浸渍法制备高选择性1-丁烯齐聚催化剂
肖林久, 陈家欢, 盛永刚, 李文泽, 王金豹
(沈阳化工大学 应用化学学院 辽宁省稀土化学及重点应用实验室, 辽宁 沈阳 110142)
肖林久, 陈家欢, 盛永刚, 李文泽, 王金豹
(沈阳化工大学 应用化学学院 辽宁省稀土化学及重点应用实验室, 辽宁 沈阳 110142)
摘要:通过分步浸渍法制备/γ-Al2O3催化剂,并用于催化1-丁烯齐聚反应,考察了催化剂制备条件和反应条件对催化性能的影响,并与Fe2(SO4)3/γ-Al2O3催化剂进行性能比较。结果表明,在0.96 MPa、60℃、液时空速为5 h-1的反应条件下,采用Fe负载量为负载量为1.2 mmol/g(γ-Al2O3)的/γ-Al2O3 催化剂,1-丁烯齐聚反应的转化率达到68.0%,二聚物选择性为95.0%。α-Fe2O3是催化1-丁烯齐聚反应的活性组分,并能提高二聚体选择性;此外,α-Fe2O3晶相与的相互作用对其催化1-丁烯齐聚反应的活性有重要影响,γ-Al2O3与的相互作用也对催化活性有一定的影响。Fe负载量为6%的/γ-Al2O3催化活性最高,此时NH3-TPD结果显示,催化剂只存在较弱的酸中心和适中的酸量。
关键词:1-丁烯齐聚; 催化剂;; 硫酸铁

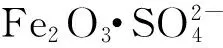
1 实验部分
