联苯甲酰桥联β-环糊精吸附U(VI)的动力学和热力学
荆鹏飞 刘慧君 张 勤 胡胜勇 雷兰林 冯志远
(南华大学化学化工学院,湖南衡阳421001)
联苯甲酰桥联β-环糊精吸附U(VI)的动力学和热力学
荆鹏飞刘慧君*张勤胡胜勇雷兰林冯志远
(南华大学化学化工学院,湖南衡阳421001)
β-环糊精与对甲苯磺酰氯在低温碱性溶液中反应合成6-对甲苯磺酰酯-β-环糊精,并利用红外光谱和核磁共振氢谱对其进行表征;联苯甲酰与6-对甲苯磺酰酯-β-环糊精以摩尔比为1:2反应合成一种新型的联苯甲酰桥联β-环糊精(BB β-CD)材料,并采用紫外可见分光光度法对其合成机理以及BB β-CD和联苯甲酰对U(VI)的吸附行为进行研究;同时采用扫描电镜对材料吸附U(VI)前后的外貌形态进行表征。通过间歇吸附法考察pH、反应时间、温度以及干扰离子等因素对吸附过程的影响。结果表明,相比联苯甲酰,BB β-CD能更有效地吸附U(VI),在pH=4.5,反应时间为60 min条件下,最大吸附量为12.16 mg·g-1,吸附率高达91.2%。动力学和热力学拟合结果表明,吸附过程更符合准二级动力学速率方程,Langmuir等温吸附模型比Freundlich等温吸附模型更适合模拟吸附过程,且吸附是自发吸热的过程。
联苯甲酰桥联β-环糊精;铀(VI)吸附;动力学;平衡;热力学
www.whxb.pku.edu.cn
1 Introduction
With the continuous development of the global atomic energy industry,uranium and its compounds are nuclear fuels in power generation,which plays an important role in the military,civilian nuclear science,and technology.But with the rapid development of the nuclear industry,a large amount of wastewater containing uranium has been discharged into the environment,which has resulted in widespread environmental contamination1-5.Therefore, the efficient separation of uranium from aqueous phase,especially from industrial effluents,has attracted high attention of researchers6-8.To remove U(VI)from aqueous solution,several methods,such as chemical precipitation9,evaporation concentration10,ion exchange11,film processing method12,adsorption and solvent extraction13-15,have been developed to date.Currently, adsorption is an attractive method due to its high efficiency and diversity of adsorption.Many different sorbents,such as alumina, sepiolite,activated carbon,carbon nanotube,silica gel,goethite, chitosan and so on,have been investigated16-22.However,how to get a quick and resultful material for the determination and adsorption of U(VI)is still a work badly in need for us to do.
β-cyclodextrins(β-CD)is a cyclic oligosaccharide with seven glucose units containing a hydrophilic exterior and hydrophobic internal cavity.The cavity structure of β-cyclodextrin can selectively form BB β-CD with other guest molecules through hostguest interactions.Sun et al.23had studied the adsorption and desorption of U(VI)on functionalized graphene oxides.Liu et al.24had studied the selective adsorption of U(VI)from acidic solution by high performance of phosphate-functionalized graphene oxide. Li et al.25had studied the adsorption and recovery of U(VI)from low concentration uranium solution by amidoxime modified Aspergillus niger.Hosseini and Abedi26had studied the adsorption of Th(IV)and U(VI)on mixed-ligands impregnated resin containing antraquinones with that conventional one.However,the study on the adsorption of U(VI)on bridged β-cyclodextrin is rarely reported.
Benzil consists of two carbonyl(C=O)groups,which can form complexes with metal ions,is a kind of good α-diketone and it is also an excellent metal-chelating agent.To the best of our knowledge,there was still no report of study on the adsorption of U(VI)by BB β-CD.Compared with the reported sorbents,BB β-CD has great application in the adsorption of U(VI)from low concentration U(VI)solution because of low toxicity,biocompatibility,biodegradability and collaborative adsorption with benzil27,28,and expands the adsorption range for U(VI).At the same time,BB β-CD is also a low-cost sorbent with high adsorption capacity for U(VI)from low concentration U(VI)solution.In our work,adsorption material of BB β-CD was prepared by the reaction of benzil and sulfated-β-CD with the molar ratio of 1:2. In order to find the optimum adsorption conditions,a series of factors,such as pH value,contact time,temperature,and interfering ions were carried out for investigating the chemical adsorption properties of the sorbent for U(VI).In addition,various kinetic and thermodynamics models are also applied to study the adsorption process.
2 Experimental
2.1Materials and methods
β-cyclodextrin(purity≥98%),benzil(purity≥98%),ptoluenesufonyl chloride(p-TsCl,99%),sodium hydroxide(purity ≥97%).Ammonium uranyl tricarbonate((NH4)4[UO2(CO3)3]), hydrochloric acid,alcohol,acetonitrile,arsenazo III,nitric acid, etc.were analytical reagent and used without further purification. All reagents were purchased fromAladdin Chemical Reagent Co. Ltd.(Shanghai,China).
U3900 UV-spectrophotometer(Hitachi Ltd,Japan),Shimadzu IR Prestige-21 FTIR(Shimadzu,Japan),Bruker AV-III 400 MHz NMR spectrometer(Bruker BioSpin,Switzerland),S-4800 Scanning Electron Microscope(Hitachi Ltd.,Japan),etc.
2.2Synthesis of sulfated-β-CD
5 g of β-CD was dissolved in 100 mL of water,3 g of sodium hydroxide,and 1.68 g of p-TsCl was also added under the condition of ice water bath.The mixture was stirred and reacted for 5 h.Then the unreacted p-TsCl was filtered,the filtrate was adjusted to pH 6-7 by 1 mol·L-1HCl and it was put into a fridge for 24 h at 4°C.The resulting precipitate was filtered and recrystallized 2 times in water,CH3CN/H2O(1/1,V/V)to give sulfatedβ-CD.
2.3Preparation of BB β-CD
BB β-CD was prepared by the reaction of benzil(0.1 g)and sulfated-β-CD(1.2374 g)with the molar ratio of 1:2 in water at 50°C for 4 h,then the mixture was put into a fridge overnight at 4°C.The resulting precipitate was filtered and washed 6 times with deionized water and ethanol and dried by vacuum evaporation at 60°C for 8 h to give BB β-CD.The synthesis routes of sulfated-β-CD and BB β-CD are showed in Fig.1.
2.4Adsorption studies
In order to obtain the optimization adsorption conditions,the effects of pH,contact time,temperature,and interfering ions were examined.In the batch adsorption experiments,15 mg BB β-CD was added to the 10 mL U(VI)solution in 25 mL flask for which concentration was 20 mg·L-1,pH value is 2.0-7.0,and time range is 20-180 min.In addition,the flasks were shaken using shakingwater bath for specified durations at desired temperatures(298-338 K).After equilibration,the residual concentration of U(VI) ions was determined by UV-spectrophotometer.The adsorption capacity Q(mg·g-1)of BB β-CD and the remove ratio R(%)of U(VI)were calculated was calculated by the following equations:

