紫外激发Ba2SiO4:Gd3+,Tb3+的发光性能
肖林久,耿艳丽,谢 颖,姜新东,崔永强,汪书东
(沈阳化工大学辽宁省稀土化学及应用重点实验室,辽宁沈阳 110142)
紫外激发Ba2SiO4:Gd3+,Tb3+的发光性能
肖林久*,耿艳丽,谢颖,姜新东,崔永强,汪书东
(沈阳化工大学辽宁省稀土化学及应用重点实验室,辽宁沈阳 110142)
采用高温固相法合成了可被紫外光激发的Ba2SiO4:Gd3+,Tb3+荧光粉。考察了激活离子掺杂量等因素对发光性能的影响。通过X射线衍射(XRD)、荧光(FL)光谱和荧光寿命曲线对所合成样品的结构和发光性能进行表征,研究了Gd3+和Tb3+的特征吸收波长激发Ba2SiO4:Gd3+,Tb3+的发光性能。在275 nm(Gd3+:8S7/2→6IJ)激发下,检测到了Tb3+的特征发射。通过对比不同Tb3+掺杂量下Gd3+:6P7/2能级的衰减曲线,发现随着Tb3+掺杂浓度的增加,该能级的荧光寿命不断缩短,表明样品中存在Gd3+→Tb3+的能量传递,传递方式为无辐射共振能量传递。在244 nm(Tb3+:4f8→4f75d1)激发下,Gd3+的掺入使得Tb3+的5D3能级的发射逐渐减弱,5D4能级的发射增强。Gd3+的掺入使得544 nm(5D4→7F5)处的特征发射增强了59%~128%,结合荧光衰减曲线得出Gd3+的掺入对Tb3+能级中5D3→5D4与7F6→7F0交叉驰豫有促进作用。
荧光粉;能量传递;交叉驰豫;Ba2SiO4:Gd3+,Tb3+
1 引 言
稀土掺杂的无机材料在照明光源、太阳能电池、X射线影像、闪烁体、生物探针等诸多领域有着广阔的应用前景,自其进入发光材料领域以来一直受到研究者们的重视[1]。白光LED以其高效节能等优点[2]吸引了世界目光,被誉为21世纪的新一代照明光源[3-5]。随着LED芯片的发展,紫外/近紫外型荧光粉越来越受到关注[6-7]。其中用于光转换的绿色荧光粉的发光效率对总的光通量影响很大,是紫外发光二极管用三基色荧光粉开发中的一个重要环节[6,8]。
以硅酸盐为基质的荧光粉由于具有良好的化学稳定性和热稳定性,而且高纯度的二氧化硅原料具有价廉、易得、烧结温度比铝酸盐体系低的优点,长期以来受到人们的重视。发射绿光的典型离子代表是Tb3+,研究发现Gd3+可有效地敏化Tb3+而增强其发光。尽管Gd3+和Tb3+共掺杂的荧光粉的发光性能及Gd3+→Tb3+的能量传递已有一些研究,如:Na3GdSiO2O7:Tb3+[9]、δ-Gd2Si2O7:Eu3+,Tb3+[10]、CdSiO3:Tb3+,Re3+(Re3+=Gd3+,Y3+,La3+)[11]等,但该离子对在Ba2SiO4基质中的研究尚鲜有报道,并且Gd3+对Tb3+间的交叉驰豫的影响也未见报道。
本文采用高温固相法制备了可被紫外光激发的Ba2SiO4:Gd3+,Tb3+荧光粉,研究了Ba2SiO4:Gd3+,Tb3+荧光粉在Gd3+特征吸收光激发下和Tb3+特征吸收光激发下的光谱性能,探讨了Gd3+→Tb3+的能量传递以及Tb3+间的交叉驰豫现象。
2 实 验
2.1样品制备
合成样品使用了氧化钆(Gd2O3,99.99%)、氧化铽(Tb4O7,99.99%)、碳酸钡(BaCO3,AR)、二氧化硅(SiO2,AR)和硼酸(H3BO3,AR)等原料。制备过程采用高温固相法,按照实验考察确定的适宜制备条件,将不同的稀土元素掺杂量按化学计量比称量放入玛瑙研钵中,充分研磨使其均匀,而后装入瓷舟放入高温管式炉中,升温至1 200℃并保温2 h,然后冷却至室温,取出样品并用玛瑙研钵研细,即得所需的Ba2-x-ySiO4:xGd3+,yTb3+样品。
2.2样品表征
样品采用Bruker D8 X射线衍射仪(XRD)确定物相,仪器经过退火的Al2O3校正,采用铜靶(λ=0.154 06 nm),电压为40 kV,电流为40 mA,扫描范围为10°~80°,步长设置为0.05°。采用Hitach公司的F-4600荧光光谱仪测试样品的激发光谱和发射光谱。采用Horiba公司的FL-3稳态/瞬态荧光光谱仪测试样品的荧光衰减曲线。所有测试都在室温条件下进行。