1.1原料和试剂
γ-Al2O3(Al2O3质量分数98%,比表面积227.41 m2/g,平均孔径5.08 nm),济南鲁淮商贸有限公司产品;Fe(NO3)3·9H2O、(NH4)2SO4,分析纯,天津市大茂化工试剂厂产品;Fe2(SO4)3,分析纯,天津市耀华化工厂产品。
1.2催化剂的制备

将载体γ-Al2O3研磨、筛分,选用粒径20~40目部分。将称量的Fe(NO3)3溶于一定量的去离子水中,待溶解后快速加入定量的γ-Al2O3,使其浸渍均匀,超声波下充分震荡1 h,放置120℃下烘干,然后移至马福炉中500℃焙烧1 h,取出冷却至室温,制得Fe负载量(质量分数,以下同)分别为2%、4%、6%、8%、10%的Fe2O3/γ-Al2O3催化剂。
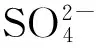
1.2.2Fe2(SO4)3/γ-Al2O3的制备
将载体γ-Al2O3研磨、筛分,选用粒径20~40目部分。将称量的Fe2(SO4)3溶于一定量的去离子水中,待溶解后快速加入定量的γ-Al2O3,使其浸渍均匀,超声波下充分震荡1 h,放置120℃下烘干,然后移至马福炉中500℃焙烧3 h,取出冷却至室温,制得Fe担载量为6%的Fe2(SO4)3/γ-Al2O3催化剂。
1.3反应装置和流程