Fig.1 Synthesis route of sulfated-β-CD and BB β-CD

where C0and Ce(mg·L-1)are the initial and equilibrium concentrations,respectively.V(L)is the volume of the testing solution,and m(g)is the mass of sorbent.
2.5Effect of interfering ions
In order to explore the selective adsorption behavior of U(VI), some important different concentrations of interfering ions such as Na+,Mg2+,Fe3+,and Cu2+were added to 20 mg·L-1U(VI)solution,pH 4.5,shaking to adsorb for 60 min.Centrifuge and UV-spectrophotometer was employed to analyze the U(VI)concentration in the adsorbed solution.
3 Results and discussion
3.1Characterization analysis
Fig.2(A)shows the FTIR spectra of β-CD,sulfated-β-CD,and BB β-CD.Compared with β-CD some new absorption peaks were found in the FTIR spectra of sulfated-β-CD.In the FTIR spectra of sulfated-β-CD,the peaks around 1177 and1364 cm-1resulted from symmetric stretching and antisymmetric stretching vibration of S=O.The peaks at 1599,1078,and 1028 cm-1were ascribed to the νC=Con benzene ring,the νC―O―Cand νC―Oof the template sulfated-β-CD.And the peaks of 837,815 cm-1were ascribed to the νC―Hon the benzene ring29.And compared with sulfated-β-CD, BB β-CD appeared characteristic bands at 1697 cm-1,which was ascribed to the νC=O.
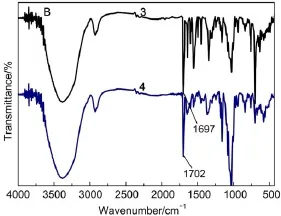
Fig.2 FTIR spectra of β-CD(1),sulfated-β-CD(2),BB β-CD(3)
The FTIR spectra of BB β-CD and BB β-CD+U(VI)are respectively shown in Fig.2(B).As shown in Fig.2(B),the FTIR spectra of BB β-CD shows that the template does not change the adsorption peak of each chemical group very much,suggesting that the template only combines with the with hydrophobic interaction and hydrogen bonding interaction,but not forming chemical bonds.The FTIR spectra of BB β-CD+U(VI)displays significant shift in some peaks.The shift of the peak from 1697 to 1702 cm-1reflects the changes in the stretching frequency of carbonyl(C=O)upon binding of U(VI).This observation indicates the involvement of carbonyl(C=O)in the adsorption process30,31.
Fig.3 shows the1HNMR(DMSO-d6,400 MHz,TMS)spectra of benzil,β-CD,sulfated-β-CD,and BB β-CD.As seen in Fig.3, the1HNMR spectra of benzil,β-CD,sulfated-β-CD,and BB β-CD have obvious difference and the change of chemical shifts of sulfated-β-CD and BB β-CD are shown in Table 1.As shown in Fig.3,the chemical shifts of sulfated-β-CD are different from those of β-CD,and the results of them are consistent with the reported sulfated-β-CD32.Compared with sulfated-β-CD,the chemical shifts of part protons(H3 and H5)of BB β-CD have obvious move and other protons have not apparent movement.Therefore,we canknow that the formation of the BB β-CD by insertion of the aromatic ring of the benzil into the sulfated-β-CD cavity can be confirmed by observing the chemical shifts induced in the H3 and H5 resonances of sulfated-β-CD due to the ring-current effects of the aromatic benzil.As shown in Table 1,the relatively large upfield shift is observed for H3 and H5 of sulfated-β-CD,which indicate that benzil molecule inserted into sulfated-β-CD cavity33. All of these proved that the synthesis of BB β-CD is reliable and successful.

Fig.3 1HNMR spectra of benzil(a),β-CD(b), sulfated-β-CD(c),and BB β-CD(d)
The UV-spectrophotometer analysis results shows the changes of absorb and wavelength about different molar ratios of benzil and sulfated-β-CD(Fig.4).Absorption wavelength moved to the maximal from 1:0 to 1:2,however,it went back when the molar ratio went to 1:2 and 1:2.5,and we can preliminarily conclude that the molar ratio of 1:2 is the best molar ratio.
As the stirring time went on,the mixture solution slowly turned to clarify while it was turbidity at the beginning.Maybe it belonged to the reason that benzil did not dissolute in the water,so it was turbidity at the beginning,but as the stirring went on,the benzil went into the cavity of sulfated-β-CD to format BB β-CD. Besides UV-spectrophotometer,it is also very important to choose the best molar ratio by determining inclusion constants under different molar ratios of benzil and sulfated-β-CD,and the resultsare reported in Table 2.Here,it is the determination and calculation process of inclusion constant under the molar ratio of 1:2 of benzil and sulfated-β-CD.UV-spectrophotometer shows adsorption of benzil in 0.05 g BB β-CD is 2.068,according to the standard concentration of benzil in Fig.5,that means the concentration of benzil in 10 mLethanol is C=1.70×10-3mol·L-1. Defining the mass ratio of benzil and sulfated-β-CD in original sample is k0,and in the BB β-CD is k1,the inclusion constant is K, k1=1.70×10-3×10×10-3×210.23/0.05=0.0714,K=(k1/k0)× 100%=0.0714/0.07477×100%=95.49%.In addition,the determination and calculation process of inclusion constants under other molar ratios of benzil and sulfated-β-CD are the same.The results of UV-spectrophotometer analysis and the determination of inclusion constants show that the molar ratio of 1:2 of benzil and sulfated-β-CD is the best molar ratio.