3 结果与讨论
3.1晶相分析
1 200℃下焙烧2 h制得的Ba1.85SiO4:0.1Gd3+,0.05Tb3+的XRD谱如图1所示。从图中可看出,样品为单一纯相,符合基质材料Ba2SiO4的JCPDS标准卡片(JCPDS No.26-1403)图谱数据,属于正交晶系。

图1 Ba2SiO4:Gd3+,Tb3+的X射线衍射谱及Ba2SiO4的标准卡片(JCPDS No.26-1403)Fig.1 XRD patterns of Ba2SiO4:Gd3+,Tb3+samples and the standard data of Ba2SiO4(JCPDS No.26-1403)
3.2激发光谱
图2(a)给出了单掺Gd3+样品的激发光谱,监测波长为313 nm(Gd3+:6P7/2→8S7/2跃迁发射),激发峰在275 nm处,来源于Gd3+的锐线型8S7/2→6I7/2能级跃迁吸收。图2(b)为单掺Tb3+样品的激发光谱,监测波长为544 nm(Tb3+:5D4→7F5跃迁发射),激发峰在244 nm处,来源于Tb3+的锐线型4f8→4f75d1跃迁吸收。图2(c)为双掺Gd3+/Tb3+样品的激发光谱。实线为313 nm(Gd3+特征发射)监测下的激发光谱,激发峰在275 nm处,来源于Gd3+的8S7/2→6I7/2能级跃迁;虚线为544 nm(Tb3+特征发射)监测下的激发光谱,从图中可同时观测到244 nm处Tb3+的4f8→4f75d1跃迁吸收和275 nm处Gd3+的8S7/2→6I7/2能级跃迁吸收,说明样品中存在Gd3+到Tb3+的能量传递。

图2 单掺Gd3+(a)、单掺Tb3+(b)及双掺Gd3+/Tb3+(c)样品的激发光谱。Fig.2 Excitation spectra of single doped Gd3+(a),single doped Tb3+(b),and double doped Gd3+/Tb3+(c)samples,respectively.
3.3发射光谱
3.3.1275 nm(Gd3+特征吸收光)激发下的发射光谱
在Gd3+特征吸收275 nm的激发下,Ba1.9-y-SiO4:0.1Gd3+,yTb3+(y=0,0.03,0.05,0.07,0.10,0.15)的发射光谱如图3所示。当Ba2SiO4中只掺Gd3+(y=0)时,仅能观测到313 nm处Gd3+的6P7/2→8S7/2跃迁发射峰;当Ba2SiO4中同时掺有Gd3+和Tb3+时,在Gd3+特征吸收光275 nm激发产生的发射光谱中,既出现了Gd3+的特征发射,还观测到了Tb3+的特征发射,证明样品中存在Gd3+到Tb3+的能量传递。随着Tb3+掺杂浓度的增大,Gd3+的发射强度降低,Tb3+的特征发射增强。当Tb3+的摩尔分数y=0.05时,Tb3+的发射达到最强,继续增加Tb3+的浓度,Gd3+和Tb3+的发光均减弱,发生了浓度猝灭。
由Gd3+的能级跃迁可知,图中313 nm的发射峰对应于Gd3+的6P7/2→8S7/2能级跃迁,381,416,437 nm的发射峰对应于Tb3+的5D3→7FJ(J=6,5,4)能级跃迁,488,544,584,616 nm的发射峰对应于Tb3+的5D4→7FJ(J=6,5,4,3)能级跃迁。
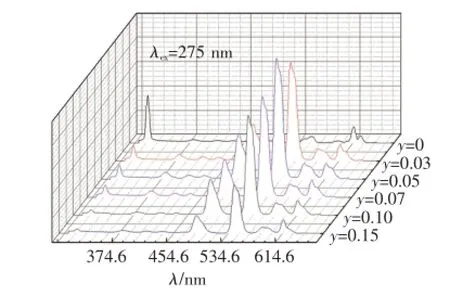
图3 在275 nm的Gd3+特征吸收光激发下的Ba1.9-ySiO4:0.1Gd3+,yTb3+(y=0,0.03,0.05,0.07,0.10,0.15)的发射光谱Fig.3 Emission spectra of Ba1.9-ySiO4:0.1Gd3+,yTb3+(y=0,0.03,0.05,0.07,0.10,0.15)under 275 nm excitation of Gd3+characteristic absorption