采用高压微型固定床连续流动的不锈钢反应器(内径8 mm,长70 mm)进行1-丁烯齐聚反应。采用北京东方仪器厂产SB-2型双柱塞微量泵将反应原料从反应器底部送入反应床层。反应温度控制在40~200℃范围,由背压调压器控制反应压力在0.8~1.8 MPa范围。反应产物从背压阀流出后,经六通阀进入气相色谱完成在线分析,随时监测反应状况。
1.4聚合产物分析
采用北京北分瑞利分析仪器公司3420A型气相色谱仪和面积归一化法分析液体产物和尾气组成。色谱仪配有30 m HJ.624毛细管柱和FID检测器;气化温度240℃,柱温220℃,检测器温度250℃;由色谱分析工作站处理数据。根据分析结果计算1-丁烯转化率和液体产物各组分的选择性。
1.5负载量计算方法
(1)
(2)

2 结果与讨论

2.1.1表面分散状态
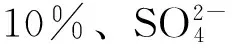

图1 不同焙烧温度下负载Fe2O3得到的的XRD谱Fig.1 XRD patterns of Fe2O3·different calcination temperatures ●Fe2(SO4)3;■α-Fe2O3;▲γ-Al2O3;★Fe2O3
2.1.2催化剂的酸性
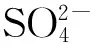
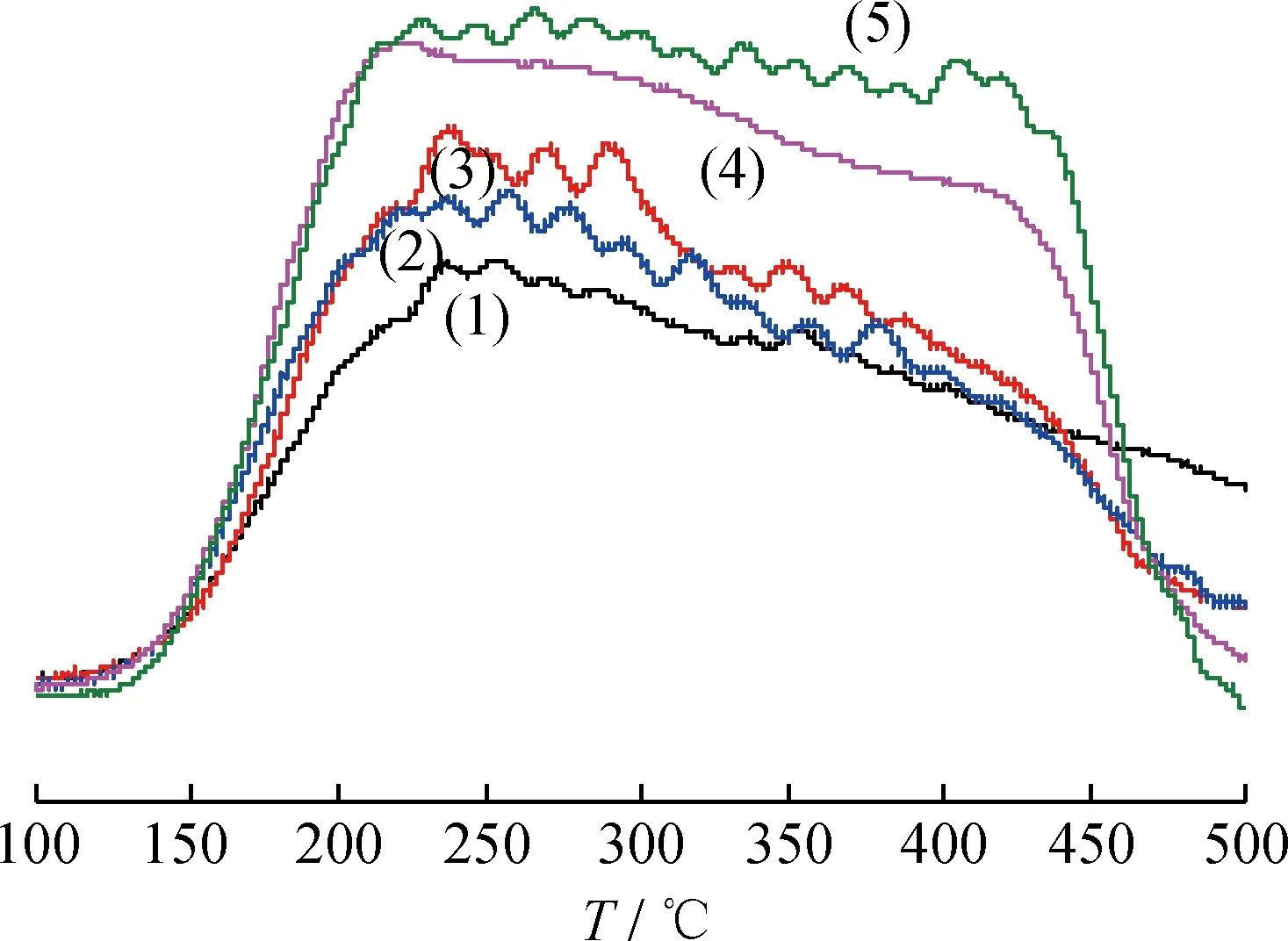
