pH和温度诱导双亲水嵌段共聚物聚甲基丙烯酸-b-聚N-(2-甲基丙烯酰氧乙基)吡咯烷酮水溶液的胶束化
张季平 程硕桢 李学丰 董金凤
(武汉大学化学与分子科学学院,武汉430072)
pH和温度诱导双亲水嵌段共聚物聚甲基丙烯酸-b-聚N-(2-甲基丙烯酰氧乙基)吡咯烷酮水溶液的胶束化
张季平程硕桢李学丰董金凤*
(武汉大学化学与分子科学学院,武汉430072)
基于可逆加成裂解链转移自由基(RAFT)聚合法开发了一系列新型双亲水嵌段共聚物——聚甲基丙烯酸-b-聚N-(2-甲基丙烯酰氧乙基)吡咯烷酮(PMAA-b-PNMP),并利用凝胶渗透色谱法(GPC)和1H NMR对其结构进行了表征。光散射和冷冻电镜的结果表明,此类双亲水嵌段共聚物的水溶液具有pH和温度诱导胶束化的现象,而且PNMP的聚合度对胶束化的pH和温度影响都非常大。一般而言,PNMP的聚合度越大,胶束化的pH值越小,胶束化的温度则越高。pD相关的1H NMR结果表明,PNMP与PMAA片段和水分子之间氢键的削弱以及PNMP与PMAA链之间相互作用的增强是pH诱导PMAA-b-PNMP胶束形成的主要原因,而PNMP片段与水分子之间氢键的削弱则是温度诱导PMAA-b-PNMP胶束形成的主要原因。此外,我们发现在PMAA-b-PNMP体系中制备的纳米金颗粒的大小可通过溶液pH进行可控调节。总体而言,pH值越高,金纳米颗粒的粒径越小。
PMAA-b-PNMP;胶束化;pH;温度;金纳米颗粒
www.whxb.pku.edu.cn
1 Introduction
Amphiphilic block copolymers show rich phase behaviors in solutions and can form spherical micelles,cylindrical micelles, vesicles,and even liquid crystalline as surfactants1,2,and thereby showing attractive applications in various fields,such as biological process,mesoporous materials,and catalysts fabrication,emulsification,and drug-controlled release3-5.Due to the diversity of block copolymer molecules,the interesting self-assembly behaviors of novel dual hydrophilic block copolymers are also reported recently6,7.Those block copolymers are a category of water soluble macromolecules in general,however,and show both amphiphilic and hydrophilic properties to favor self-assembly through changing the environmental conditions such as solution pH or temperature8-10.In other words,the self-assembly behaviors of dual hydrophilic block polymers are stimuli-responsive. Polymeric monomers such as acrylic acid(AA),methacrylic acid (MAA),and poly(ethylene glycol)(PEG)are most used in constructing those copolymers because the hydrophilicity of AA and MAA can be adjusted by pH whereas that of PEG relates to temperature highly,and thereby altering the hydrophilic-hydrophobic balance of polymers.
Pyrrolidone chemicals including N-alkyl pyrrolidone and polyvinylpyrrolidone(PVP)are well-known for their low vapor pressure,low toxicity and biocompatibility,which have important application in many fields11.For example,PVP are critical in controlling the shape of novel metal nanomaterials12,13.However, only several types of pyrrolidone containing polymers are available commercially,mainly PVP with different polymerization degrees,the construct of pyrrolidone containing polymers is of great importance especially in extending their application fields. Studies on poly(N-(2-methacryloylxyethyl)pyrrolidone)(PNMP) showed that PNMP not only reserved the functions of PVP but also showed thermo-responsive property14,15.We have reported a series of diblock copolymers poly(laurylmethacrylate)-b-poly[N-(2-methacryloylxyethyl)pyrrolidone](PLMA64-b-PNMPm,m= 251,393,484,512)16,which can form self-organized micelles in tetrahydrofuran(THF)and can be used to fabricate gold nanoparticles with well-defined size.Recently,the assembly behaviors of similar block copolymers,poly(stearylmethacrylate)-poly(N-2-(methacryloyloxyethyl)pyrrolidone)17,in dodecane was reported,which were used to stabilize Pickering emulsions. However,those two categories of amphiphlic copolymer are water insoluble that limited their further applications strongly.Fabricating pyrrolidone containing dual hydrophilic block polymers might be a suitable way to solve the problem,and is also the major motivation of this work.
