敌百虫与敌敌畏的吸收光谱和发射光谱特征*
李丽清,程学礼,赵燕云
(泰山学院化学化工学院, 山东 泰安 271000)
敌百虫与敌敌畏的吸收光谱和发射光谱特征*
李丽清,程学礼,赵燕云
(泰山学院化学化工学院, 山东泰安271000)
用G09程序包研究了敌百虫和敌敌畏的吸收光谱及荧光/磷光光谱,并从分子轨道角度解释了吸收光谱特征。含时密度泛函结果揭示了:① 水能够明显地增强敌百虫和敌敌畏的吸收和发射光谱;②由于缺乏nπ*或ππ*跃迁,无论在水中还是在气相中敌百虫的吸收和发射光谱都很弱,因此需要引入印迹分子以改变其光谱特征;③ 敌敌畏的S0→S3跃迁产生了在184.38 nm处的最强吸收峰,被指认为从HOMO到LUMO的ππ*跃迁。
敌百虫;敌敌畏;荧光;发射光谱
Dipterex and dichlorvos are the most commonly used organophosphorous pesticides. It is also a broad-spectrum insecticide with various action modes, short half lives, low dosage but high toxicity[1-10]. Although the agricultural use of most organochlorine pesticides has been diminishing gradually, significant levels of dipterex and dichlorvos are still in the environment due to their persistence[11]. However, the most serious issue is the abuse of dipterex and dichlorvos. Nowadays, dipterex and dichlorvos are still used widely in most rural and Western areas of China. As a result, pesticide residues attracted more and more attention[12-14], especially after the export of Chinese agricultural products has been influenced significantly since the Japanese Positive list system for Agricultural Chemical Residues in Foods was put in practice in 2006[15]. Therefore it is crucial to improve the ultrasensitive techniques to detect the residue levels of dipterex in agricultural products[16-18].
The determination of ultra trace organophosphorus pesticides in farm produce is a great challenge. At present, multifarious spectroscopic techniques are still the most effective detecting methods, and are the staple for analyzing pesticide residues[19-21]. Due to its simplicity, low cost, high sensitivity and selectivity, chemiluminescence-based detection has become a quite useful detecting tool in flow injection, column liquid chromatographic and in capillary electrophoresis systems[22-23]. In the detection of trace amounts of dipterex and dichlorvos in agricultural products, it is essential to explore the absorption and fluorescence emission spectroscopic characters of dipterex and dichlorvos. We have reported a density functional theory (DFT) investigation on the spectroscopic characteristics and absorption spectra of dipterex and dichlorvos without considering any solvents[24]. In nature, dipterex and dichlorvos diffuse into surface water and groundwater at first before contaminating our environment, and most of the on-site detective operations about dipterex and dichlorvos will be implemented under aqueous solution, so the effect of water must be considered. At the present work, we will carry out further theoretical explorations into the absorption and fluorescence emission spectroscopic characters of dipterex and dichlorvos, which are very helpful to understand and interpret the available experimental data, and will provide new insights and theoretical bases on the detection of ultra trace dipterex and dichlorvos.
1 Computational methods
All calculations were performed with G09 program package at 6-31G(d,p) basis set level. First of all, we oriented the methyl, trichloromethyl and hydroxyl groups in dipterex and dichlorvos to optimize various geometries in gas phase at M062X/6-31G(d,p) level to search the most stable ground-state configurations. The reason we selected the M06-2X[25]hybrid exchange-correlation meta-GGA functionals is that this functional suite has good performance in main-group thermochemistry[26-27].Then the most stable ground states of dipterex and dichlorvos after re-optimization with the conductor-like screening model (CPCM)[28-29]were selected in this study to investigate the absorption and fluorescence emission spectroscopic characters. In other words, in combination with the CPCM solvation model to consider the effect of bulk water in aqueous solutions, the M06-2X functionals were employed to re-optimize the ground states, and to compute the absorption and emission spectra, while the configuration interaction single-excitation (CIS)[30-31]was utilized to locate the structural geometries of singlet and triplet excited states of dipterex and dichlorvos. We employed time-dependent M06-2X functionals to calculate the absorption and emission spectra because time-dependent density functional theory (TD-DFT) methods have been proved to be reliable[32]. In this study, the input parameters and settings were the default if not specified. The computational results will be compared with our previous work[24]without considering water as solvent.