Table 1 Chemical shifts of part protons of sulfated-β-CD and BB β-CD
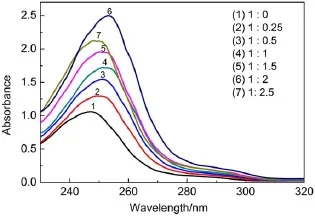
Fig.4 UV absorption of different molar ratios of benzil and sulfated-β-CD
SEM shows that the surface of BB β-CD was uneven and rough while that of BB β-CD absorbed U(VI)was homogeneous.The reason is that pores in BB β-CD provides necessary channel and adsorption space for the adsorption.Therefore,BB β-CD can effectively adsorb U(VI)(Fig.6).
3.2Effect of pH
pH is one of the important factors that affect the adsorption efficiency,and the effect of pH on the adsorption of U(VI)from aqueous solutions is showed in Fig.7.The results showed that the adsorption of U(VI)increased gradually as pH increases from 2.0 to 4.5,then decreases when the pH value is higher than 4.5.Because at low pH,it is difficult for diketone to chelate metal ions and there are two reasons to explain it.On the one hand,the lower uptake at low pH may be attributed to the higher acidities which made the protonation of O in BB β-CD on benzil by H+34,and formed positively charged BB β-CD surface which prevent the adsorption of metal ions due to electrostatic repulsion35-37.On the other hand,the low adsorption can be due to the competition of H+and metal ions in the solution for the adsorption sites of BB β-CD38.And when the pH continues to increase,U(VI)may hydrolyse to UO2OH+and(UO2)2(OH)22+)or precipitation39,resultingin a false impression or adsorption error40.In order to get quantitative adsorption of U(VI)at higher pH values while avoid hydrolysis and precipitation,pH 4.5 was considered as the optical value,and the adsorption capacity of U(VI)was 12.16 mg·g-1.

Fig.5 Standard concentration of benzil

Table 2 Inclusion constants under different molar ratios of benzil and sulfated-β-CD

Fig.6 SEM spectra of BB β-CD(a)and BB β-CD+U(VI)(b) (a)BB β-CD;(b)BB β-CD+U(VI)
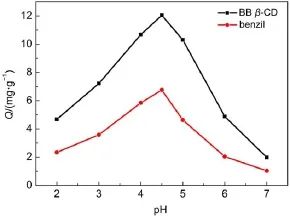
Fig.7 Effect of pH on the adsorption of U(VI)
3.3Effect of contact time and kinetic studies
The effect of contact time was investigated to determine the equilibrium point,and the result was given in Fig.8.The results showed that the adsorption capacity of U(VI)gradually increased during the 20-120 min and then tended to equilibrate in the following contact time for benzil.However,the sorbent BB β-CD tended to equilibrate in 60 min.This observation is due to the fact that the hydrophobic space of β-CD inclusion hydrophobic benzene ring of benzil,two oxygen atoms of benzil exposed and U (VI)adsorbed quickly and fully.Therefore,the U(VI)can be easier adsorbed on BB β-CD than benzil.The BB β-CD in this study had good adsorption capacity at pH 4.5,and the adsorption equilibrium could reach a balance in 60 min.

Fig.8 Effect of contact time on the adsorption of U(VI)
To analyze the kinetic adsorption behaviors of U(VI)on BB β-CD,two kinetic models namely pseudo-first-order and pseudosecond-order models were used to fit the adsorption process.The pseudo-first-order kinetic model is given by the following equation41:
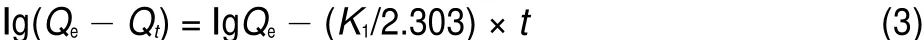
where Qeand Qt(mg·g-1)are the amount of U(VI)adsorbed at equilibrium and at time t(min),respectively.K1(min-1)is the rate constant of pseudo-first-order,and t(min)is the reaction time. Values of Qeand K1were calculated from the intercept and slope values of the straight line by plotting lg(Qe-Qt)versus t are reported in Table 3 and as shown in Fig.9.The results showed that the linear plot of lg(Qe-Qt)and time followed pseudo-first-order kinetic model of U(VI)adsorption on BB β-CD.
At the same time,the kinetic adsorption behaviors of U(VI)on BB β-CD was also described according to the pseudo-secondorder kinetic using the following equation42:

where K2(mg·g-1·min-1)is the rate constant of pseudo-secondorder,and t(min)is the reaction time.Values of Qeand K2were calculated from the slope and intercept values of the straight line by plotting t/Qtversus t are reported in Table 3 and as shown in Fig.10.The results showed that the linear plot of t/Qtand time followed pseudo-second-order kinetic model of U(VI)adsorption on BB β-CD.The calculated Qevalue from pseudo-second-order kinetic equation agreed very well with the experimental Qevalue. The kinetic data showed that the adsorption of U(VI)followed pseudo-second-order kinetic model(R2=0.9944),and the experimental Qe(exp)value(12.16 mg·g-1)was close to the model Qevalue(12.165 mg·g-1).

