一种偶氮四唑衍生物的合成、结构和光学性质
董 文,邱燕璇,蔡嘉伟
(广州大学 化学化工学院,广东 广州 510006)
一种偶氮四唑衍生物的合成、结构和光学性质
董 文,邱燕璇,蔡嘉伟
(广州大学化学化工学院,广东广州 510006)
合成一种偶氮四唑衍生物4-偶氮四唑苯酚(H2ATP),培养该化合物的晶体,X-射线单晶衍射分析表明,该化合物的晶体含有1个H2ATP分子和3个结晶水分子[(H2ATP)(H2O)3](1),1属于三斜晶系,Pī空间群,其晶胞参数:a=6.816(3)Å,b=9.406(4)Å,c=9.628(3)Å,α=72.42(2)o,β=75.65(2)o,γ=79.84 (3)o,Mr=244.23,V=566.6(4)Å3,Z=2,Dc=1.431 g·cm-3,μ=0.118 mm-1,F(000)=256.0,R= 0.112 3,wR=0.209 3.在[(H2ATP)(H2O)3]中发现了π-π堆积相互作用和O-H…O、O-H…N 2种氢键相互作用.并研究了H2ATP的光致变色和荧光性质.
偶氮衍生物;晶体结构;光致变色;荧光
1 Experimental section
1.1Materials and physical measurements
All the commercial reagents and solvents were used without further purification unless otherwise stated.IR spectrum was recorded as pressed KBr pellets on a Bruker Tensor 27 spectrophotometer with an average of 64 scans.Elemental analysis was carried out using a Perkin-Elmer analyzer model 240.UV-Vis absorption spectra were collected on U-2550 Ultraviolet-Visible Spectrophotometer.Luminescence spectra were recorded with an F-4500 fluorescence spectrophotometer.
1.2X-ray crystallography and data collection
The crystals were filtered from the solution and immediately coated with hydrocarbon oil on the microscope slide.Suitable crystals were mounted on glass fibers with silicone grease and placed in a Bruker Smart APEX(II)area detector using graphite monochromated Mo-Kα radiation(λ=0.710 73 Å)at 296(2)K.The structures were solved by direct methods and successive Fourier difference syntheses (SHELXS-97)and refined by full-matrix leastsquares procedure on F2with anisotropic thermal parameters for all non-hydrogen atoms(SHELXL-97). Hydrogen atoms were located from difference maps and refined with isotropic temperature factors.Table 1 gives the crystallographic data for[(H2ATP)(H2O)3].The bond lengths and angles for 1 are listed in Table 2.

Table 1 Crystallographic parameters of structure 1
1.3Synthesis of[(H2ATP)(H2O)3](1)
Compound H2ATP was synthesized in good yields(90%)via 5-aminotetrazole with NaNO2in HCl at 0~5℃ and further coupling with phenol at room temperature(Scheme 1)[18].The crude product of H2ATP(38.0 mg,0.2 mmol)was dissolved in 5 mL of ethanol and 15 mL of water,and then three drops of dilute NH3·H2O(2 mol·dm-3)were added to the above mixture solution and stirred till the precipitates dissolved.Then 1 mol·dm-3of HCl was added to the above mixture to adjust pH to 7 and reacted under refluxing condition for 2 hours.The resulting mixture was filtered and yellow crystals were obtained by slow evaporation after one day and washed with water.Yield:75.6%.Elemental analysis:Anal.Calcd(%)for[(H2ATP)(H2O)3]of C7H12N6O4:C,34.39;H,4.91;N,34.39. Found(%):C,34.54;H,4.74;N,34.55.FTIR(KBr,cm-1):3 532 s,3 015 s,1 863 w,1 714 w,1 607 m,1 588 m,1 472 m,1 412 m,1 309 w,1 250 m,1 202 m,1 147 m,1 106 m,923 w,845 m,756 w,719 w,641 w,539 m,441 w.