2 Results and discussion
2.1Ground state structures

Fig.1 Structural parameters and frontier orbitals (represented as 0.02e isosurfaces) of the most stable trichlorphon and dichlorvos obtained by full optimization with CPCM solvation model at M062X/6-31G (d,p) level(Bond lengths are in 10-1 nm and bond angles in degrees)
The structural parameters as well as the highest occupied molecular orbitals (HOMO) and lowest unoccupied molecular orbitals (LUMO) of ground-state dipterex and dichlorvos are depicted in Fig.1. In comparison with our previous results[24], solvent water has minor influence on the optimized geometric configurations and frontier orbitals. Fig.1 shows that the formation of a hydrogen bonding between P=O and O-H stabilizes trichlorphon, and in the most stable structure the O-C-C-Cl dihedral angle is nearly 180 degrees. Compared with dipterex, dichlorvos has an apparent delocalized bond, and its LUMO and HOMO on the C=C double bond are of π*anti-bonding and π bonding characters, respectively.
2.2Absorption spectra
The predicted UV-Vis spectra of dipterex and dichlorvos in aqueous solution are illustrated in Fig.2 and Fig.3, wherein the spectra of natural dipterex and dichlorvos in gas phase are also listed. Their molar absorbance coefficients (ε) and oscillator strengths (f) in water are much higher than those in natural states. However, our simulations also reveal that water as solvent can not alter the shapes of frontier orbitals, but can strengthen the molar absorbance and make the absorption red-shifted. In this work, we will use the results and molecular orbitals obtained in water to discuss the absorption and emission mechanisms.
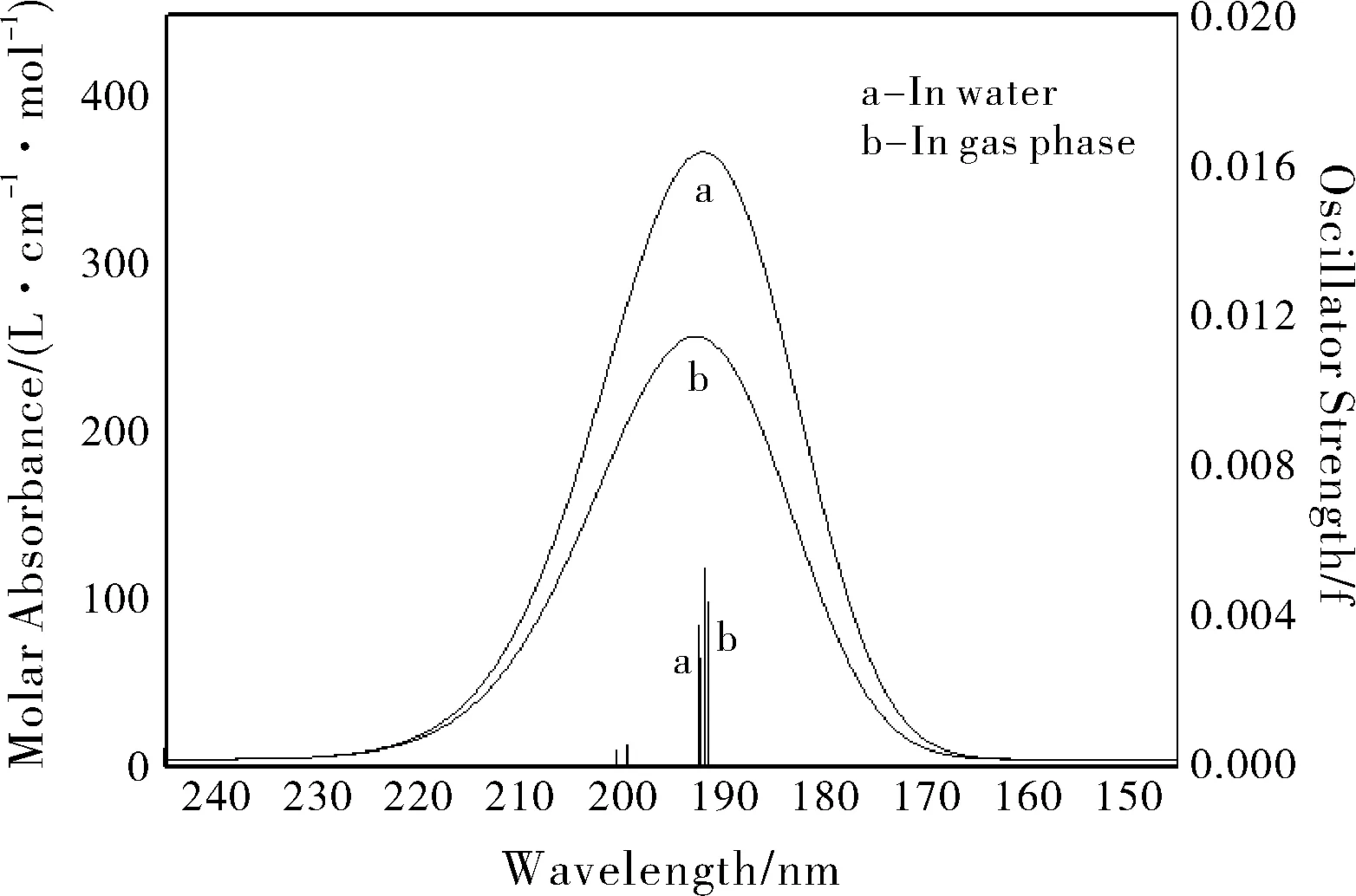