图2 不同Fe负载量的的 NH3-TPD曲线Fig.with different Fe loadings
w(Fe)/%: (1) 2; (2) 4; (3) 6; (4) 10; (4) 12
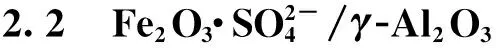
2.2.1负载Fe2O3焙烧温度的影响

图3 负载Fe2O3的焙烧温度对所得催化剂催化1-丁烯齐聚反应转化率的影响Fig.3 Effect of calcination temperature for loading Fe2O3 of/γ-Al2O3 catalyst on oligomerization conversion of 1-butene over the catalyst
2.2.2Fe负载量的影响
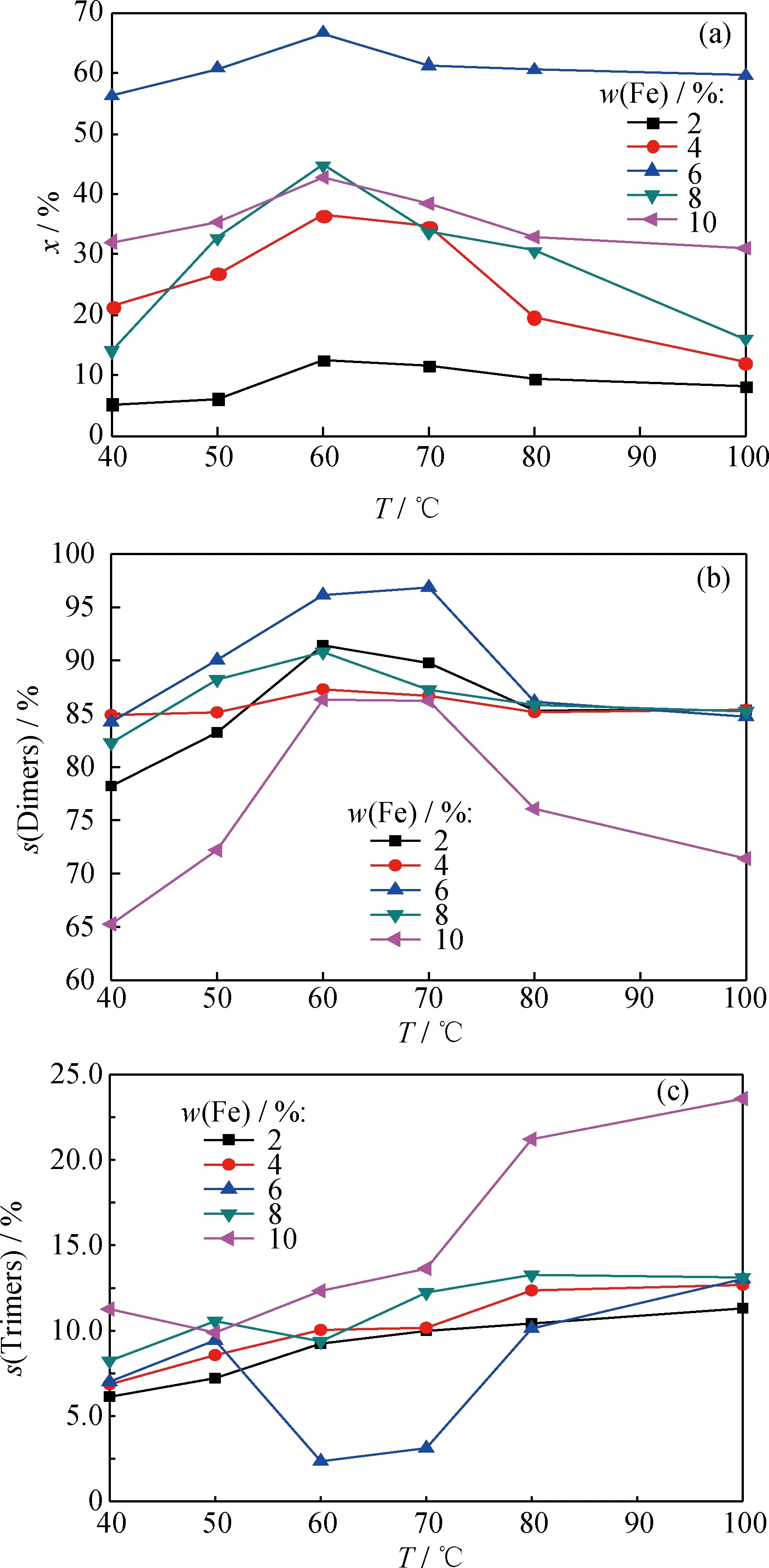
图4 Fe负载量对催化剂催化 1-丁烯齐聚反应的影响Fig.catalyst on oligomerization of 1-butene at different temperatures
(a)x; (b)s(Dimers); (c)s(Trimers)
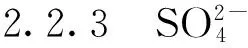
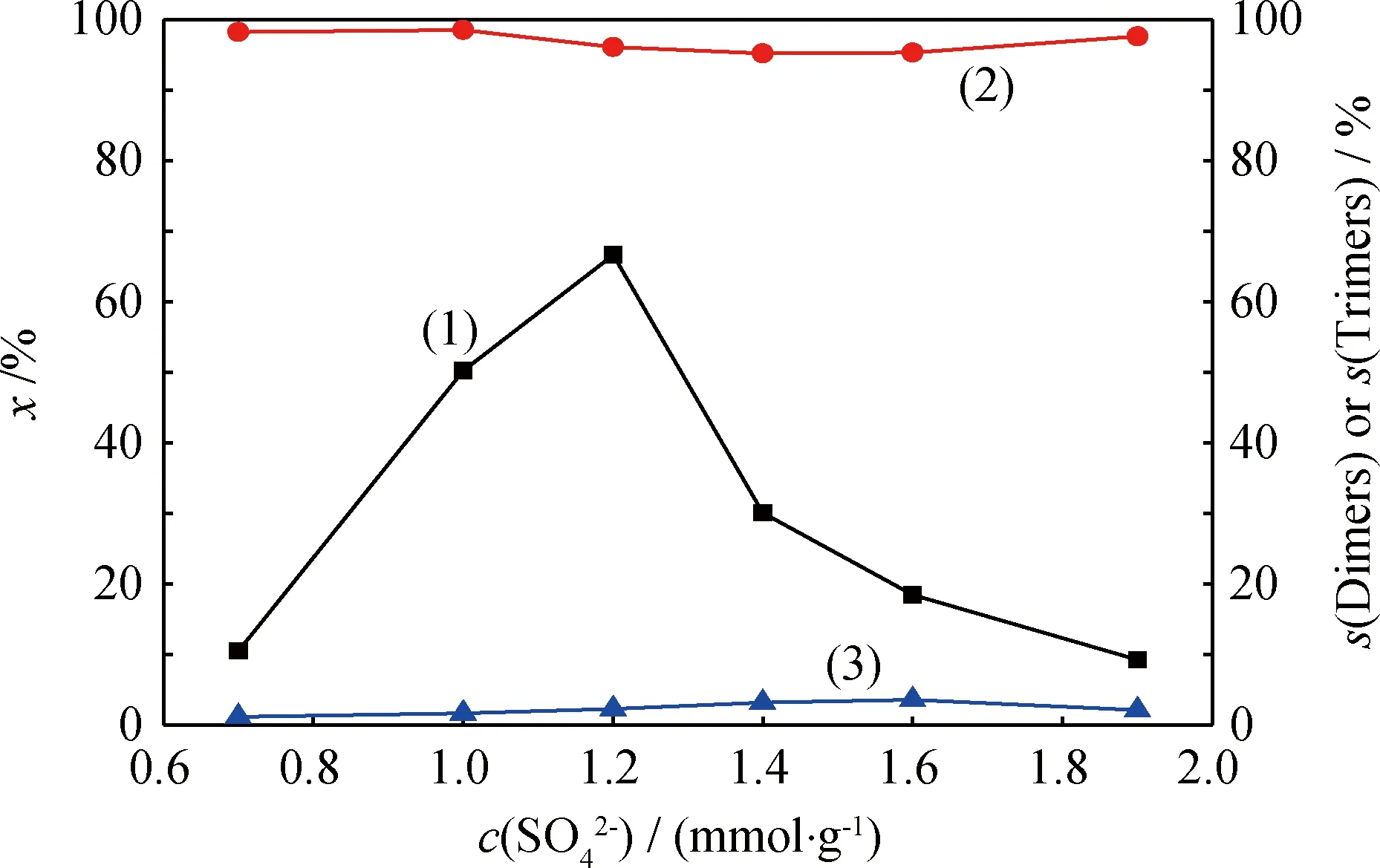
