饲料缬氨酸水平对军曹鱼鱼体脂肪含量、血浆生化指标和肝脏脂肪代谢基因表达的影响
王 震 徐 玮 麦康森 路 凯 刘迎隆 艾庆辉
(中国海洋大学教育部海水养殖重点实验室, 农业部海水养殖重点实验室, 青岛 266003)
饲料缬氨酸水平对军曹鱼鱼体脂肪含量、血浆生化指标和肝脏脂肪代谢基因表达的影响
王 震 徐 玮 麦康森 路 凯 刘迎隆 艾庆辉
(中国海洋大学教育部海水养殖重点实验室, 农业部海水养殖重点实验室, 青岛 266003)
实验旨在研究饲料缬氨酸水平对军曹鱼(Rachycentron canadum)[初始体质量为(40.9±0.8) g]鱼体脂肪含量、血浆生化指标和肝脏脂肪代谢基因表达的影响。在基础饲料中梯度添加晶体缬氨酸, 配制出缬氨酸含量分别为1.26% (缺乏组)、2.21% (适量组)和2.62% (过量组)3种等氮等脂饲料, 饲喂养殖在海水浮式网箱的军曹鱼10周, 每天饱食投喂2次。结果表明, 缬氨酸缺乏组的军曹鱼鱼体和肌肉脂肪含量显著低于缬氨酸适量组和过量组(P<0.05)。肝脏脂肪含量随着饲料中缬氨酸含量从1.26%升高到2.21%而显著升高(P<0.05), 然后随之而逐渐下降(P>0.05)。军曹鱼血浆总蛋白和总胆固醇含量在缬氨酸缺乏饲料组显著低于其他各处理组(P<0.05)。饲料缬氨酸水平对军曹鱼血浆谷草转氨酶和谷丙转氨酶均无显著影响(P>0.05)。军曹鱼肝脏固醇调节元件结合蛋白-1 (Sterol regulatory element binding protein-1, SREBP-1)基因表达水平和肝脏脂肪酸合成酶(FAS)表达量, 均随着饲料缬氨酸水平增加而显著升高(P<0.05)。军曹鱼肝脏过氧化物酶体增殖物激活受体α (peroxisome proliferator activated receptor, PPARα)表达量在缬氨酸适量组, 显著低于过量组(P<0.05), 而与缺乏组差异不显著(P>0.05)。而随着缬氨酸含量升高, 肉毒碱棕榈酰转移酶-1 (CPT-1, Carnitine palmitoyl transferase-1)表达量逐渐下降(P<0.05)。总之, 饲料缺乏缬氨酸可减少军曹鱼鱼体脂肪积累。饲料中缬氨酸水平对军曹鱼鱼体脂肪沉积的影响, 可能是通过调控脂肪合成和β-氧化相关基因表达而实现的。
军曹鱼; 缬氨酸; 脂肪含量; 生化指标; 脂肪代谢
缬氨酸属于支链氨基酸, 作为鱼类十种必需氨基酸之一, 有着重要的生理功能[1—3]。有鱼类缬氨酸研究主要集中在缬氨酸对鱼类生长、饲料利用以及鱼体组成的影响等方面。研究表明, 缬氨酸缺乏导致鱼体生长下降、饲料利用率降低和蛋白质合成减少[4—9]。然而, 缬氨酸对于鱼体脂肪沉积的研究结果, 在不同鱼类上差异比较大。研究发现,饲料中缺乏缬氨酸能够显著降低鱼体、肝脏和血浆脂肪含量[5, 6, 8, 10]。也有报道发现, 鱼体脂肪含量随着饲料缬氨酸含量增加而显著减少[6]。
在哺乳动物上研究发现, 支链氨基酸对于脂肪代谢具有重要的调控作用[11—13]。饲料中添加支链氨基酸能够显著降低小鼠体内甘油三酯含量, 上调过氧化物酶体增殖物激活受体α (Peroxisome proliferator activated receptor, PPARα)基因表达, 表明脂肪降解相关基因被上调表达[12, 14]。研究发现, 食物缺乏缬氨酸显著降低了小鼠脂肪组织含量, 并证明脂肪组织含量降低是由于机体能量消耗增加和脂肪合成相关基因表达下调的结果[15]。然而, 在鱼类上关于缬氨酸如何调控脂代谢的研究尚未见报道。
本研究拟通过配制不同缬氨酸水平饲料投喂军曹鱼, 以探讨饲料缬氨酸水平对于军曹鱼鱼体脂肪含量、血浆生化指标和肝脏脂肪代谢基因表达的影响, 为军曹鱼高效功能性配合饲料开发提供理论依据。
1 材料与方法
1.1 饲料配方和制备