Fig.2 Absorption spectra of trichlorphon in water and in gas phase in ultraviolet region 150-240 nm determined at TD-M062X/6-31G(d,p) level (The half-bandwidths were set as 3000 cm-1)

Fig.3 Absorption spectra of dichlorvos in water and in gas phase in ultraviolet region 150-230 nm determined at TD-M062X/6-31G (d,p) level(The half-bandwidths were set as 3000 cm-1)
The absorption spectra of dipterex in water and gas phase are very weak. Our calculations predicted that the S1absorption at 199.50 nm is very weak (f=0.0006), and the excitation from ground state (S0) to S2at 191.86 nm (f=0.0053) is vey close to the S0→S3excitation at 191.48 nm (f=0.0044), compared with those excitations in gas phase at 200.58 (f=0.0005), 192.42 (f=0.0038) and 192.32 nm (f=0.0029), respectively, as shown in Fig.2. The S0→S1excitation is assigned to the nσ*(C-Cl bonds) transition, but S0→S2and S0→S3excitations are designated as nσ*and σσ*transitions. The weak absorption spectra of dipterex may be attributed to the fact that there are no nπ*or ππ*transitions.
Molecular orbitals involved in the lowest transitions of dichlorvos absorption spectra are illustrated in Fig.4, and their vertical transition energies (E), absorption wavelengths (λ), oscillator strengths (f) and transition state assignments are listed in Table 1. From Fig.4 and Table 1 it can be seen that, the first two singlet transitions are of πσ*characters, so they both have very low oscillator strengths. The strongest absorption peak at 184.38 nm is assigned to the S3state involving the ππ*transition from HOMO to LUMO. Compared with the results in gas phase[24], the wavelength of this absorption peak is only slightly blue-shifted, but the oscillator strength is increased significantly from 0.4827 to 0.6125.