Table 3 Kinetic data for adsorption of U(VI)

Fig.9 Pseudo-first-order plot for adsorption of U(VI)
3.4Adsorption isotherms
Generally speaking,adsorption isotherms can provide some significant information in optimizing the application of BB β-CD, Langmuir and Freundlich isotherms were used to simulate the adsorption isotherms of U(VI).According to the Langmuir isotherm model,adsorption process commonly occurs on the surface of sorbent until monolayer coverage is obtained.The linear equation of the Langmuir adsorption model can be expressed as follows43:
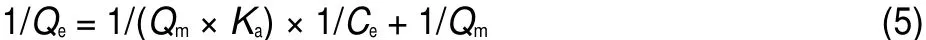
where Qe(mg·g-1)and Qm(mg·g-1)are the equilibrium and maximum adsorption capacities,respectively.Ce(mg·L-1)is the equilibrium concentration of metal ions in solution,Ka(L·mg-1) is the Langmuir constant related to energy of adsorption.The values of Qmand Kacalculated from the intercept and slope values of the straight line by plotting 1/Qeversus 1/Ceare reported in Table 4 and as shown in Fig.11.The results showed that the linear plot of 1/Qeand 1/Cefollowed the Langmuir adsorption model of U(VI)adsorption on BB β-CD.

Fig.10 Pseudo-second-order plot for adsorption of U(VI)
Unlike the Langmuir adsorption model,the Freundlich adsorption model is an empirical model,which is based on heterogeneous surfaces and allows for several kinds of adsorption sites on the surface of adsorption material.The model can be represented by the following equation44,45:

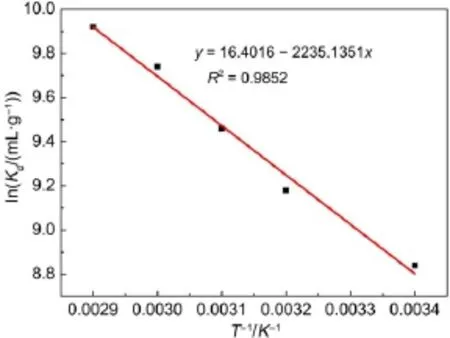
where Qe(mg·g-1)and Ce(mg·L-1)are the equilibrium concentrations of metal ions in solution,respectively.and KFand n are Freundlich constants,which mean adsorption capacity and adsorption intensity,respectively.The values of KFand n calculated from the intercept and slope values of the straight line by plotting lnQeversus lnCeare reported in Table 4 and as shown in Fig.12. The values of KFand n were found to be 1.01 and 1.35.The value of 1 3.5Effect of temperature and adsorption thermodynamics The effect of temperature on the adsorption of U(VI)on thestudied BB β-CD were investigated at 298,308,318,328,and 338 K,respectively.Thermodynamic parameters were calculated to confirm the thermodynamic feasibility and the nature of the adsorption process.The thermodynamic parameters corresponding toU(VI)adsorptionontheBB β-CDcanbeexpressedusingvan′t Hoff equation46: Table 4 Isotherm model constant parameters for adsorption of U(VI) Fig.11 Langmuir plots for adsorption of U(VI) Fig.12 Freundlich plots for adsorption of U(VI) where C0and Ce(mg·L-1)are the initial and equilibrium concentrations,respectively.V(mL)is the volume of the testing solution,m(g)is the mass of sorbent,Kd(mL·g-1)is the distribution coefficient,ΔS0(J·mol-1·K-1)is standard entropy,ΔH0(kJ·mol-1) is the standard enthalpy,ΔG0(kJ·mol-1)is the standard Gibbs free energy,T(K)is the absolute temperature,and R(8.314 J·mol-1· K-1)is the gas constant. The curve of temperature and distribution coefficient is reported in Table 5 and as shown in Fig.13.As shown in Table 5,ΔH0is positive because the adsorption of U(VI)on BB β-CD is endothermic.The values of free energy are negative,and the decrease in the value of ΔG0with increase in temperature shows that the reaction is spontaneous and more favorable at higher temperature. Table 5 Thermodynamic parameters for the adsorption of U(VI) Fig.13 van′t Hoff plots for the adsorption of U(VI) Fig.14 Infection on the adsorption of U(VI)by interfering irons Fig.15 Possible adsorption mechanism of U(VI) 3.6Interfering ions analysis In order to evaluate the selective adsorption of U(VI)by the BB β-CD,the effect of interfering ions on adsorption of U(VI)were carried out(Fig.14).The results showed that interfering ions had different influence on adsorption capacity of U(VI).Na+didn′t obviously affect the adsorption of U(VI).The adsorption of U(VI) could have the similar capacity when the concentration of Mg2+, Fe3+,and Cu2+were lower than 10 mg·L-1.The possible adsorption mechanism of U(VI)is shown in Fig.15. Anovel BB β-CD was synthesised by the reaction of benzil and sulfated-β-CD with the molar ratio of 1:2,and it was