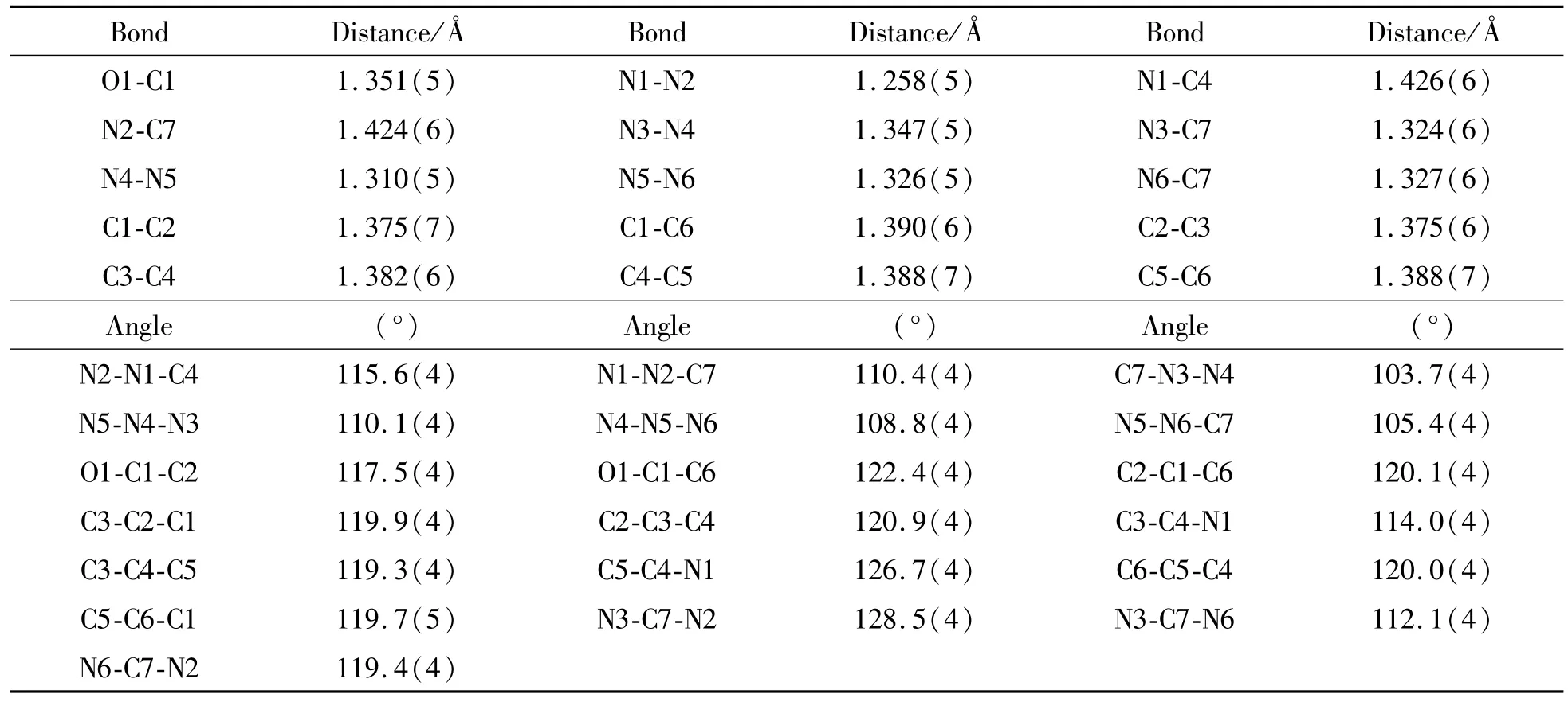
Table 2 Bond lengths and angles for[(H2ATP)(H2O)3]
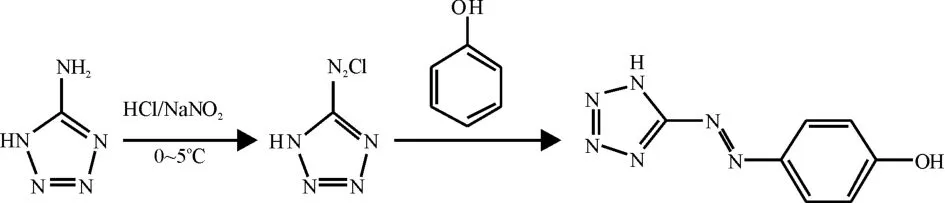
Scheme 1 Synthesis of H2ATP
2 Results and discussion
2.1The crystal structure of 1
The atomic labeling diagram for 1 is shown in Fig.1a.Each unit cell of[(H2ATP)(H2O)3]contains a fundamental H2ATP molecule and three lattice water molecules.The neutral H2ATP molecules show a trans-enol isomer with the bond length for C1(O1 (phenol)being 1.351(5)Å.The N1-N2 bond length is 1.258(5)Å.The dihedral angle of 2.89° between the tetrazole and phenyl rings of H2ATP molecule displays a coplanar structure.Two face-to-face π-π stacking interactions are found between tetrazolate and phenolic rings with separated shortest interplanar C…center(zole)and N…center(phen)distance of 3.370 and 3.509Å,respectively(Fig.1b). Two O-H…O and O-H…N hydrogen bonding interactions are found from the phenolic and tetrazole moieties of H2ATP molecule with O…O and O…N distances arrangement from 2.664 to 2.951 Å.A 3D supramolecular structure is generated by the π-π stacking and hydrogen bonding interactions(Fig. 1c).