图负载量对催化剂催化1-丁烯齐聚反应的影响Fig.catalyst on oligomerization of 1-buteneT=60℃; p=0.96 MPa; LHSV=5 h-1; w(Fe)=6% (1) x; (2) s(Dimers); (3) s(Trimers)

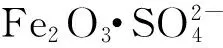
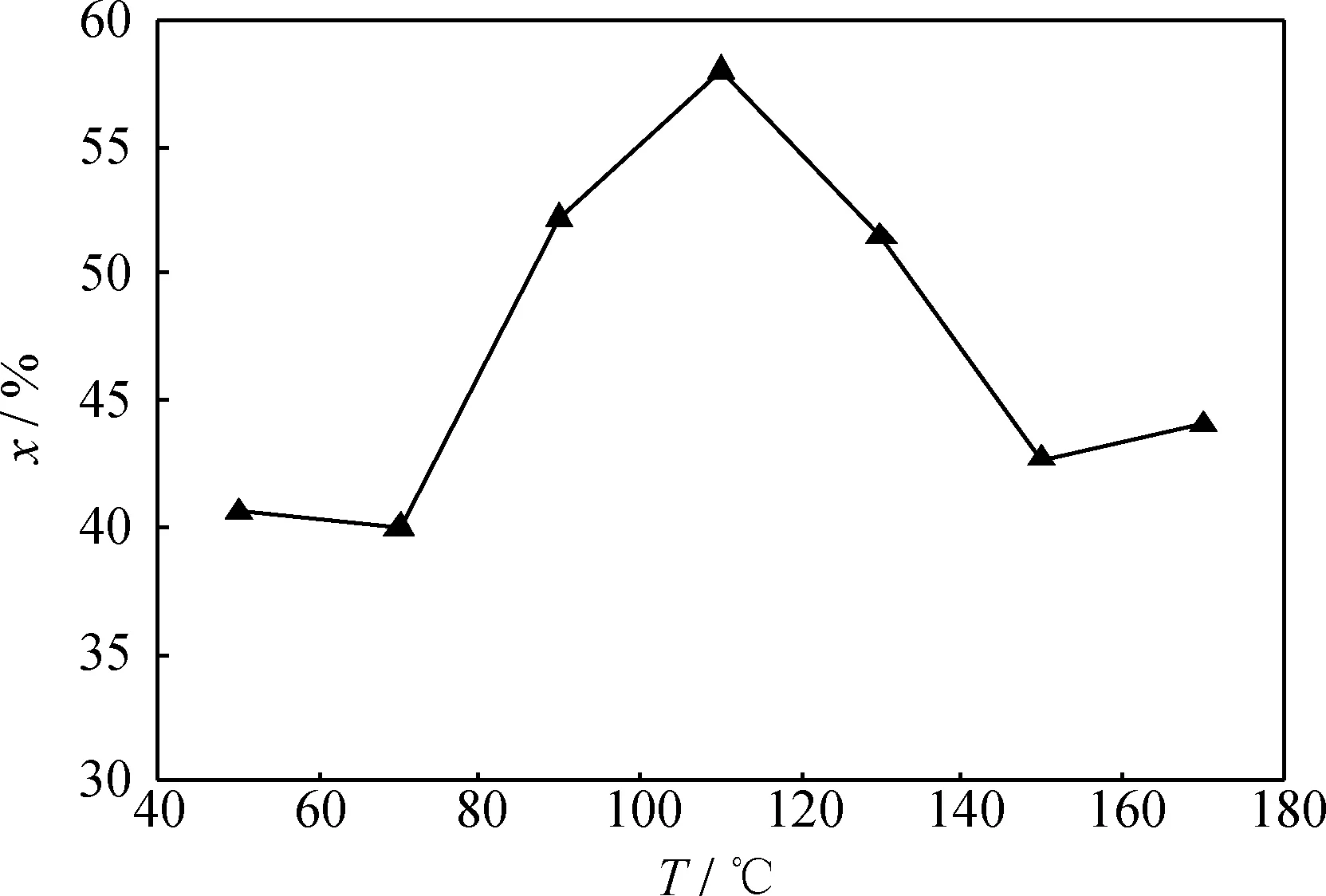
图6 不同反应温度下Fe负载量为6%的Fe2(SO4)3/γ-Al2O3催化剂催化1-丁烯齐聚反应的转化率Fig.6 Conversion of 1-butene oligomerization vs reaction temperatures over Fe2(SO4)3/γ-Al2O3catalyst with 6% Fe loadingp=1.2 MPa; LHSV=1 h-1
2.4.1反应温度的影响
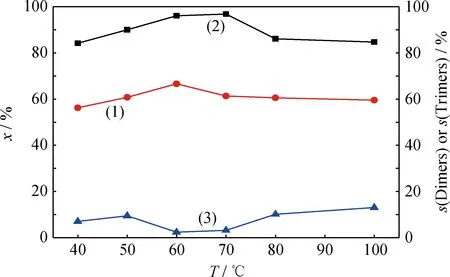
图7 反应温度对催化剂催化 1-丁烯齐聚反应的影响Fig.7 Effects of reaction temperature on oligomerization of
(1)x; (2)s(Dimers); (3)s(Trimers)