In this work,four well-defined diblock copolymers PMAA-b-PNMP,poly(methacrylic acid)-b-poly[N-(2-methacryloylxyethyl) pyrrolidone],were synthesized by reversible addition-fragmentation chain transfer(RAFT)polymerization method and were characterized by1H NMR and gel permeation chromatography (GPC).Since PMAA-b-PNMP is composed of temperature-responsive PNMP and pH-responsive PMAA,and thereby showing pH-and temperature-responses in the self-assembling behaviors, which were investigated by employing static light scattering (SLS),dynamic light scattering(DLS),NMR,and cryo-TEM.Due to the pH and thermal responses of PMAA and PNMP segments, respectively,the self-assembly behaviors of PMAA-b-PNMP are responded to both pH and temperature stimuli.Specifically,they show pH and temperature-induced micellization in aqueous solution.According to structural similarity to PVP and the application in nanomaterial fabrication,PMAA-b-PNMP is also used to control the size of gold nanoparticles(Au NPs)with stimuliresponsive factors.This work not only provides new insights into the stimuli-responsive assembly behavior of pyrrolidone containing dual hydrophilic block polymers,but also shows a solid evidence in nano-material fabrications of them.
2 Materials and methods
2.1Materials
2-Cyanoprop-2-yl dithiobenzoate(CPDB)was synthesized according to the reported scheme16.2,2′-Azobisisobutyronitrile (AIBN,99%,Shanghai HATECH Co.Ltd.)was recrystallized from ethanol.Methacryloyl chloride(98%,Shanghai HATECH Co.Ltd.),N-hydroxyethyl pyrrolidone(98%,TCI),and methacrylate acid(MAA,98%,Shanghai HATECH Co.Ltd.)were distilled under reduced pressure.Dimethylformamide(DMF,99%, Shanghai HATECH Co.Ltd.)was distilled under reduced pressure,mixed with 0.05 mol·L-1NaNO3(99%,Shanghai HATECH Co.Ltd.)and then filtered on 0.2 μm polytetrafluoroethylene filters prior to use in GPC.N-(2-Methacryloyloxyethyl)pyrrolidone(NMP)was synthesized by the method described elsewhere16.All other solvents and reagents were purchased from commercial sources and were used as received.
2.2Synthesis of PMAA-b-PNMP diblock copolymers
Diblock copolymer PMAA-b-PNMP was synthesized by RAFT polymerization method as shown in Scheme 1.Firstly,the welldefined MAA homopolymer(PMAA macro-CTA)was prepared: MAA(15.0 g,174 mmol),CPDB(0.4 g,1.81 mmol),AIBN (0.059 g,0.36 mmol),and DMF(40 mL)were charged in 100 ml schlenk tube capped with rubber septa.Subsequently,the homogenous solution was degassed with N2for 30 min and heated at 60°C for 10 h under stirring.The final mixture was diluted by methanol,and then precipitated in the mixture of diethyl ether/ethyl acetate(V/V=1/1)twice,and dried in a vacuum oven at 30°C to give a pink powder.Yield:14.2 g(92.2%),number average molecular weight(Mn)=8700 g·mol-1,weight average molecular weight(Mw)=8961 g·mol-1,Mw/Mn=1.03.
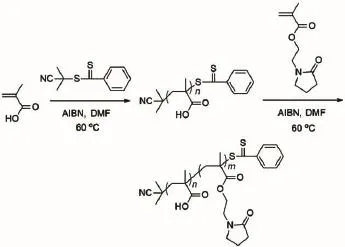
Scheme 1 Synthetic route of PMAA-b-PNMPby RAFT polymerization method
The resulting PMAA macro-CTA was used to synthesize PMAA-b-PNMP by the typical procedure as following:PMAA macro-CTA(1.25 g,0.143 mmol),NMP(3.75 g,17 mmol),AIBN (0.015 g,0.09 mmol),and DMF(15 mL)were charged in 25 ml schlenk tube capped with rubber septa.Subsequently,the solution was degassed with N2for 30 min,and heated at 60°C for 10 h under stirring.The final reaction mixture was diluted by DMF,and then precipitated into the mixture of diethyl ether/ethyl acetate(V/ V=1/1)at least twice,and dried in a vacuum oven at 30°C to give a pink powder.Yield:2.8 g(56.2%),Mn=38900 g·mol-1,Mw/Mn= 1.08.