successfully used for the adsorption of U(VI).The BB β-CD used as sorbent had good adsorption capacity(12.16 mg·g-1)and remove ratio (91.2%)of U(VI)at the optimum conditions.The adsorption capacity of U(VI)showed no obvious change in the presence of Na+,Mg2+,Fe3+,and Cu2+when concentration was lower than 10 mg·L-1.Kinetic study showed that the pseudo-second-order model was appropriate to describe the adsorption process,indicating the chemical adsorption.Among different models used for describing equilibrium isotherm data,Langmuir model is in good agreement with the experimental data with high R2(0.9907).The adsorption of U(VI)dependence on temperature was investigated and the thermodynamic parameters DH0,DS0,and DG0were calculated. The results showed that it was a feasible,spontaneous and endothermic adsorption process.In this paper,the raw materials are commercially available,the experimental method for the adsorption of U(VI)is reliable and feasible and it can provide certain reference value for future research. References (1)Olszewski,G.;Boryło,A.;Skwarzec,B.J.Environ.Radioactiv. 2015,146,56.doi:10.1016/j.jenvrad.2015.04.001 (2)Liu,P.H.;Wei,C.S.;Zhang,S.M.;Zhu,C.M.;Xie,S.R. Asian J.Chem.2015,27,1049.doi:10.14233/ ajchem.2015.18056 (3)Cesare,M.D.;Cesare,N.D.;D'Onofrio,A.Appl.Radiat. Isotopes.2015,103,166.doi:10.1016/j.apradiso.2015.06.011 (4)Bourgeois,D.;Burt-Pichat,B.;Goff,X.L.Anal.Bioanal. Chem.2015,407(22),6619.doi:10.1007/s00216-015-8835-7 (5)Bonato,M.;Ragnarsdottir,K.V.Wat.Air Soil.Pollut.2012,223 (7),3845.doi:3846.10.1007/s11270-012-1153-1 (6)Gu,Z.X.;Tu,C.N.;Wang,Y.;Yang,J.J.;Liu,N.;Liao,J.L.; Yang,Y.Y.;Tang,J.Acta Phys.-Chim.Sin.2015,31(Suppl), 95.[顾泽兴,涂昌能,王云,杨吉军,刘宁,廖家莉,杨远友,唐军.物理化学学报,2015,31(Suppl),95.]doi:10.3866/ PKU.WHXB2014Ac13 (7)Yousif,A.M.;El-Afandy,A.H.;AbdelWahab,G.M.;Mubark, A.E.;Ibrahim,I.A.J.Radioanal.Nucl.Chem.2015,303(3), 1821.doi:10.1007/s10967-014-3688-7 (8)Sun,T.X.;Shen,X.H.;Chen,Q.D.Acta Phys.-Chim.Sin. 2015,31(Suppl),32.[孙涛祥,沈兴海,陈庆德.物理化学学报,2015,31(Suppl),32.]doi:10.3866/PKU.WHXB2014Ac10 (9)Mellah,A.;Chegrouche,S.Barkat,M.Hydrometallurgy 2007, 85,163.doi:10.1016/j.hydromet.2006.08.011 (10)Duff,M.C.;Morris,D.E.;Hunter,D.B.;Bertsch,P.M. Geochim.Cosmochim.Ac.2000,64(9),1535.doi:10.1016/ S0016-7037(99)00410-X (11)Zou,W.H.;Zhao,L.;Han,R.P.Chin.J.Chem.Eng.2009,17, 586.doi:10.1016/S1004-9541(08)60248-7 (12)John,A.M.S.;Cattrall,R.W.;Kolev,S.D.J.Memb.Sci.2012, 409(4),242.doi:10.1016/j.memsci.2012.03.061 (13)Gok,C.;Aytas,S.J.Hazard.Mater.2009,168(1),369.doi: 10.1016/j.jhazmat.2009.02.063 (14)Joseph,C.;Schmeide,K.;Sachs,S.;Brendler,V.;Geipel,G.; Bernhard,G.Chem.Geol.2011,284(3),240.doi:10.1016/j. chemgeo.2011.03.001 (15)Oshita,K.;Sabarudin,A.;Takayanagi,T.;Oshima,M.; Motomizu,S.Talanta 2009,79(2),1031.doi:10.1016/j. talanta.2009.03.035 (16)Qian,L.;Ma,M.;Cheng,D.J.Radioanal.Nucl.Chem.2015, 303,161.doi:10.1007/s10967-014-3352-2 (17)Branislava,M.M.;Milijan,J.;Mirjana,L.M.Radiat.Environ. Bioph.2015,54(2),217.doi:10.1007/s00411-015-0589-2 (18)Ahmed,S.H.;Sharaby,C.M.;Gammal,E.M.E. Hydrometallurgy 2013,134,150.doi:10.1016/j. hydromet.2013.02.003 (19)Tan,L.;Liu,Q.;Jing,X.Chem.Eng.J.2015,273,307. doi:10.1016/j.cej.2015.01.110 (20)Basu,H.;Singhal,R.K.;Pimple,M.V.Int.J.Environ.Sci. Technol.2015,12,1899.doi:10.1007/s10967-014-3677-x (21)Sun,Y.;Yang,S.;Wang,Q.Radiochim.Acta 2014,102,797. doi:10.1515/ract-2013-2204 (22)Chao,X.;Wang,J.;Yang,T.Carbohyd.Polym.2015,121,79. doi:10.1016/j.carbpol.2014.12.024 (23)Sun,Y.B.;Yang,S.B.;Chen,Y.;Ding,C.C.;Cheng,W.C.; Wang,X.K.Environ.Sci.Technol.2015,49(7),4255. doi:10.1021/es505590j (24)Liu,X.;Li,J.;Wang,X.J.Nucl.Mater.2015,466(45),56. doi:10.1016/j.jnucmat.2015.07.027 (25)Li,L.;Hu,N.;Ding,D.X.;Xin,X.;Wang,Y.D.;Xue,J.H.; Zhang,H.;Tan,Y.RSC Adv.2015,5,65827.doi:10.1039/ C5RA13516H (26)Hosseini,M.S.;Abedi,F.J.Radioanal.Nucl.Chem.2015,303, 2173.doi:10.1007/s10967-014-3366-9 (27)Mirzajani,R.;Pourreza,N.;Najjar,S.S.A.Res.Chem. Intermediat.2014,40(8),2667.doi:10.1007/s11164-013-1120-5 (28)Ogoshi,T.;Harada,A.Sensors 2008,8,4961.doi:10.3390/ s8084961 (29)Wang,Y.L.;Feng,R.S.;Guo,Y.J.Chin.J.Appl.Chem.2011, 28,1269.doi:10.3724/SP.J.1095.2011.00680 (30)Xiao,Y.Q.;Xia,L.S.;Li,R.R.;Li,G.;Huang,X.Atom Energy Science and Technology 2015,49,2130.doi:10.7538/ yzk.2015.49.12.2130 (31)Wang,J.S.;Zou,X.L.;Jia,L.Atom Energy Science and Technology 2015,49,255.doi:10.7538/yzk.2015.49.02.0255 (32)Huang.Y.;Fan,X.D.Journal of Northwest University(Natural Science Edition)2003,33,41.doi:1000-274X(2003)01-0041-04 (33)Ding,H.;Chao,J.;Zhang,G.Spectrochim.Acta A 2003,59, 3421.doi:10.1016/S1386-1425(03)00176-8 (34)Ji,X.Z.;Liu,H.J.;Wang,L.L.J.Radioanal.Nucl.Chem. 2013,295,265.doi:10.1007/s10967-012-1979-4 (35)Chen,S.P.;Hong,J.X.;Yang,H.X.J.Environ.Radioactiv. 2013,126,253.doi:10.1016/j.jenvrad.2013.09.002 (36)Huang,G.L.;Zou,L.X.;Su,Y.;Lv,T.T.;Wang,L.L. J.Radioanal.Nucl.Chem.2016,307(2),1135.doi:10.1007/ s10967-015-4275-2 (37)Hosseini,S.H.;Rahmanisani,A.;Jalalabadi,Y.Int.J.Environ. Anal.Chem.2015,95(4),277.doi:10.1080/ 03067319.2015.1016009 (38)Chen,F.;Tan,N.;Long,W.;Yan,X.M.;Chen,F.Mar.Pollut. Bull.2013,74,213.doi:10.1016/j.marpolbul.2013.06.055 (39)Long,D.J.;Liu,J.H.;Wang,X.M.Nuclear Power Engineering 2012,33,1.doi:10.1128/JVI.06957-11 (40)Tong,K.S.;Kassim,M.J.;Azraa,A.Chem.Eng.J.2011,170, 145.doi:10.1016/j.cej.2011.03.044 (41)Starvin,A.M.;Rao,T.P.Talanta 2004,63(2),225. doi:10.1016/j.talanta.2003.11.001 (42)Li,Z.;Chen,F.;Yuan,L.;Liu,Y.;Zhao,Y.;Chai,Z.;Shi,W. Chem.Eng.J.2012,210,539.doi:10.1016/j.cej.2012.09.030 (43)Zhou,L.M.;Shang,C.;Liu,Z.R.;Huang,G.L.Adesina,A.A. J.Colloid Interface Sci.2012,366(1),165.doi:10.1016/j. jcis.2011.09.069 (44)Mellah,A.;Chegrouche,S.;Barkat,M.J.Colloid Interface Sci. 2006,296(2),434.doi:10.1016/j.jcis.2005.09.045 (45)Oguz,E.J.Colloid Interface Sci.2005,281(1),62. doi:10.1016/j.jcis.2004.08.074 (46)Aksoyoglu,S.J.Radioanal.Nucl.Chem.1989,134(2),393. doi:10.1007/BF02278276 Kinetics and Thermodynamics of Adsorption of Benzil-Bridged β-Cyclodextrin on Uranium(VI) JING Peng-FeiLIU Hui-Jun*ZHANG QinHU Sheng-Yong LEI Lan-LinFENG Zhi-Yuan Sulfated β-cyclodextrin(β-CD)was prepared by the reaction of β-CD with p-toluenesulfonyl chloride at low temperature in aqueous sodium hydroxide.The product was analyzed by Fourier transform infrared spectroscopy(FTIR)and proton nuclear magnetic resonance(1H NMR).The novel benzil-bridged β-CD(BB β-CD)was acquired by the reaction of benzil with sulfated β-CD at a molar ratio of 1:2.UV spectrophotometry was used to study the synthetic mechanism of BB β-CD and benzil and their adsorption onto U(VI).Scanning electron microscopy(SEM)was used to analyze the surface properties of the materials.The adsorption of BB β-CD onto U(VI)was investigated as a function of pH,contact time, temperature,and interfering ions using the batch adsorption technique.It was found that the adsorption equilibrium of BB β-CD was reached faster than that of benzil.The optimum experimental conditions were pH=4.5 and shaking for 60 min,achieving the maximum adsorption capacity of 12.16 mg·g-1and a U(VI)removal ratio of 91.2%.Kinetic studies revealed that the adsorption reached equilibrium within 60 min for U(VI)and followed a pseudo-second-order rate equation.The isothermal data correlated with the Langmuir model better than with the Freundlich model.The thermodynamic data indicated the spontaneous and endothermic nature of the process. BB β-CD;Uranium(VI)adsorption;Kinetics;Equilibrium;Thermodynamics January 4,2016;Revised:April 20,2016;Published on Web:April 21,2016. O642;O643 10.3866/PKU.WHXB201604212 *Corresponding author.Email:liuhuijun@usc.edu.cn;Tel:+86-13607341186. The project was supported by the National Natural Science Foundation of China(11375084)and Hunan Provincial Innovation Foundation for Postgraduate,China(CX2015B399). 国家自然科学基金(11375084)和湖南省研究生科研创新项目(CX2015B399)资助 ©Editorial office ofActa Physico-Chimica Sinica [Article]


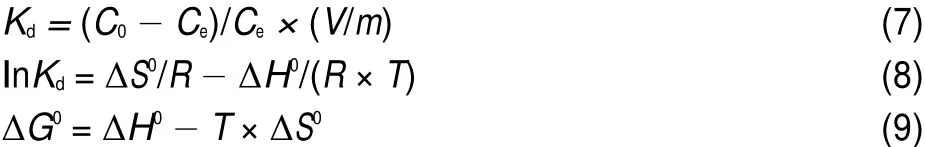


4 Conclusions
(College of Chemistry and Chemical Engineering,University of South China,Hengyang 421001,Hunan Province,P.R.China)