2.4.2反应压力的影响
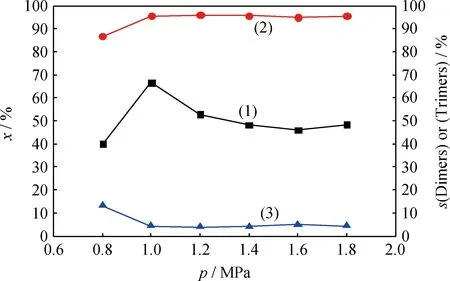
图8 反应压力对催化剂催化 1-丁烯齐聚反应的影响Fig.8 Effects of reaction pressure on oligomerization of
(1)x; (2)s(Dimers); (3)s(Trimers)
2.4.3液时空速的影响
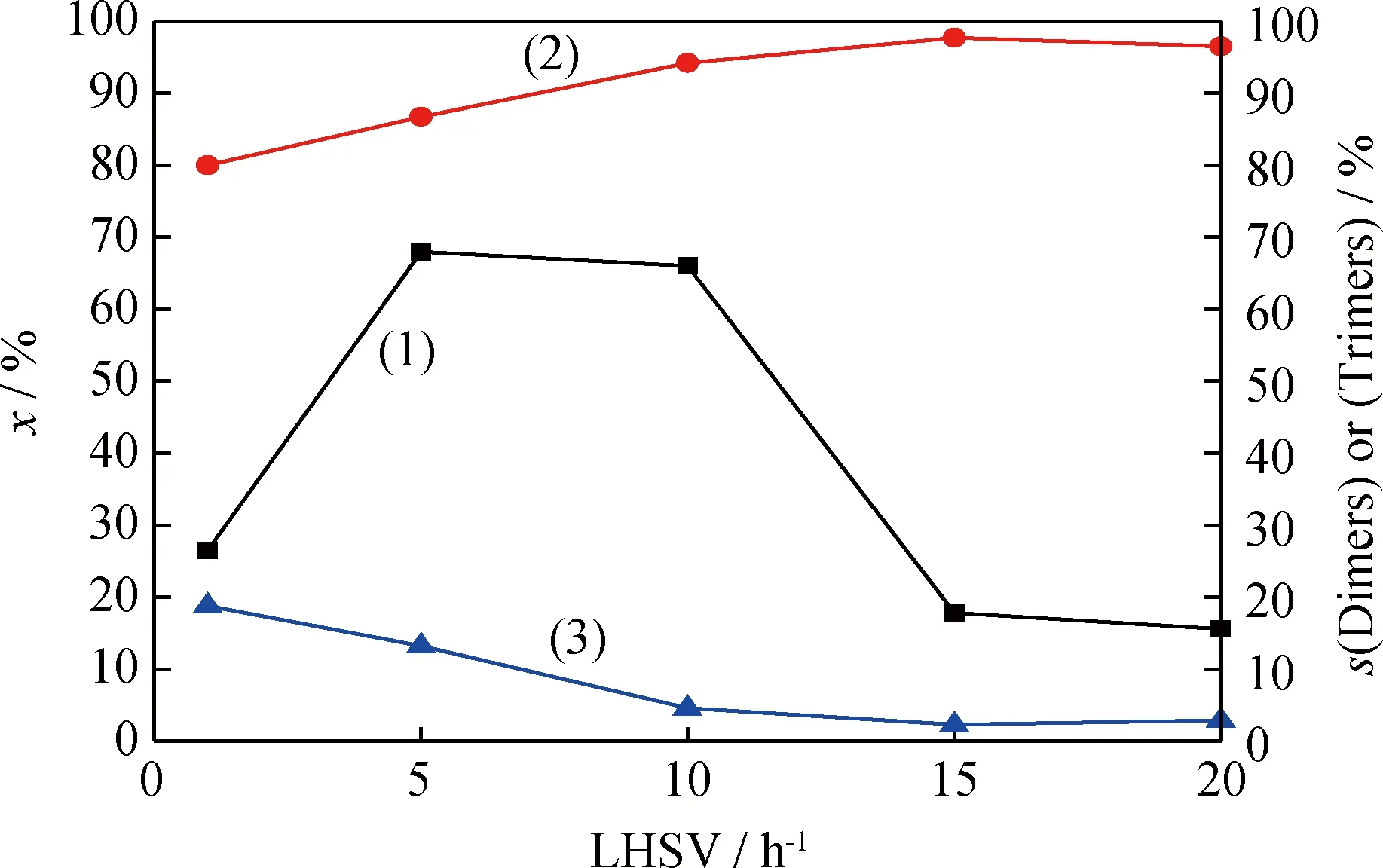
图9 反应液时空速对催化剂催化 1-丁烯齐聚反应的影响Fig.9 Effects of liquid hourly space velocity on oligomerization of 1-butene over
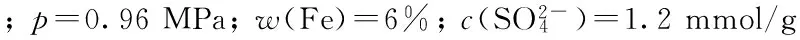
(1)x; (2)s(Dimers); (3)s(Trimers)
3 结 论
参考文献
[1] 陈启智,黄杰.国内乙烯工业中副产品的利用途径[J].乙烯工业,2011, 23(1): 20-25.(CHEN Qizhi, HUANG Jie. Utilization of domestic ethylene industry byproducts[J].Ethylene Industry, 2011, 23(1): 20-25.)