2.3Polymer characterization
The1H NMR spectra were recorded on a Varian Mercury 300 MHz spectrometer in d6-DMSO.The GPC measurements were performed at 35°C using DMF(containing 0.05 mol·L-1NaNO3) as eluent with a flow rate of 1.0 mL·min-1.The column set is consisted of two MZ-SD plus 5 μm columns(50 nm and Linear); Wyatt Optilab DSP Interferometric refractometer and Wyatt DAWN EOS multi-angle laser light scattering detectors with a helium-neon laser light source(λ=685 nm),K5-flow cell,and a broad range of scattering angles from 45°-160°were employed. The molecular weight and polydispersity data were determined using the Wyatt ASTRA software package.The refractive index increment(dn/dc)of polymer solution was measured using an Optilab DSP refractometer at a wavelength of 685 nm,where n is the refractive index and c is the polymer concentration(g·mL-1). 2.4Sample preparation
The PMAA-b-PNMP copolymers(0.1 g)were dissolved in 100 mL water(pH=10)at room temperature under vigorous stirring. Samples with different pH were adjusted by the addition of the HCl or NaOH aqueous.Those with the required pD for NMR measurements were prepared in D2O and titrated with DCl or NaOD,in which 3-(trimethylsilyl)propionic acid-d4sodium salt (TMSP)was used as the internal standard.The pH or pD of solutions was measured using the Rex model PHSJ-5 digital pH meter(Leici,China)with a temperature sensor using 201 D combination pH electrode,and the pD values were recalculated as pD=pHmeasurement+0.418.
2.5Static and dynamic light scattering measurements
The static light scattering(SLS)and dynamic light scattering (DLS)of samples were performed on Zetasizer instrument ZEN3600(Malvern,UK)with a 173°back scattering angle and He-Ne laser(λ=633 nm).To obtain the apparent hydrodynamic diameter(Dh),the intensity autocorrelation functions were analyzed using CONTIN.
2.6Preparation of PMAA101-b-PNMP539coated gold
nanoparticles
The aqueous solution of HAuCl4·4H2O(2.5 mg·mL-1,0.2 mL) was added into 4.5 mL aqueous solution containing PMAA101-b-PNMP539copolymer(1 mg·mL-1)at room temperature and stirred for 20 min.Then,0.1 mL of freshly prepared aqueous solution of NaBH4(10 mg·mL-1)as a reducing agent was rapidly added into the mixture with vigorous stirring for 30 min,and the mixture was left undisturbed for 12 h at room temperature.
2.7UV-Vis measurements
UV-Vis spectra were carried out on a UV-Vis Tu-1901 spectrophotometer(Pgeneral,China)using ultrapure water as a blank at 25°C.
2.8TEM measurements
Samples for cryogen transmission electron microscopy(cryo-TEM)observation of PMAA-b-PNMP aggregates were prepared as follows:one drop of the sample dispersion was deposited on a carbon-coated copper grid.After blotting the excess dispersion away to form a thin liquid film,the grid was immediately plunged into liquid ethane.The specimens were maintained at approximately-173°C and imaged in a transmission electron microscopy(JEOL 1400)at an accelerating voltage of 200 kV under low dose conditions.
Samples for TEM observation of PMAA-b-PNMP stabilized gold nanoparticles were prepared by placing one drop of the gold dispersion on a carbon-coated copper grid,and then dried at room temperature.The morphologies were observed on a JEM-2100 operated at an acceleration voltage of 200 kV.
3 Results and discussion
3.1Synthesis and characteristics of PMAA-b-PNMP diblock copolymers
PMAA-b-PNMP is synthesized according to the methodologies as described in the experimental section(Scheme 1).Four diblock copolymers with the constant polymerization degree of PMAA and different polymerization degree of PNMP,namely PMAA101-b-PNMP153,PMAA101-b-PNMP240,PMAA101-b-PNMP420, and PMAA101-b-PNMP539,are synthesized.The details of PMAA-b-PNMP are summarized in Table 1.
The GPC trace of each diblock copolymer shows an unimodalcharacteristics(Fig.1a),indicating the narrow Mwdistribution (polydispersity<1.3).Simultaneously,the significant shift toward the higher molecular weight confirms the chain extension of the PMAA with PNMP successfully19.The molecular structure of the PMAA-b-PNMP was further confirmed by the1H NMR spectrum as shown in Fig.1b.1H NMR(δ,d6-DMSO):0.9-1.9(CH3CCH2in backbone of both MAAand NMP units),2.1(NCH2CH2CH2CO in pyrrolidone ring),2.48(NCH2CH2CH2CO in pyrrolidone ring), 3.51-3.56(CH2NCH2in NMP unit),4.05(COOCH2in NMP unit).Thus,the GPC and1H NMR results confirm that diblock copolymers PMAA-b-PNMP are synthesized by the RAFT polymerization method successfully.