Fig.4 Excitation states and molecular orbitals depicted as 0.02e isosurface involved in the lowest transitions of dichlorvos absorption spectra at TD-M062X/6-31G(d,p) level

TransitionstatesE/eVλ/nmfStateassignmentS1(HOMO→LUMO+1)6.0439205.140.0024πσ*(95.6%)S2(HOMO→LUMO+2)6.3029196.710.0007πσ*(85.9%)S3(HOMO→LUMO)6.7244184.380.6125ππ*(98.6%)
2.3Emission spectra
The CIS-optimized singlet and triplet excited states of dipterex and dichlorvos combined with CPCM solvation model are shown in Fig.5 and Fig.6. The theoretical calculation will predict the fluorescence and phosphorescence spectra in water and in gas phase. In theses excited states, a C-Cl bond is extended significantly and highly activated. In fact, dipterex and dichlorvos are almost destroyed. Due to the fact that the strongest absorption of dipterex is assigned to the S0→S3excitation, we optimized the singlet S1-S3and T1, T2triplet excited states to ascertain its emission spectra. We also located the T3excited state of dipterex, but from the molecular orbitals of emission spectra it can be seen that this state is a resonance structure of T2.

Fig.5 Structural geometries of the excited states of dipterex optimized at CIS/6-31G(d,p) level in aqueous solutions

Fig.6 Structural parameters of S1 and T1 states of dichlorvos optimized at CIS/6-31G(d,p) level in aqueous solutions
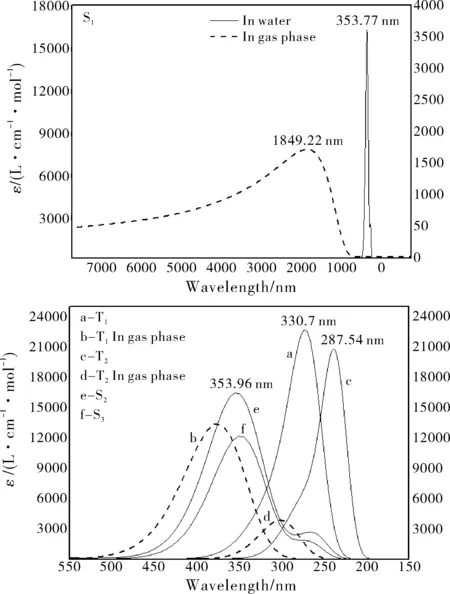
Fig.7 Emission spectra of dipterex in gas phase (dashed lines) and in water (solid lines). The half-bandwidths were set as 3000 cm-1
Based on the singlet and triplet geometries, the fluorescence and phosphorescence characters of dipterex and dichlorvos in gas phase and in water were calculated and compared, as illustrated in Fig.7 and Fig.8. The S1fluorescence of dipterex in water differs markedly with that in gas phase: the emission spectrum in gas phase is a broad band in the infrared region, while in water it is a sharp peak. Compared with the T1and T2emission spectra of dipterex in water, the peaks in gas phase is red-shifted distinctly, and the emission intensities are much lower.

Fig.8 S1 and T1 emission spectra of dichlorvos in gas phase (dashed lines) and in water (solid lines)(The half-bandwidths were set as 3000 cm-1)
The lowest-lying fluorescence and phosphorescence intensities of dichlorvos in water and in gas phase are much lower than the corresponding ones of dipterex. Fig.8 shows that the fluorescence spectrum of dichlorvos in gas phase is very weak, and differs distinctly from the spectrum in water.
3 Conclusion
The absorption and emission spectra of dipterex and dichlorvos in water were investigated systematically with TD-DFT method, and compared with those in gas phase. Water as solvent significantly enhances the absorption and fluorescence/phosphorescence intensities, and the peaks are red-shifted in comparison with those in gas phase. Moreover, the S1fluorescence spectra of dipterex and dichlorvos in water are different notably from those in gas phase. Our calculations predicted that the absorption of dipterex is relatively weak due to lack of nπ*or ππ*transitions, but its fluorescence and phosphorescence spectra are very strong. However, in contrast to dipterex, dichlorvos has strong absorption spectra but low emission spectra. The strongest absorption peak of dichlorvos at 184.38 nm is the S3excitation involving the ππ*transition from HOMO to LUMO.
References
[1]Mattson A M, Spillane J T, Pearce G W. Organophosphorous insecticides, dimethyl 2,2-dichlorovinyl phosphate (DDVP), organic phosphorus compound highly toxic to insects[J]. J. Agr. Food Chem, 1955, 3: 319-321.
[2]Westlake W E. Pesticides. Anal. Chem, 1957, 29: 679-683.