Fig.1 The crystal structures for 1
2.2UV-Vis absorption of 1
Fig.2a gives the pH-dependent UV-vis spectra of aqueous solution of H2ATP with concentration of 8 ×10-5mol·dm-3.The pH of this solution is 7.56. For the H2ATP solution,there are two obvious absorption peaks centered at 246 and 350 nm and a weaker shoulder peak around 430 nm.When the pH increase from 3.35 to 7.56 by titration with 2 mol· dm-3HCl solution,the strength of two main absorption peaks at 246 and 350 nm keep almost no change.When the pH increases from 7.56 to 10.58 by titration with 2 mol·dm-3NaOH aqueous solution,the absorption peaks at 246 and 350 nm decrease in intensity and two added new red-shifted absorption peaks around 267,430 nm accompanying with a weak shoulder peak around 400 nm appear and increase gradually in intensity.Three regions with three isosbestic points at 250,290 and 373 nm can be distinguished.The absorption peaks for aqueous solution of H2ATP in pH scale of 3.35~10.58 should originate from the π or n electronic excitation from the intrinsic chromophoric phenolic,tetrazolate azo moieties of H2ATP or its deprotonated anions. Fig.2b gives the photochromic property for the neutral aqueous solution of H2ATP with concentration of 8×10-5mol·dm-3under 365 nm UV light irradiation at room temperature.Upon irradiation for 95 min,the intense peak at 350 nm decreases slightly,which is accompanied by an increase at 430 nm until a stationary state is reached.The spectral changes should be attributed to the reversible trans-enol to cisenol photochromic property of H2ATP.The photochromic property for the alkaline aqueous solution of H2ATP is measured,as can be seen in Fig.2c.Upon irradiation of the aqueous solution for 85 minutes with 365 nm UV light,two absorption peaks around 267 and 430 nm decrease in intensity and two new blueshifted absorption peaks around 246 and 350 nm are noted and three regions with three isobestic points at 255,290 and 375 nm can be distinguished.
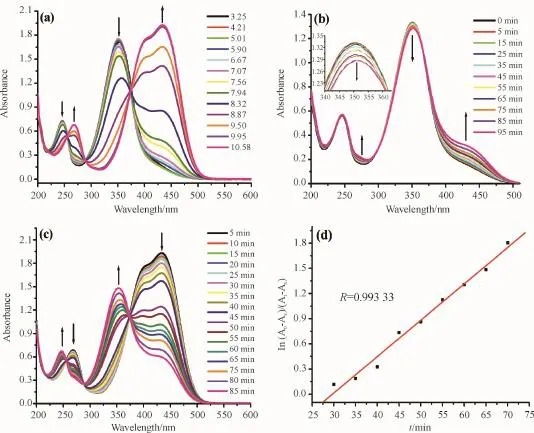
Fig.2 The UV-vis spectral changes for H2ATP
Moreover,the photochromism of the basic aqueous solution of H2ATP is in accordance with the firstorder kinetics(Fig.2d),and the photochromism rate constant is about 4.333×10-2min-1[19].