为进一步分析Gd3+→Tb3+的能量传递,对比图3中Gd3+的特征发射(313 nm)和Tb3+的特征发射(544 nm)荧光强度随Tb3+掺杂浓度的变化情况,得到图4。随着Tb3+浓度的增加,Gd3+的特征发射峰的强度(实线)逐渐降低,而Tb3+的特征发射峰的强度(虚线)先增大后减小,由此可看出Gd3+发射出的能量并未转换成光释放出来,而是通过无辐射共振的方式作为Tb3+的吸收光谱,从而被Tb3+吸收。而随着Tb3+掺入量的增加,较多的Tb3+原子吸收的能量也就越多,从而使得Gd3+以发光形式放出的能量进一步减少,所以Gd3+的313 nm发射峰的强度随着Tb3+掺入量的增加而逐渐降低,Tb3+的544 nm特征发射峰的强度增加[12]。但是当y(Tb3+)>0.05时,Tb3+的544 nm(5D4→7F5)处的发光强度反而降低。Tb3+的能级结构表明,5D4没有能量匹配的交叉弛豫途径,因此5D4(544 nm)发光的浓度猝灭主要是由于Tb3+离子间交换相互作用引起的,使得能量的损失超过了能量的发射,发生浓度猝灭,继而发光强度降低[13]。

图4 Ba1.9-ySiO4:0.1Gd3+,yTb3+(y=0,0.03,0.05,0.07,0.10,0.15)在313 nm和544 nm处的发射强度(λex=275 nm)Fig.4 Emission intensity of Ba1.9-ySiO4:0.1Gd3+,yTb3+(y=0,0.03,0.05,0.07,0.10,0.15)at 313 nm and 544 nm(λex=275 nm)
图5是不同Tb3+掺杂浓度样品在275 nm激发下,监测Gd3+:6P7/2→8S7/2(313 nm)处的衰减曲线。从拟合曲线得出,Gd3+单掺样品的荧光寿命为63.80 μs。随着样品中Tb3+掺杂浓度的增加,Gd3+的衰减明显变快,当y(Tb3+)为10%时,Gd3+:6P7/2的寿命为46.40 μs。荧光寿命缩短的原因是随着样品中Tb3+的加入,Gd3+存在了一种新的衰减路径,在6P7/2能级布居的电子通过能量传递,将能量传递给Tb3+使其达到5H7能级,造成了Gd3+:6P7/2能级衰减加速。根据Dexter能量传输理论[14],这说明Gd3+和Tb3+之间存在的能量传递是无辐射能量传递。

图5 275 nm激发下的Gd3+:6P7/2→8S7/2(313 nm)的发光衰减曲线与Tb3+掺杂量的关系Fig.5 Decay time of Gd3+:6P7/2emission(313 nm)under the excitation of 275 nm as a function of Tb3+content
根据Gd3+和Tb3+能级数据画出Gd3+→Tb3+的能量传递示意图,如图6所示。由于Gd3+的8S7/2→6P7/2与Tb3+的5H7→7F6能量间距相近,均约为32 000 cm-1,因此Gd3+→Tb3+的能量传递可通过共振无辐射形式实现。
在275 nm激发下,Gd3+的基态8S7/2能级吸收能量激发到6IJ能级,然后快速无辐射弛豫(NR)到6P7/2能级。6P7/2能级的电子可跃迁回到基态产生Gd3+的特征发射(313 nm),或者通过共振能量传递将激发能传递给Tb3+,使得Tb3+从基态跃迁到5H7能级,再无辐射弛豫到Tb3+的较低激发态5D3和5D4能级,最后跃迁至不同的基态能级产生Tb3+的特征发射[15]。

图6 Gd3+和Tb3+离子的能级结构(λex=275 nm)Fig.6 Schematic energy-level diagram of selected states of Gd3+and Tb3+ions(λex=275 nm)
3.3.2244 nm(Tb3+特征吸收光)激发下的发射光谱
图7是在Tb3+特征吸收244 nm激发下的Ba1.95-xSiO4:xGd3+,0.05Tb3+(x=0,0.05,0.10,0.15,0.20)的发射光谱。由图可知,Gd3+的掺入使样品在381,416,437 nm处(5D3→7FJ(J=6,5,4))的发射减弱,而488,544,584,616 nm处(5D4→7FJ(J=6,5,4,3))的发射增强。分析图中不同Gd3+掺杂量引起Tb3+的荧光强度变化可知,5%~15%Gd3+的掺入使得掺杂5%的Tb3+在544 nm处的发射增强了59%~128%,而416 nm处的发射强度单调降低。