[3]Ishmael J, MacGregor J A, Manley A. Dichlorvos-a comprehensive review of 11 rodent carcinogenicity studies[J]. Regul. Toxicol. Pharm., 2006, 44: 238-248.
[4]Guo J-X, Wu J J-Q, Wright J B, et al. Mechanistic insight into acetylcholinesterase inhibition and acute toxicity of organophosphorus compounds: a molecular modeling study[J]. Chem. Res. Toxicol., 2006, 19: 209-216.
[5]Kim N, Park I-S, Kim D-K. High-sensitivity detection for model organophosphorus and carbamate pesticide with quartz crystal microbalance-precipitation sensor[J]. Biosens. Bioelectron., 2007, 22: 1593-1599.
[6]Zhou S, Wang L, Li L, et al. Stereoisomeric separation and bioassay of a new organophosphorus compound, O,S-Dimethyl-N-(2,2,2-trichloro-1-methoxyethyl)phosphoramidothioate: some implications for chiral switch[J]. J. Agr. Food Chem., 2009, 57: 6920-6926.
[7]Xu Z, Fang G, Wang S. Molecularly imprinted solid phase extraction coupled to high-performance liquid chromatography for determination of trace dichlorvos residues in vegetables[J]. Food Chem., 2010, 119: 845-850.
[8]Meng L, Qiao X, Song J, et al. Study of an online molecularly imprinted solid phase extraction coupled to chemiluminescence sensor for the determination of trichlorfon in vegetables[J]. J. Agr. Food Chem., 2011, 59: 12745-12751.
[9]Schäfer R B, von der Ohe P C, Kühne R, Schüürmann G, Liess M. Occurrence and toxicity of 331 organic pollutants in large rivers of North Germany over a decade (1994 to 2004)[J]. Environ. Sci. Technol., 2011, 45: 6167-6174.
[10]Shamsipur M, Sarkouhi M, Hassan J. Selective monitoring of organophosphorus pesticides by31P-NMR spectroscopy: application to purity assay of technical products and concentration determination of formulated samples[J]. Appl. Magn. Reson., 2012, 42: 227-237.
[11]Sherma J, Zweig G. Pesticides. Anal. Chem., 1983, 55: 57R-70R.
[12]Schöning M J, Krause R, Block K, et al. A dual amperometric/potentiometric FIA-based biosensor for the distinctive detection of organophosphorus pesticides[J]. Sensor. Actuat. B: Chem., 2003, 95: 291-296.
[13]Cheng X, Zhang Z, Tian S. A novel long path length absorbance spectroscopy for the determination of ultra trace organophosphorus pesticides in vegetables and fruits[J]. Spectrochim. Acta A, 2007, 67: 1270-1275.
[14]Zhang H-X, Wei R-B, Chen C-Z, et al. A novel fluorescent epoxy resin for organophosphate pesticide detection[J]. Chin. Chem. Lett., 2015, 26: 39-42.
[15]Wang S, Wang Z, Zhang Y, et al. Pesticide residues in market foods in Shaanxi Province of China in 2010[J]. Food Chem., 2013, 138: 2016-2025.
[16]Hall G L, Mourer C R, Shibamoto T, Fitzell D. Development and validation of an analytical method for naled and dichlorvos in air[J]. J. Agr. Food Chem., 1997, 45: 145-148.
[17]Guan H, Zhang F, Yu J, et al. The novel acetylcholinesterase biosensors based on liposome bioreactors-chitosan nanocomposite film for detection of organophosphates pesticides[J]. Food Res. Int., 2012, 49: 15-21.
[18]Yang L, Li H, Zeng F, et al. Determination of 49 organophosphorus pesticide residues and their metabolites in fish, egg, and milk by dual gas chromatography-dual pulse flame photometric detection with gel permeation chromatography cleanup[J]. J. Agr. Food Chem., 2012, 60: 1906-1913.
[19]Feigenbrugel V, Loew C, Le Calvé S, et al. Near-UV molar absorptivities of acetone, alachlor, metolachlor, diazinon and dichlorvos in aqueous solution[J]. J. Photochem. Photobio. A, 2005,174: 76-81.