[2] 付玉川,陈翠翠,杨俊伟.1-丁烯生产工艺及其应用概述[J].山西化工,2013, 33(6): 21-24.(FU Yuchuan, CHEN Cuicui, YANG Junwei. Overview of the 1-butene production process and application[J].The Shanxi Chemical Industry, 2013, 33(6): 21-24.)
[3] 罗文国,温朗友,俞芳,等.Ziegler-Natta型催化剂上异辛烯加氢制异辛烷的研究[J].石油学报(石油加工),2007, 23(6): 86-90.(LUO Wenguo, WEN Langyou, YU Fang, et al. Study on the hydrogenation of isooctene to isooctane over Ziegler-Natta catalyst[J].Acta Petrolei Sinica(Petroleum Processing Section), 2007, 23(6): 86-90.)
[4] MCGUINNESS D S. Olefin oligomerization via metallacycles: Dimerization, trimerization, tetramerization, and beyond[J].Chemical Reviews, 2011, 111(3): 2321-2341.
[5] TZOMPANTZI F J, MANRíQUEZ M E, PADILLA J M, et al. One pot preparation of NiO/ZrO2sulfated catalysts and its evaluation for the isobutene oligomerization[J].Catalysis Today, 2008, 133-135: 154-159.
[6] NAWORSKI J S. PETER H. Oligomerization of 1-butene in sulfuric acid[J].Mechanisms and Rates, 1969, 8(3): 397-401.
[7] VILLEGAS J I, KUMAR N, HEIKKILT, et al. A study on the dimerization of 1-butene over Beta zeolite[J].Topics in Catalysis, 2007, 45(1): 187-190.
[8] LAVRNOV A V, DUPLYAKIN V K. Butene oligomerization over phosphoric acid: Structural characterization of products[J].Kinetics and Catalysis, 2009, 50(2): 235-240.
[9] 蔡天锡,曹殿学,齐爱华,等.NiSO4/γ-Al2O3对低级烯烃齐聚反应的催化作用[J].催化学报,1994, 15(1): 23-27.(CAI Tianxi, CAO Dianxue, QI Aihua, et al. Catalytic behavior of NiSO4/γ-Al2O3in alkene oligometization[J].Chinese Journal of Catalysis, 1994, 15(1): 23-27.)
[10] 严志宇,唐晓东,曹殿学,等.负载型硫酸铁对烯烃齐聚催化作用的研究ⅡFe2(SO4)2/γ-Al2O3对1-丁烯齐聚反应[J].石油学报(石油加工),1997, 13(1): 13-17.(YAN Zhiyu, TANG Xiaodong, CAO Dianxue, et al. Studies on catalysis of olefin oligomerization on supported ferric sulfateⅡA study on the catalytic behavior of Fe2(SO4)2/γ-Al2O3for 1-butene oligomerization[J].Acta Petrolei Sinica(Petroleum Processing Section), 1997, 13(1): 13-17.)
[11] 王丽,丁洪生,赵春明,等.[Emim]HSO4-FeCl3催化1-丁烯齐聚反应研究[J].华工科技,2012, 20(1): 10-12.(WANG Li, DING Hongsheng, ZHAO Chunming, et al. Study on oligomerization of 1-butene catalyzed by ionic liquid[Emim]HSO4-FeCl3[J].Science & Technology in Chemical Inductry, 2012, 20(1): 10-12.)
[12] RIAAN B, PRINSLOOB N M. Butene oligomerization over phosphoric acid: Structural characterization of products[J].Industrial & Engineer Chemistry Research, 2009, 48(22): 10156-10162.
[13] 严志宇,曹殿学,蔡天锡.负载型硫酸铁对烯烃齐聚催化作用的研究Ⅳ不同方法制备的Fe2(SO4)2/γ-Al2O3的丙烯齐聚反应的考察[J].石油学报(石油加工),1997, 13(2): 41-45.(YAN Zhiyu, CAO Dianxue, CAI Tianxi. Studies on olefins oligomerization catalyzed by supported ferric sulfate Ⅳ A study of propene oligomerization on Fe2(SO4)2/γ-Al2O3prepated by different procedures[J].Acta Petrolei Sinica(Petroleum Processing Section), 1997, 13(2): 41-45.)
[14] ZBORIL R, MASHLAN M, PEDRIDIS D. Iron(Ⅲ) oxides from thermal processess-synthesis, structural and magnetic properties, moessbauer spectroscopy characterization, and applications[J].Chemistry of Materials, 2002, 14(3): 969-982.