对单掺0.05Tb3+和双掺0.1Gd3+、0.05Tb3+样品在244 nm激发下的Tb3+:5D3→7F5(416 nm)处的荧光衰减进行监测,得到图8。当离子间相互作用不明显时,衰减曲线可使用单指数函数拟合。单掺0.05Tb3+样品的衰减曲线稍微偏离单指数衰减,这是因为样品中存在Tb3+间交叉驰豫过程[16]。随着样品中Gd3+的掺入,衰减曲线偏离程度加大,且Tb3+:5D3能级的荧光寿命从8.50μs减小到7.28 μs,说明Gd3+的掺入增大了Tb3+间交叉驰豫的几率,使Tb3+:5D3能级布居的电子数减少。

图7 244 nm Tb3+特征吸收光激发下的Ba1.95-xSiO4:xGd3+,0.05Tb3+(x=0,0.05,0.10,0.15,0.20)的发射光谱Fig.7 Emission spectra of Ba1.95-xSiO4:xGd3+,0.05Tb3+under the excitation of 244 nm(x=0,0.05,0.10,0.15,0.20)

图8 244 nm激发下的Tb3+:5D3→7F5(416 nm)的发光衰减曲线Fig.8 Decay time of Tb3+:5D3emission(416 nm)under the excitation of 244 nm
在Tb3+发射过程中,5D3→5D4与7F6→7F0由于能级差相近,存在交叉驰豫现象(图9),使得Tb3+中处于5D3激发态上的电子跃迁到5D4能级,而另一个处于基态的Tb3+(7F6)被激发到7F0能级。结合图7、8可知,Gd3+的掺入增大了Tb3+间交叉驰豫的几率,从而使得5D3能级发射减弱,5D4能级发射增强。
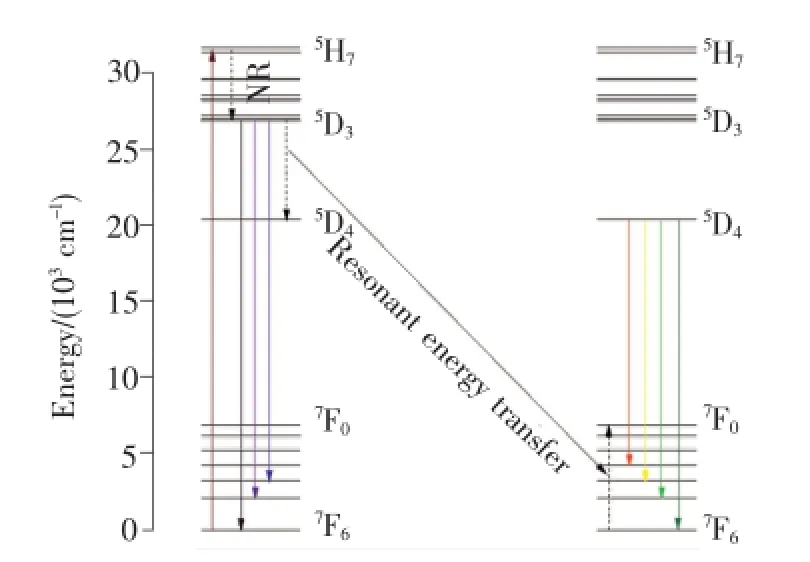
图9 Ba2SiO4晶体中Tb3+离子的交叉驰豫Fig.9 Cross-relaxation process between Tb3+ions in Ba2SiO4
4 结 论
采用高温固相法合成的Ba2SiO4:Gd3+,Tb3+荧光粉,在275 nm和244 nm激发下均可产生544 nm的Tb3+特征发射光。在275 nm的Gd3+特征吸收光的激发下,检测到Tb3+的特征发射,通过对比不同Tb3+掺杂量下Gd3+:6P7/2能级的衰减曲线,发现随着Tb3+掺杂浓度的增加,该能级的荧光寿命由63.80 μs衰减到46.40 μs,表明样品中存在Gd3+→Tb3+的能量传递,传递方式为无辐射共振能量传递,可归结为Gd3+的6P7/2能级和Tb3+的5H7能级间的共振转移。在244 nm的Tb3+特征吸收光的激发下,Gd3+的掺入可增强Tb3+的发射,使得掺杂5%的Tb3+在544 nm处的发射增强了59%~128%,结合荧光衰减曲线得出Gd3+的掺入对Tb3+能级中5D3→5D4与7F6→7F0交叉驰豫有促进作用。
[1]刘春旭,张家骅,吕少哲,等.纳米Gd2O3:Eu3+中Judd-Ofelt参数的实验确定[J].物理学报,2004,53(11):3945-3949. LIU C X,ZHANG J H,LÜ S Z,et al..Judd-Ofelt parameters determined experimentally for nanoparticles Gd2O3:Eu3+[J].Acta Phys.Sinica,2004,53(11):3945-3949.(in Chinese)
[2]ZHANG Y,GENG D L,SHANG M M,et al..Single-composition trichromatic white-emitting Ca9MgNa(PO4)7:Ce3+/ Tb3+/Mn2+phosphors-soft chemical synthesis,luminescence,and energy-transfer properties[J].Eur.J.Inorg. Chem.,2013,2013(25):4389-4397.