2.3Luminescence for the aqueous solution of 1
Photoluminescent spectrum for the aqueous solution of H2ATP is depicted in Fig.3.The aqueous solution of H2ATP exhibits a green emissive peak at 510 nm under 300 nm UV light excitation.The luminescence should be attributed to the π-π*transition of H2ATP ligand[20].

Fig.3 Photoluminescent spectrum for the aqueous solution of[(H2ATP)(H2O)3]
3 Conclusion
Compound 1 includes π-system molecule of H2ATP and three crystal water molecules that are capable of forming π-π stacking interactions between tetrazolate and phenolic rings and formation intermolecular hydrogen bonds.These O-H…O and O-H …N hydrogen bonding interactions together with ππ stacking interactions are responsible for the stabilization of the 3D supramolecular structure of 1.Both the neutral and alkaline aqueous solution of H2ATP show trans-enol to cis-enol photochromic properties. The aqueous solution of 1 exhibits an emission maximum at 510 nm under UV light excitation.H2ATP may be used as an optical and color-changing material.
References:
[1] HUNGER K.Industrial dyes[M].Weinheim,FRG:Wiley-VCH Verlag GmbH&Co.KGaA,2003.
[2] DUARTE L,GIULIANO B M,REVA I,et al.Tautomers and UV-Induced photoisomerization of a strongly intramolecularly H-bonded aromatic azo-dye:1-(Cyclopropyl)diazo-2-naphthol[J].J Phys Chem A,2013,117(41):10671-10680.
[3] FERREIRA G R,GARCIA H C,COURI M R C,et al.On the azo/hydrazo equilibrium in sudan I azo dye derivatives[J]. J Phys Chem A,2013,117(3):642-649.
[4] GARCIA-AMORóS J,CASTRO M C R,COELHO P,et al.New heterocyclic systems to afford microsecond green-light isomerisable azo dyes and their use as fast molecular photochromic switches[J].Chem Commun,2013,49:11427-11429.
[5] FREEMAN H S,POSEY J R J C.Analogs of disperse red 167 containing a built-in photostabiliser moiety[J].Dyes Pigmen,1992,20(3):147-169.
[6] WANG S Q,SHEN S Y,XU H J.Synthesis,spectroscopic and thermal properties of a series of azo metal chelate dyes[J]. Dyes Pigmen,2000,44(3):195-198.
[7] FERINGA B L.Molecular switches[M].Weinheim,FRG:Wiley-VCH Verlag GmbH&Co.KGaA,2001.
[8] KUMAR A S,YE T,TAKAMI T,et al.Reversible photo-switching of single azobenzene molecules in controlled nanoscale environments[J].Nano Lett,2008,8(6):1644-1648.
[9] SASAKI T,TOUR J M.Synthesis of a new photoactive nanovehicle:A nanoworm[J].Org Lett,2008,10(5):897-900.
[10]BROWNE W R,FERINGA B L.Light switching of molecules on surfaces[J].Annu Rev Phys Chem,2009,60:407-428.
[11]ISAK S,EYRING E,SPIKES J,et al.Direct blue dye solutions:photo properties[J].J Photochem Photobiol A,2000,134(1/2):77-85.
[12]LI Y,PATRICK B O,DOLPHIN D.Near-infrared absorbing azo dyes:Synthesis and X-ray crystallographic and spectral characterization of monoazopyrroles,bisazopyrroles,and a boron-azopyrrole complex[J].J Org Chem,2009,74(15):5237-5243.
[13]KANIS D R,RATNER M A,MARKS T J.Design and construction of molecular assemblies with large second-order optical nonlinearities:Quantum chemical aspects[J].Chem Rev,1994,94(1):195-242.
[14]YU H,IKEDA T.Photocontrollable liquid-crystalline actuators[J].Adv Mater,2011,23(19):2149-2180.
[15]SAMANTA S,GHOSH P,GOSWAMI S.Recent advances on the chemistry of transition metal complexes of 2-(arylazo)pyridines and its arylamino derivatives[J].Dalton Trans,2012,41:2213-2226.