Table 1 Molecular information of PMAA-b-PNMP
3.2Stimuli-responsive assembly behaviors of PMAA-b-PNMP
It is noticed that the dual hydrophilic diblock copolymers PMAA-b-PNMP are responded to both pH and temperature as shown in Fig.2.Initially,the sample is clear and transparent at pH=5.3 and 25°C,whereas it transforms into a turbid one upon decreasing pH(pH=4.8)or heating(T=60°C)and then returns to its original state through increasing solution pH or cooling, indicating the pH-and temperature-induced reversible micellization.
3.2.1pH-induced micellization
Generally speaking,all samples are optical transparent at about pH>5 whereas the detailed pH value relates to the polymerization degree of PNMP strongly,and they would transform into turbid ones when the solution pH is decreased.Fig.3a shows the pH-dependent light scattering intensities of 1 mg·mL-1PMAA-b-PNMP aqueous solutions.It′s clear that the light scattering intensity is increased dramatically when the solution pH is lower than a critical pH value for each PMAA-b-PNMP,indicating the formation of pH-induced aggregates.It is noticed that the pH value,where the light scattering intensity begins to increase,is decreasedfrom5.28to5.05fromPMAA101-b-PNMP153toPMAA101-b-PNMP539.In other words,the larger the polymerization degree of PNMP,the lower the transition pH.Fig.3b shows the typical pH-dependent size distribution of PMAA101-b-PNMP539that the aggregate size is increased when the solution pH is decreased.That's to say,the lower the pH,the larger the aggregate.

Fig.1 (a)GPC traces of macro-CTAagent PMAA101and diblock copolymers PMAA-b-PNMP; (b)1H NMR spectrum of PMAA101-b-PNMP539in d6-DMSO

Fig.2 pH-and temperature-induced reversible micellization in 1 mg·mL-1PMAA101-b-PNMP420aqueous solution

Fig.3 (a)pH-dependent scattering intensities of 1 mg·mL-1PMAA-b-PNMPaqueous solutions at 25°C and (b)pH-dependent size distribution of 1 mg·mL-1PMAA101-b-PNMP539at 25°C
In order to confirm the pH-induced micelles formation in PMAA-b-PNMPaqueous solutions,cryo-TEM is employed.Fig.4a and 4b show the cryo-TEM images of 1 mg·mL-1PMAA101-b-PNMP539at pH=2.1 and pH=3.9,respectively.Clearly,spherical micelles are formed in both conditions.It should be mentioned that micelles with a diameter larger than 200 nm are dominant at pH=2.1,whereas those are mainly about 100 nm at pH=3.9.The results are consistent with those obtained from DLS(Fig.3b)well.1H NMR technique is powerful in understanding the interaction and microenvironment variation of micelle formation process in the molecular level20,21.Fig.5a shows the1H NMR spectra of PMAA101-b-PNMP539at six typical pD values.On the whole,all the proton signals of PMAA101-b-PNMP539show high resolution in1H NMR spectra regardless of the solution pD,and the effect of pD on1H NMR can be observed clearly.For one thing,all the NMR signal peaks are compressed upon decreasing pD by comparing with that at pD=8.4.In the amphiphilic molecule systems,the compressed and broadened NMR signal peaks are often attributed to the formation of larger aggregates22.Herein,the compressed NMR signal peaks confirm the pH-induced micellization.Another, all the NMR signal peaks shift upfield slightly upon decreasing pD.Since the chemical shifts(δ)of molecular protons often highly relate to their location microenvironment.To make the shift more distinguishable,the shifts of two typical protons of Hgand Hcthat locate in the pyrrolidone ring and the main chain(Fig.5b),respectively,are studied.Fig.5b shows the pD-dependent chemical shifts of Hgand Hc.It is clear that the chemical shifts of Hgand Hcshow similar dependence on pD.The chemical shifts keep nearly constant when pD is above about 6.5,further decreasing pD results in the chemical shifts shifting upfield significantly until pD 5.1 and then keep constant again when pD is lower than 5.1.