[3]侯青月,李金凯,段广彬,等.白光发光二极管用荧光粉的研究进展[J].中国粉体技术,2015,21(5):44-47. HOU Q Y,LI J K,DUAN G B,et al..Progress of research on phosphors for white light emitting diodes[J].China Powder Sci.Technol.,2015,21(5):44-47.(in Chinese)
[4]YE S,XIAO F,PAN Y X,et al..Phosphors in phosphor-converted white light-emitting diodes:recent advances in materials,techniques and properties[J].Mater.Sci.Eng.,2010,71(1):1-34.
[5]曾琦华,张信果,梁宏斌,等.白光LED用荧光粉的研究进展[J].中国稀土学报,2011,29(1):8-17. ZENG Q H,ZHANG X G,LIANG H B,et al..Progress of research on phosphors for white-light emitting diodes[J].J. Chin.Rare Earth Soc.,2011,29(1):8-17.(in Chinese).
[6]LIU Y,LIU G X,WANG J X,et al..Single-component and warm-white-emitting phosphor NaGd(WO4)2:Tm3+,Dy3+,Eu3+:synthesis,luminescence,energy transfer,and tunable color[J].Inorg.Chem.,2014,53(21):11457-11466.
[7]ZHANG X G,ZHOU L Y,PANG Q,et al..Tunable luminescence and Ce3+→Tb3+→Eu3+energy transfer of broadband-excited and narrow line red emitting Y2SiO5:Ce3+,Tb3+,Eu3+phosphor[J].J.Phys.Chem.C,2014,118(14):7591-7598.
[8]谢飞燕,董志月,刁贵强,等.一种新型的白光LED用绿色荧光粉Ca8MgLu(PO4)7:Tb3+[J].发光学报,2015,36(10):1132-1136. XIE F Y,DONG Z Y,DIAO G Q,et al..A novel green phosphor Ca8MgLu(PO4)7:Tb3+for near ultraviolet white lightemitting diodes[J].Chin.J.Lumin.,2015,36(10):1132-1136.(in Chinese)
[9]倪海勇,梁宏斌,王灵利,等.Na3GdSi2O7:Tb3+荧光粉发光特性及Gd3+→Tb3+之间的能量传递[J].发光学报,2013,34(8):970-975. NI H Y,LIANG H B,WANG L L,et al..Luminescent properties of phosphor Na3GdSi2O7:Tb3+and Gd3+→Tb3+energy transfer[J].Chin.J.Lumin.,2013,34(8):970-975.(in Chinese)
[10]FERNÁNDEZ-CARRIÓN A J,ÓCAÑA M,GARC I'A-SEVILLANO J,et al..New single-phase,white-light-emitting phosphors based on δ-Gd2Si2O7for solid-state lighting[J].J.Phys.Chem.C,2014,118(31):18035-18043.