[15] 刘科伟,陈天朗.硫酸铵的热分解[J].化学研究与应用,2002, 14(6): 737-738.(LIU Kewei, CHEN Tianlang. Studies on the thermal decomposition of ammonium sulfate[J].Chemical Research and Application, 2002, 14(6): 737-738.)
[16] 许越,夏海涛.催化剂设计与制备工艺[M].北京:化学工业出版社,2003: 27.
[17] 刘冰.Al-TiO2系的机械力化学和固相反应动力学[D].济南:山东大学,2005.
[18] 唐晓东,严志宇,曹殿学,等.负载型硫酸铁对烯烃齐聚催化作用的研究ⅠFe2(SO4)2/γ-Al2O3对丙烯齐聚反应催化作用的考察[J].石油学报(石油加工),1996, 12(4): 36-43.(TANG Xiaodong, YAN Zhiyu, CAO Dianxue, et al. Studies on catalysis of olefin oligomerization on supported ferric sulfateⅠ A study on the catalytic behavior of Fe2(SO4)2/γ-Al2O3for propene oligomerization[J].Acta Petrolei Sinica(Petroleum Processing Section), 1996, 12(4): 36-43.)
[19] CAI T X, ZHANG L Y, QI A H, et al. Propene oligomerization catalyst derived from nikel sulfate supported onγ-alumina[J].Catalysis Today, 1991, 69(1): 1-13.
[20] ADRIEN B, PIERRE-ALAIN R B, LIONEL M, et al. Novel catalytic system for ethylene oligomerization: An iron(Ⅲ) complex with an anionicN,N,Nligand[J].Inorganic Chemicals and Reactions, 2011, 30(10): 2640-2642.
[21] ROMAN R, THEODORUS DE B, PASCAL R, et al. Theoretical unraveling of selective 1-butene oligomerization catalyzed by iron- bis(arylimino)pyridine[J].Organometallics, 2009, 28(18): 5358-5367.
[22] 唐晓东,严志宇,曹殿学,等.负载型硫酸铁对烯烃齐聚催化作用的研究Ⅲ Fe2(SO4)2/γ-Al2O3的表征[J].石油学报(石油加工), 1997, 13(1): 13-17.(TANG Xiaodong, YAN Zhiyu, CAO Dianxue, et al. Studies on catalysis of olefin oligomerization on supported ferric sulfate Ⅲ Characterization of Fe2(SO4)2/γ-Al2O3catalyst[J].Acta Petrolei Sinica (Petroleum Processing Section), 1997, 13(1): 13-17.)
收稿日期:2015-05-18
基金项目:辽宁省科技技术攻关项目(2011223006)资助
文章编号:1001-8719(2016)04-0695-08
中图分类号:O643.32
文献标识码:A
doi:10.3969/j.issn.1001-8719.2016.04.006
XIAO Linjiu, CHEN Jiahuan, SHENG Yonggang, LI Wenze, WANG Jinbao
(LiaoningProvince,KeyLaboratoryforRare-EarthChemistryandApplication,CollegeofAppliedChemistry,ShenyangUniversityofChemicalTechnology,Shenyang110142,China)
Abstract:/γ-Al2O3 catalyst was prepared by step-impregnation method, and its catalytic performance for 1-butene oligomerization was studied and compared with Fe2(SO4)3/γ-Al2O3 catalyst. The influences of the preparation and reaction conditions on the catalytic activity of /γ-Al2O3 for 1-butene oligomerization were investigated. The results showed that, on the reaction condition of pressure of 0.96 MPa, reaction temperature of 60℃ and liquid hourly space velocity of 5 h-1, with the /γ-Al2O3 of 6% Fe loading and 1.2 mmol/g(γ-Al2O3) loading as catalyst, the conversion rate of 1-butene oligomerization reached 68.0% and the dimerization selectivity was 95.0%. α-Fe2O3 is an active component in catalytic 1-butene oligomerization and high dimerization selectivity can be obtained. In addition, the interaction between α-Fe2O3 crystal phase and had an important influence on the catalytic activity of /γ-Al2O3 in 1-butene oligomerization and the interaction between γ-Al2O3and also had some influence. Catalyst activity of /γ-Al2O3 was maximum with 6% Fe loading, and its NH3-TPD results showed there existed weak acid sites and only a moderate amount of acid.
Key words:1-butene oligomerization; catalyst; /γ-Al2O3; ferric sulfate
通讯联系人: 肖林久,男,教授,博士,从事精细化工、稀土材料、催化等方面的研究;Tel:024-89381616;E-mail:x109@163.com