Fig.4 Cryo-TEM images of PMAA101-b-PNMP539at pH=2.1(a)and pH=3.9(b)at 25°C
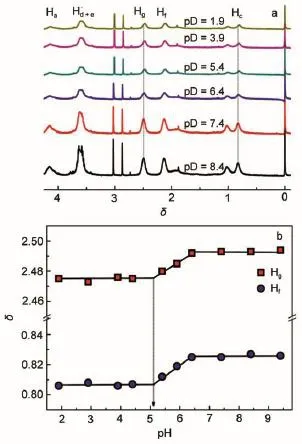
Fig.5 pD-dependent1H NMR spectra of PMAA101-b-PNMP539at 25°C(a)and pD-dependent chemical shifts of Hgand Hc(b)
During the pH-induced micelle formation process,the effect of pH on the chemical shifts of PMAA101-b-PNMP539protons might come from the following reasons.It is well-known that PMAAis pH sensitive with an apparent pKavalue of about 5.3523therefore, it mainly appears as nonionic type polymethacrylic acid at the acidic condition instead of the ionic type sodium salt at the neutral condition.Since apparent precipitate could be observed in the mixture of PMAA and PNMP homopolymers whereas they are both water soluble individually at the same condition,indicatingthe presence of inter-and intra-chain interactions between PMAA and PNMP segments.In other words,the binding mixtures of PMAA and PNMP segments should be formed.Accordingly,the hydrogen bond interaction between water and PMAA or PNMP should be weakened,which causes the chemical shifts shifting toward upfield24.Simultaneously,the binding mixture formation of PMAAand PNMP segments could increase the hydrophobicity of PMAA-b-PNMP diblock copolymers,and thereby endowing them micellization ability.Moreover,the binding mixture is the hydrophobic part that locates in the micellar core,resulting in the chemical shifts of PMAA-b-PNMP shifting toward upfield22,25. Therefore,the pD-dependent chemical shifts of Hgand Hcshown in Fig.5b should be attributed to the mentioned factors,however, support the pH-induced micellization well.In addition,the increase in the polymerization degree of PNMP could strength the hydrophilicity of PMAA-b-PNMP,and thereby resulting in the micellization pH shifting toward lower pH value.
3.2.2Temperature-induced micellization
It is also noticed that the aqueous solutions of PMAA-b-PNMP could transform from clear and transparent into turbid upon heating,indicating larger aggregates formation.The temperaturedependent scattering intensity of PMAA101-b-PNMP153at different pH values(the insect image in Fig.6a)shows that the scattering intensity would increase significantly above a critical temperature at a given pH,i.e.,5.4,5.5,and 5.6,suggesting the temperatureinduced micellization.The temperature where scattering intensity beginstoincreaseisthemicellizationtemperature.Fig.6ashowsthe pH-dependentmicellizationtemperatureof1mg·mL-1PMAA101-b-PNMP153,PMAA101-b-PNMP240andPMAA101-b-PNMP420aqueous solution.The higher the solution pH,the higher the micellization temperature.It is also noticed that the micellization temperature of PMAA-b-PNMP is increased with the increase of the polymerization degree of PNMP for a given pH.Generally,the larger the polymerization degree of PNMP,the higher the micellization temperature.For example,those of PMAA101-b-PNMP153,PMAA101-b-PNMP240and PMAA101-b-PNMP420are 56,59,and 64°C at pH 5.4,respectively.The results suggest that the high polymerization degree of PNMP blocks micelles formation.
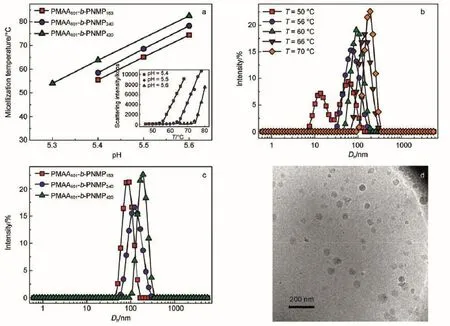
Fig.6 (a)pH dependent micellization temperature of PMAA-b-PNMP(The insert figure represents the temperature dependent intensity of PMAA101-b-PNMP153at different pH values);(b)temperature-dependent size distribution of PMAA101-b-PNMP420;(c)size distribution of PMAA-b-PNMPat pH 5.4 and 70°C;(d)cryo-TEM image of PMAA101-b-PNMP420at pH 5.3 and 60°C
The temperature-dependent size distribution of PMAA101-b-PNMP420at pH=5.3(Fig.6b)shows that it exhibits two distinct peaks at 50°C but only one peak above the micellization temperature,i.e.,56°C for PMAA101-b-PNMP420at pH 5.4.Upon further increasing temperature from 56 to 70°C,the micellar size is increased from 66 to 179 nm,respectively,indicating that high temperature favors micelle formation with larger size.Fig.6c shows the size distribution of 1 mg·mL-1PMAA101-b-PNMP153,PMAA101-b-PNMP240and PMAA101-b-PNMP420at pH 5.4 and 70°C,respectively.It's clear that self-organized micelles with diameter about 100 nm are formed in them.In addition,micellar size is increased slightly upon increasing the polymerization degree of PNMP from 153 to 420,i.e.,those are 83,119,and 179 nm for PMAA101-b-PNMP153,PMAA101-b-PNMP240,and PMAA101-b-PNMP420,respectively.The results suggest that PMAA-b-PNMP with a higher polymerization degree of PNMP favors larger micelle formation.It should be mentioned that no stable micelle phase can be observed in 1 mg·mL-1PMAA101-b-PNMP539from pH 5.1 to pH 5.6,however,precipitation appears rapidly upon heating instead due to the formation of micelles with very large sizes or the aggregates of micelles.To prove self-organized assemblies formation,cryo-TEM is employed as shown in Fig.6d, and spherical micelles with a diameter about tens of nanometers can be observed in 1 mg·mL-1PMAA101-b-PNMP420at pH=5.3 and 60°C,confirming temperature-induced micelles formation.