[11]ZENG W,WANG Y H,HAN S C,et al..Enhancement of CdSiO3:Tb3+green long-lasting phosphors by co-doping with Re3+(Re3+=Gd3+,Y3+,La3+)ions[J].J.Lumin.,2014,152:210-213.
[12]SUN X Y,HUANG S M,GU M,et al..Enhanced Tb3+luminescence by non-radiative energy transfer from Gd3+in silicate glass[J].Phys.B,2010,405(2):569-572.
[13]杨志平,杨富,侯春彩,等.新型绿色荧光粉BaLa2ZnO5:Tb3+的合成与发光性质[J].发光学报,2014,35(10):1153-1157. YANG Z P,YANG F,HOU C C,et al..Synthesis and luminescence properties of novel green emitting phosphor BaLa2ZnO5:Tb3+[J].Chin.J.Lumin.,2014,35(10):1153-1157.(in Chinese)
[14]许少鸿.固体发光[M].北京:清华大学出版社,2011. XU S H.Luminescence of Solids[M].Beijing:Tsinghua University Press,2011.(in Chinese)
[15]RAJU G S,PARK J Y,JUNG H C,et al..Gd3+sensitization effect on the luminescence properties of Tb3+activated calcium gadolinium oxyapatite nanophosphors[J].J.Electrochem.Soc.,2011,158(2):J20-J26.
[16]VAN DER ENDE B M,AARTS L,MEIJERINK A.Near-infrared quantum cutting for photovoltaics[J].Adv.Mater.,2009,21(30):3073-3077.

肖林久(1958-),男,辽宁庄河人,博士,教授,2005年于东北大学获得博士学位,主要从事稀土发光材料、精细化工、催化和反应技术等方面的研究。
E-mail:x109@163.com
Luminescence Properties of Ba2SiO4:Gd3+,Tb3+Under UV Excitation
XIAO Lin-jiu*,GENG Yan-li,XIE Ying,JIANG Xin-dong,CUI Yong-qiang,WANG Shu-dong
(Key Laboratory of Rare-earth Chemistry and Applications of Liaoning Province,Shenyang University of Chemical Technology,Shenyang 110142,China)
*Corresponding Author,E-mail:x109@163.com
A series of Ba2SiO4:Gd3+,Tb3+green phosphors were synthesized by solid state reaction.The influence of the factors such as the amount of ion doping on the luminescence properties was investigated.Their structure and luminescence properties were characterized by X-ray diffraction(XRD)analysis,fluorescence spectrometry(FL),and decay curves.The spectroscopic properties of Ba2SiO4:Gd3+,Tb3+under the excitation of the characteristic absorption wavelength of Gd3+and Tb3+were investigated. The emission of Tb3+was observed under 275 nm(Gd3+:8S7/2→6IJ)excitation.By comparing Gd3+:6P7/2energy decay curve with different Tb3+doping amount,it is found that the level of fluorescence continuously shorten the service life with the increasing of Tb3+concentration,indicating the occurrence of energy transfer from Gd3+to Tb3+with the mode of nonradiative resonance transfer.Under the excitation of 244 nm(Tb3+:4f8→4f75d1),the emission intensity of5D3level decreases,but the5D4level increases with the doping of Gd3+.The emission intensity at 544 nm(5D4→7F5)increases 59%~128%with the doping of Gd3+.Combining with the fluorescence decay curves,it is found that the doping of Gd3+can promote the cross relaxation of5D3→5D4and7F6→7F0in Tb3+.
phosphors;energy transfer;cross relaxation;BaSiO:Gd3+,Tb3+24
O482.31
A
10.3788/fgxb20163706.0644
1000-7032(2016)06-0644-06
2016-02-03;
2016-03-13
辽宁省教育厅科研项目(L2012149,L2015420)资助

