4):240-245.
[11]Cooke JR, Loredo JS, Liu L, et al. Acetylcholinesterase inhibitors and sleep architecture in patients with Alzheimer's disease[J].Drugs Aging,2006,23(6):503-511.
[12]Mizuno S, Kameda A, Inagaki T, et al. Effects of donepezil on Alzheimer's disease: the relationship between cognitive function and rapid eye movement sleep[J].Psychiatry Clin Neurosci,2004,58(6):660-665.
[13]García-Alberca JM, Lara JP, Cruz B, et al. Sleep disturbances in Alzheimer's disease are associated with neuropsychiatric symptoms and antidementia treatment[J].J Nerv Ment Dis,2013,201(3):251-257.
[14]Brusco LI, Fainstein I, Márquez M, et al. Effect of melatonin in selected populations of sleep-disturbed patients[J].Biol Signals Recept,1999,8(1-2):126-131.
[15]Serfaty M, Kennell-Webb S, Warner J, et al. Double blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementia[J].Int J Geriatr Psychiatry,2002,17(12):1120-1127.
[16]Singer C, Tractenberg RE, Kaye J, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer's disease[J].Sleep,2003,26(7):893-901.
[17]Rocha FL, Hara C, Ramos MG, et al. An exploratory open-label trial of ziprasidone for the treatment of behavioral and psychological symptoms of dementia[J].Dement Geriatr Cogn Disord,2006,22(5-6):445-448.
[18]Rocca P, Marino F, Montemagni C,et al.Risperidone, olanzapine and quetiapine in the treatment of behavioral and psychological symptoms in patients with Alzheimer's disease: preliminary findings from a naturalistic, retrospective study[J]. Psychiatry Clin Neurosci,2007, 61(6):622-629.
[19]Camargos EF, Pandolfi MB, Freitas MP, et al. Trazodone for the treatment of sleep disorders in dementia: an open-label, observational and review study[J]. Arq Neuropsiquiatr, 2011,69(1):44-49.
[20]McCurry SM, Gibbons LE, Logsdon RG, et al. Nighttime insomnia treatment and education for Alzheimer's disease: a randomized, controlled trial[J].J Am Geriatr Soc,2005,53(5):793-802.
[21]Teri L, Gibbons LE, McCurry SM, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial[J].JAMA,2003,290(15):2015-2022.
[22]Moraes W, Poyares D, Sukys-Claudino L, et al. Donepezil improves obstructive sleep apnea in Alzheimer disease: a double-blind, placebo-controlled study[J].Chest, 2008,133(3):677-683.
[23]Wurtman R. Ramelteon: a novel treatment for the treatment of insomnia[J]. Expert Rev Neurother,2006,6(7):957-964.
[24]Yin Y, Liu Y, Zhuang J, et al. Low-dose atypical antipsychotic risperidone improves the 5-year outcome in Alzheimer's disease patients with sleep disturbances[J]. Pharmacology,2015,96(3-4):155-162.
[25]Camargos EF, Louzada LL, Quintas JL,et al. Trazodone improves sleep parameters in Alzheimer disease patients: a randomized, double-blind, and placebo-controlled study[J].Am J Geriatr Psychiatry,2014,22(12):1565-1574.
[26]Grippe TC, Gonçalves BS, Louzada LL, et al. Circadian rhythm in Alzheimer disease after trazodone use[J].Chronobiol Int,2015,32(9):1311-1314.
[27]Raji MA, Brady SR. Mirtazapine for treatment of depression and comorbidities in Alzheimer disease[J].Ann Pharmacother,2001,35(9):1024-1027.
[28]Defrancesco M, Marksteiner J, Fleischhacker WW, et al. Use of Benzodiazepines in Alzheimer's Disease: A Systematic Review of Literature[J].Int J Neuropsychopharmacol, 2015,18(10):pyv055.
[29]Werth E, Savaskan E, Knoblauch V, et al. Decline in long-term circadian rest-activity cycle organization in a patient with dementia[J].J Geriatr Psychiatry Neurol,2002,15(1):55-59.
[30]Clancy JA, Mary DA, Witte KK, et al. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity[J].Brain Stimul,2014,7(6):871-877.
[31]Spence DW, Kayumov L, Chen A, et al. Acupuncture increases nocturnal melatonin secretion and reduces insomnia and anxiety: a preliminary report[J].J Neuropsychiatry Clin Neurosci,2004,16(1):19-28.
[32]Van Someren EJ. More than a marker: interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities[J].Chronobiol Int,2000,17(3):313-354.
[本文编辑]廖晓瑜,姬静芳
Mechanism and treatment of sleep disorders in Alzheimer’s disease
SUN Wen-jing, HE Bin, YIN You*
Department of Neurology, Changzheng Hospital, Second Military Medical University, Shanghai 200003, China
Alzheimer's disease (AD) is a neurodegenerative disease highly correlated with age and characterized by progressive impairment in memory and cognition, also known as senile dementia. Survey shows more than 40% of AD patients with varying degrees of sleep problems (sleep structure changes, circadian rhythm disorder, sleep apnea and so on), which can lead to accelerated cognitive and behavioral deterioration, the nerve-endocrine system dysfunction, emotional irritability and depression, and even death. Due to the poor efficacy of first-line treatment drugs, and no significant positive results were achieved in AD drug development over the past ten years, how to effectively improve the associated symptoms of AD has become one of the therapeutic targets for delaying the progression of AD. In the past 5 years, research on the relationship between sleep and AD, related drugs and non-drug therapy has attracted increasing attention. In this paper, a review of the pathogenesis and clinical treatment of sleep disorders in patients with AD will be presented.
sleep disorders; Alzheimer's disease; pathogenesis; treatment
2016-01-28[接受日期]2016-07-25
国家自然科学基金(30900473,81371459,81171252), 国家科技部重大专项(2011ZXJ09202-015),国家科技支撑计划项目(2015BAI13B01),上海市科学技术委员会重点项目(11411950203),上海市自然科学基金(15ZR1414600). Supported by National Natural Science Foundation of China(30900473,81371459,81171252), National Ministry of Science and Technology Major Project(2011ZXJ09202-015), National Key Technology Research and Development Program of Ministry of Science and Technology of China (2015BAI13B01), Medical Key Project of Shanghai Municipal Science and Technology Commission(11411950203)and Natural Science Foundation of Shanghai(15ZR1414600).
孙文静,硕士生. E-mail:sunwj1121@163.com
Corresponding author). Tel: 021-64041990, E-mail:yinyou179@163.com
10.12025/j.issn.1008-6358.2016.20160098
R 749.1+6
A