Fig.7 Proposed mechanism of pH-and temperature-induced micellization of PMAA-b-PNMP
It is well-known that PNMP is thermally sensitive and presents distinct cloud point,which relates to the molecular weight strongly17.Generally,the larger the molecular weight,the lower the cloud point.The cloud point suggests that the hydrogen bonds between PNMP and water is weakened upon heating.In other words,the hydrophilic PNMP segment shows hydrophobicity atthe high temperature.Thus,PMAA-b-PNMPbecomes amphiphilic molecular with its PNMPand PMAAsegments as the hydrophobic and hydrophilic parts,respectively,and thereby resulting in the temperature-induced micellization.

Fig.8 (a)UV-Vis spectra of PMAA101-b-PNMP539stabilizedAu NPs at different pH values;TEM images of PMAA101-b-PNMP539stabilizedAu NPs and the corresponding size distribution histograms of more than 200 particles at(b)pH=6 and(c)pH=3
3.2.3Mechanisms of pH-and temperature-induced micellization
Based on the experimental results and the analysis mentioned above,it is clear that micelles with the binding mixture of PMAA and PNMP as the hydrophobic tail and the unbinding part of PNMP as the hydrophilic headgroup are formed upon decreasing solution pH.However,those with PNMP as the hydrophobic tail and PMAAas the hydrophilic headgroup are formed upon heating instead.Therefore,the pH-and temperature-induced micellization of diblock copolymers PMAA-b-PNMP might be represented as shown in Fig.7.
3.3PMAA-b-PNMP stabilized glod nanoparticles
Since pyrrolidone-based polymer such as PVP is critical in fabricating metal nanostructures with special shapes because of the strong interactions between the O atom in the pyrrolidone ring and metal nanocrystals.13The diblock copolymer PMAA-b-PNMP might shield some new findings in this field due to its stimuliresponses.Herein,we focus on the effect of solution pH on gold nanostructures.Fig.8 shows the representative photographs of gold nanoparticles(Au NPs)and the corresponding absorbance spectra of Au NPs in aqueous solution.Macroscopically,the color of the solution is changed from brown to purple gradually when the solution pH is varied from 6 to 3.In the UV-Vis absorption spectra,the characteristic surface plasmon resonance(SPR)bands ofAu NPs appear concomitantly in the range of 500-600 nm.For one thing,the characteristic absorbance peak of Au NPs shifts from about 510 to 540 nm.Another,the absorbance peak intensity near 520 nm is increased gradually upon decreasing pH from 6 to 3.The results suggest that Au NPs are successfully synthesized in the mentioned pH region.Both the red shift and intensity increase of the characteristic absorbance peak of Au NPs indicate that largerAu NPs are synthesized at the lower solution pH.
In order to clarify the size and shape of the resultant Au NPs, TEM is employed to directly observe the colloidal particles as shown in Fig.8(b,c).It is clear that spherical Au NPs are formed in both conditions.The size distribution histograms show that the average diameter of Au NPs synthesized at pH 3 is about 6 nm, which is far larger than that prepared at pH 6(3.3 nm).The TEM results are consistent with those of UV-Vis spectra.The nucleation and growth processes are the two key factors,relating to the type and concentration of precursors,reaction time,capping agent,and so on,in controlling gold nanoparticle size26-28.Herein,the relative concentration of precursors should play an important role because PMAA101-b-PNMP539mainly presents as monomers and micelles at pH 6 and pH 3,respectively.Due to the interactions between pyrrolidone ring and AuCl4-ions,the relative concentration of AuCl4-ions binding on PMAA101-b-PNMP539micelles at pH 3,or the relative concentration of precursors,should be much higher than that on monomers at pH 6,which favors the growth of Au NPs strongly,resulting in the larger sizes.The results suggest that pH is one tool to control the size of Au NPs using diblock copolymer PMAA-b-PNMP as stabilizers.