[20]Xue L, Cai J, Li J, et al. Application of particle swarm optimization (PSO) algorithm to determine dichlorvos residue on the surface of navel orange with Vis-NIR spectroscopy[J]. Procedia Eng., 2012, 29: 4124-4128.
[21]Guo X, Zhang X, Cai Q, et al. Developing a novel sensitive visual screening card for rapid detection of pesticide residues in food[J]. Food Control, 2013, 30: 15-23.
[22]Noda I. Recent developments in two-dimensional (2D) correlation spectroscopy[J]. Chin. Chem. Lett., 2015, 26: 167-172.
[23]Fang M, Lei F, Zhou J, et al. Rapid, simple and selective determination of 2,4-dinitrophenol by molecularly imprinted spin column extraction coupled with fluorescence detection[J]. Chin. Chem. Lett., 2014, 25: 1492-1494.
[24]Li L-Q, Cheng X-L, Zhao Y-Y, et al. Density functional theory investigations on the spectroscopic characteristics and luminescent mechanisms of dipterex and dichlorvos[J]. Spectrosc. Spect. Anal., 2014, 34: 122-127.
[25]Zhao Y, Truhlar D G. Comparative DFT study of van der Waals complexes: rare-gas dimers, alkaline-earth dimers, zinc dimer, and zinc-rare-gas dimers[J]. J. Phys. Chem. A, 2006, 110: 5121-5129.
[26]Zhao Y, Truhlar D. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals[J]. Theor. Chem. Acc., 2008, 120: 215-241.
[27]Hohenstein E G, Chill S T, Sherrill C D. Assessment of the performance of the M05-2X and M06-2X exchange-correlation functionals for noncovalent interactions in biomolecules[J]. J. Chem. Theory Comput., 2008, 4: 1996-2000.
[28]Cossi M, Scalmani G, Rega N, et al. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model[J]. J. Comp. Chem., 2003 24: 669-681.
[29]Ho J, Coote M L. A universal approach for continuum solvent pKa calculations: are we there yet?[J]. Theor. Chem. Acc., 2010, 125: 3-21.
[30]Foresman J B, Head-Gordon M, Pople J A et al. Toward a systematic molecular orbital theory for excited states[J]. J. Phys. Chem., 1992, 96: 135-149.
[31]Zhang L, Li H, Chen X, et al. Absorption and fluorescence emission spectroscopic characters of size-expanded yDNA bases and effect of deoxyribose and base pairing[J]. J. Phys. Chem. B, 2009, 113: 1173-1181.
[32]Liu H, Li G, Zhang L, et al. Electronic promotion effect of double proton transfer on conduction of DNA through improvement of transverse electronic communication of base pairs[J]. J. Chem. Phys., 2011, 135: 134315-134325.
Absorption and Emission Spectroscopic Characters of Dipterex and Dichlorvos*
LILi-qing,CHENGXue-li,ZHAOYan-yun
(School of Chemistry and Chemical Engineering, Taishan University, Shandong Tai’an 271000, China)
The absorption and fluorescence/phosphorescence spectra of dipterex and dichlorvos were explored with G09 program package, and the absorption characters were interpreted by molecular orbitals. The TD-DFT results revealed that water can observably strengthen the absorption and emission spectra of dipterex and dichlorvos. The absorption and emission spectra of dipterex in water and in gas phase were both very weak because there are no nπ*or ππ*transitions, so imprinted molecules should be introduced to alter its spectroscopic characters. The S0→S3excitation caused the strongest absorption of dichlorvos at 184.38 nm, which was assigned to the ππ*transition from HOMO to LUMO.
dipterex; dichlorvos; fluorescence; emission spectra
国家自然科学基金资助项目(11174215和21203084);山东省自然科学基金联合专项(ZR2012BL03和ZR2012BL10);山东省高校科研发展计划项目(J13LD05)。
李丽清(1963-), 女,博士,教授。
程学礼(1975-),男,博士,副教授。
O611.3; TP183
A
1001-9677(2016)010-0052-05