[16]LIN J M,YANG M,QIU Y X,et al.Synthesis,structure,and halo-,photo-,and thermochromism properties of 5-azotriazolyl salicylic acid and its CdIIcomplex[J].Chem Plus Chem,2013,78(6):598-604.
[17]CHEN W B,LI Z X,YU X W,et al.Syntheses,structures and properties of 5-azotetrazolyl salicylic acid and its dilanthanide complexes[J].Dalton Trans,2014,43:9090-9097.
[18]SHEGAL I L,STANOVKINA K V,KOVALENKO N G,et al.Synthesis of azo compounds containing triazole and tetrazole residues[J].Chem Heterocycl Compd,1974,10(3):369-371.
[19]LI Y C,QI C,LI S H,et al.1,1′-Azobis-1,2,3-triazole:A high-nitrogen compound with stable N8structure and photochromism[J].J Am Chem Soc,2010,132(35):12172-12173.
[20]YIN P X,CHENG J K,LI Z J,et al.Ag(I)-mediated in situ dehydrogenative coupling of 3-amino-1,2,4-triazole into 3,3′-azobis(1,2,4-triazole)in Cd(II)coordination polymers[J].Inorg Chem,2009,48(23):10859-10861.
【责任编辑:周 全】
Synthesis,crystal structure and optical properties of an azo tetrazolate derivative
DONG Wen,QIU Yan-xuan,CAI Jia-wei
(School of Chemistry and Chemical Engineering,Guangzhou University,Guangzhou 510006,China)
An azo tetrazolate derivative of 4-azotetrazolyl phenol(H2ATP)was synthesized and the crystal structure of[(H2ATP)(H2O)3](1)was characterized by single crystal X-ray diffraction analysis.1 crystallizes in the triclinic system,space group Pī with a=6.816(3),b=9.406(4),c=9.628(3)Å,α=72.42 (2)o,β=75.65(2)o,γ=79.84(3)o,Mr=244.23,V=566.6(4)Å3,Z=2,Dc=1.431 g·cm-3,μ= 0.118 mm-1,F(000)=256.0,R=0.112 3 and wR=0.209 3.The π-π stacking interaction and two O-H …O and O-H…N hydrogen bonding interactions were found in 1.The photochromic and photoluminescent properties for 1 were investigated and demonstrated.
azo derivative;crystal structure;photochromism;luminescence
O614 Document code:A
0 Introduction
Aromatic azo compounds are an interesting class of organic compounds that have been found wide application as dyes in optical and color-changing materials such as sunglasses,textiles,paints,cosmetics, and food additives[1-4].Specifically,aromatic heterocyclic azo dyes have shown brilliant color and chromophoric strength[4-6].About 50%of the dyes produced in the world are azo derivatives[3].The main characteristic of such types of compounds is the presence of the azo group(-N=N-),which usually undergo reversible trans-cis photoisomerization thatcan also be exploited in the design of photoswitches,molecular motors,etc[7-10],and even in the development of potential photothermal sensitizers in the photodynamic therapy of some forms of cancer and other diseases[11-12].Because azo linker is an efficient electronic bridge,which is expected to promote the creation of extended-conjugated systems and increase of the absorption strength,various photo responsive azo based materials have been thus prepared to date by the incorporation of azo dyes into different matrices with dispersed,covalently linked or coordinated methods[4,12-15].Detailed knowledge on the structures and photochemical behavior of aromatic azo compounds is crucial for understanding their reactivity and applications[2].In our previous work,several aromatic heterocyclic azo compounds and their azometal complexes have been reported[16-17].Here,an azo tetrazolate derivative of H2ATP was synthesized and the photochromism and photoluminescent properties were investigated and discussed.
date:2016-04-09; Revised date:2016-04-25
O 614
A
1671-4229(2016)03-0013-05
Foundation items:This work was supported by the National Natural Science Foundation of China(21271052);Science and Technology Program Foundation of Guangdong(2015A030313502)and Program Foundation of the second batch of innovation teams of Guangzhou Bureau of Education(13C04)
Biography:DONG Wen(1965-),male,professor,Ph.D.E-mail:dw320@aliyun.com