4 Conclusions
In summary,we have synthesized a new series of structurally dual hydrophilic diblock copolymers PMAA-b-PNMP,by RAFT polymerization method.Compared with the previously studies on pyrrolidone containing polymers14-17,PMAA-b-PNMP homologues are responded to both pH and temperature.In specifically, they show pH-and temperature-induced micellization in aqueous solution.For one thing,the binding mixture of PMAAand PNMP endows the hydrophobicity of PMAA-b-PNMP and the micellization ability as well because of inter-and intra-chain interactions between PMAA and PNMP segments,which can be controlled by pH conventionally.Another,the dehydration of PNMP due to the weakness in the hydrogen bond interactions between PNMP and water upon heating endows the hydrophobicity of PMAA-b-PNMP,resulting in the temperature-induced micelles formation.We also provide a solid evidence in controlling the size of Au NPs through pH adjusting in the presence of PMAA-b-PNMP.According to the pH-and temperature-induced micelles formation,especially the distinguished micellar core composition at low pH and high temperature of diblock copolymers PMAA-b-PNMP in aqueous solution,they might have some potential applications in those of industrial technologies such as stimuli-responsive emulsification,controlled drug delivery,and nano-material development.
References
(1)Yin,L.;Lodge,T.P.;Hillmyer,M.A.Macromolecules 2012,45, 9460.doi:10.1021/ma302069s
(2)López-Barrón,C.R.;Li,D.;Wagner,N.J.;Caplan,J.F. Macromolecules 2014,47,7484.doi:10.1021/ma501238w
(3)Chang,X.;Dong,R.;Ren,B.;Cheng,Z.;Peng,J.;Tong,Z. Langmuir 2014,30,8707.doi:10.1021/la501652r
(4)Wan,Y.;Shi,Y.;Zhao,D.Chem.Mater.2008,20,932. doi:10.1021/cm7024125
(5)Groison,E.;Brusseau,S.;D′Agosto,F.;Magnet,S.;Inoubli,R.;Couvreur,L.;Charleux,B.ACS Macro Lett.2012,1,47. doi:10.1021/mz200035b
(6)Xuan,J.;Han,D.;Xia,H.;Zhao,Y.Langmuir 2014,30,410. doi:10.1021/la404493n
(7)Zhang,Y.;Yin,Q.;Lu,H.;Xia,H.;Lin,Y.;Cheng,J.ACS Macro Lett.2013,2,809.doi:10.1021/mz4003672
(8)Agut,W.;Brûlet,A.;Taton,D.;Lecommandoux,C.Langmuir 2007,23,11526.doi:10.1021/la701482w
(9)Qiao,Z.;Ji,R.;Huang,X.;Du,F.;Zhang,R.;Liang,D.;Li,Z. Biomacromolecules 2013,14,1555.doi:10.1021/bm400180n
(10)Guinaudeau,A.;Coutelier,O.;Sandeau,A.;Mazières,S.;Thi, H.D.N.;Drogo,V.L.;Wilson,D.J.;Destarac,M. Macromolecules 2014,47,41.doi:10.1021/ma4017899
(11)Jouyban,A.;Fakhree,M.A.;Shayanfar,A.J.Pharm.Pharm. Sci.2010,13,524.doi:10.18433/J3P306
(12)Tsunoyama,H.;Ichikuni,N.;Sakurai,H.;Tsukuda,T.J.Am. Chem.Soc.2009,131,7086.doi:10.1021/ja810045y
(13)Xian,J.;Hua,Q.;Jiang,Z.;Ma,Y.;Huang,W.Langmuir 2012, 28,6736.doi:10.1021/la300786w
(14)Deng,J.;Shi,Y.;Jiang,W.;Peng,Y.;Lu,L.;Cai,Y. Macromolecules 2008,41,3007.doi:10.1021/ma800145s
(15)Sun,J.;Peng,Y.;Chen,Y.;Liu,Y.;Deng,J.;Lu,L.;Cai,Y. Macromolecules 2010,43,4041.doi:10.1021/ma100133q
(16)Zhang,J.;Zou,M.;Dong,J.;Li,X.Colloid Polym.Sci.2013, 291,2653.doi:10.1007/s00396-013-3020-z
(17)Cunningham,V.J.;Armes,S.P.;Musa,O.M.Polym.Chem. 2016,7,1882.doi:10.1039/C6PY00138F
(18)Arthur,K.;Paabo,C.M.;Robinson,R.A.;Bates,R.G.Anal. Chem.1968,40,700.doi:10.1021/ac60260a013
(19)Convertine,A.J.;Lokitz,B.S.;Vasileva,Y.;Myrick,L.J.; Scales,C.W.;Lowe,A.B;McCormick,C.L.Macromolecules 2006,39,1724.doi:10.1021/ma0523419
(20)Jiang,Z.;Jia,K.;Liu,X.;Dong,J.;Li,X.Langmuir 2015,31, 11760.doi:10.1021/acs.langmuir.5b02312
(21)Zou,M.;Dong,J.;Yang,G.;Li,X.Phys.Chem.Chem.Phys. 2015,17,10265.doi:10.1039/C5CP00180C
(22)Chaghi,R.;Ménorval,L.;Charnay,C.;Derrien,G.;Zajac,J. Langmuir 2009,25,4868.doi:10.1021/la803451q
(23)Merle,Y.J.Phys.Chem.1987,91,3092.doi:10.1021/ j100295a089
(24)Ma,J.;Guo,C.;Tang,Y.;Chen,L.;Bahadur,P.;Liu,H.J.Phys. Chem.B 2007,111,5155.doi:10.1021/jp070887m
(25)Vamvakaki,M.;Palioura,D.;Spyros,A.;Armes,S.P.; Anastasiadis,S.H.Macromolecules 2006,39,5106. doi:10.1021/ma0605595
(26)Cheng,L.;Li,X.;Dong,J.J.Mater.Chem.C 2015,3,6334. doi:10.1039/C5TC00624D
(27)Sardar,R.;Funston,A.M.;Mulvaney,P.;Murray,R.W. Langmuir 2009,25,13840.doi:10.1021/la9019475
(28)Han,Y.;Zhu,L.;Shen,M.;Li,H.H.Acta Phys.-Chim.Sin. 2013,29(1),131.[韩莹,朱露,沈明,李恒恒.物理化学学报,2013,29(1),131.]doi:10.3866/PKU.WHXB201210082
pH-and Temperature-Induced Micellization of the Dual Hydrophilic Block Copolymer Poly(methacrylate acid)-b-poly(N-(2-methacryloylxyethyl) pyrrolidone)in Aqueous Solution
ZHANG Ji-PingCHENG Shuo-ZhenLI Xue-FengDONG Jin-Feng*
(College of Chemistry and Molecules Sciences,Wuhan University,Wuhan 430072,P.R.China)
Anew series of structurally controllable dual hydrophilic diblock copolymers poly(methacrylate acid)-b-poly(N-(2-methacryloylxyethyl)pyrrolidone),PMAA-b-PNMP including PMAA101-b-PNMP153,PMAA101-b-PNMP240,PMAA101-b-PNMP420,and PMAA101-b-PNMP539,were synthesized by reversible addition-fragmentation chain transfer(RAFT)polymerization and characterized by gel permeation chromatography(GPC)and1H nuclear magnetic resonance(NMR).The pH-and temperature-induced micellization behavior of PMAA-b-PNMP in aqueous solution was confirmed by static light scattering(SLS)and dynamic light scattering(DLS)and cryogen transmission electron microscopy(cryo-TEM)techniques.The polymerization degree of PNMP strongly affects the micellization behavior.Generally,with higher polymerization degree,the micellization pH was lower and the micellization temperature was higher.During the micellization processes,the weakness of the hydrogen bond interactions between water and PNMP or PMAAand the strength in the inter-and intra-chain interactions between PNMP and PMAAsegments are dominant during the pH-induced micellization,as revealed by the pD-dependent1H NMR spectra.However,the weakness in the hydrogen bond interactions between water and PNMP is the major cause of the temperature-induced micellization process.We also provide solid evidence forthe ability to control the size ofAu NPs by adjusting the pH in the presence of PMAA-b-PNMP.Specifically,with higher pH,the size of theAu NPs was smaller.
PMAA-b-PNMP;Micellization;pH;Temperature;Gold nanoparticle
April 25,2016;Revised:May 26,2016;Published on Web:May 27,2016.
O648
10.3866/PKU.WHXB201605271
*Corresponding author.Email:jfdong@whu.edu.cn;Tel:+86-27-63587628.
The project was supported by the National Natural Science Foundation of China(21273165,21573164).
国家自然科学基金(21273165,21573164)资助项目
©Editorial office ofActa Physico-Chimica Sinica
[Article]