以鱼粉、豆粕、明胶和啤酒酵母为蛋白源, 以鱼油、豆油和卵磷脂为脂肪源, 实验设计3种等氮等脂饲料, 缬氨酸含量别为1.26% (缺乏组)、2.21%(适量组) 和2.62% (过量组)(干物质), 以甘氨酸为缬氨酸的等氮替代物(表 1)。以军曹鱼, 添加相对应的晶体氨基酸, 使基础饲料中除了缬氨酸以外, 其他各种氨基酸含量达到在军曹鱼鱼体中的含量(表2)。所有蛋白源原料均过60目筛, 按梯度混合均匀,混匀后与鱼油和豆油充分混合, 以6.0 mol/L NaOH调节饲料的pH达到7.0左右, 并添加适量水搅拌混匀, 用F(Ⅱ)-26型双螺杆挤条机(华南理工大学, 广州)加工成硬颗粒饲料(4.0 mm×8.0 mm和6.0 mm× 8.0 mm), 然后置于45°C鼓风烘箱烘干至饲料水分含量10%以下, 冷却后用塑料袋包装后保存于-20°C冰箱中备用。

表 1 实验饲料的配方以及营养组成(%干物质)Tab. 1 Formulation and chemical proximate composition of the experimental diets (% dry matter)
1.2 养殖实验和样品采集
健康无病的军曹鱼幼鱼从广东湛江附近一个商业鱼苗场中购买。实验开始之前, 所有鱼均被暂养(2周)在海水浮动网箱(4.5 m×4.5 m×9.0 m), 暂养期间, 投喂3种试验料的混合物(混合比例1∶1∶1)使之适用养殖环境和实验饲料。在实验开始前, 所有实验鱼饥饿24h, 用丁香酚(1∶10000)麻醉后称体质量。挑选规格均匀军曹鱼幼鱼[平均初始体质量(40.9±0.8) g], 分别随机分配到9个海水网箱(1.5 m× 1.5 m×2.5 m)中, 每个网箱放养20尾。实验设计3个重复, 实验为期10周。养殖实验期间, 所有军曹鱼每天饱食投喂两次(7:00和18:00), 记录每天摄食量、死鱼数量和重量, 监测海水温度、盐度和溶解氧整个, 实验期间, 海水水温为26—32°C, 盐度27‰—23‰, pH 7.1—7.4, 溶解氧在6.5 mg/L左右。
实验结束前, 所有实验鱼饥饿24h, 然后分别对每个网箱的实验鱼麻醉、计数并称体质量。每个实验网箱, 随机选取3尾鱼用于鱼体粗脂肪。随机取4尾后用于静脉取血, 随后解剖取其肝脏样品快速放于1.5 mL无RNAase离心管(RNAase-Free;Axygen), 并迅速放于液氮中冷冻并于-80°C冰箱保存。另取6尾鱼, 取肝脏、内脏团和肌肉置于10 mL离心管, 保存于-20°C冰箱, 用于后续分析脂肪含量。
1.3 化学分析
饲料原料和饲料粗蛋白(凯氏定氮法)和全鱼粗脂肪(索氏抽提测定法)的检测方法均参考AOAC (1990)标准方法[16]。肝脏和肌肉脂肪含量, 以氯仿/甲醇(2∶1, v/v)方法提取[17, 18]。饲料及原料氨基酸测定参照国标测定方法(GB/T5009.124-2003), 采用日立L-8900全自动氨基酸测定仪(Hitachi L-8900 automatic amino acid analyzer, Hitachi, Japan)测得。血浆总蛋白(Total plasma protein, TP)、谷草转氨酶活力(Aspartate aminotransferase, AST)、谷丙转氨酶活力(Alanine aminotransferase, ALT)、血浆甘油三脂(Total triglyceride, TG)、血浆胆固醇(Total cholesterol, TC)、高密度脂蛋白胆固醇(High-density lipoproteincholesterol, HDL-C)和低密度脂蛋白胆固醇(Low-density lipoprotein cholesterol,LDL-C)均用全自动生化分析仪测定(迈瑞医疗国际股份有限公司, BS-200)使用其配套南京建成商业试剂盒测定。
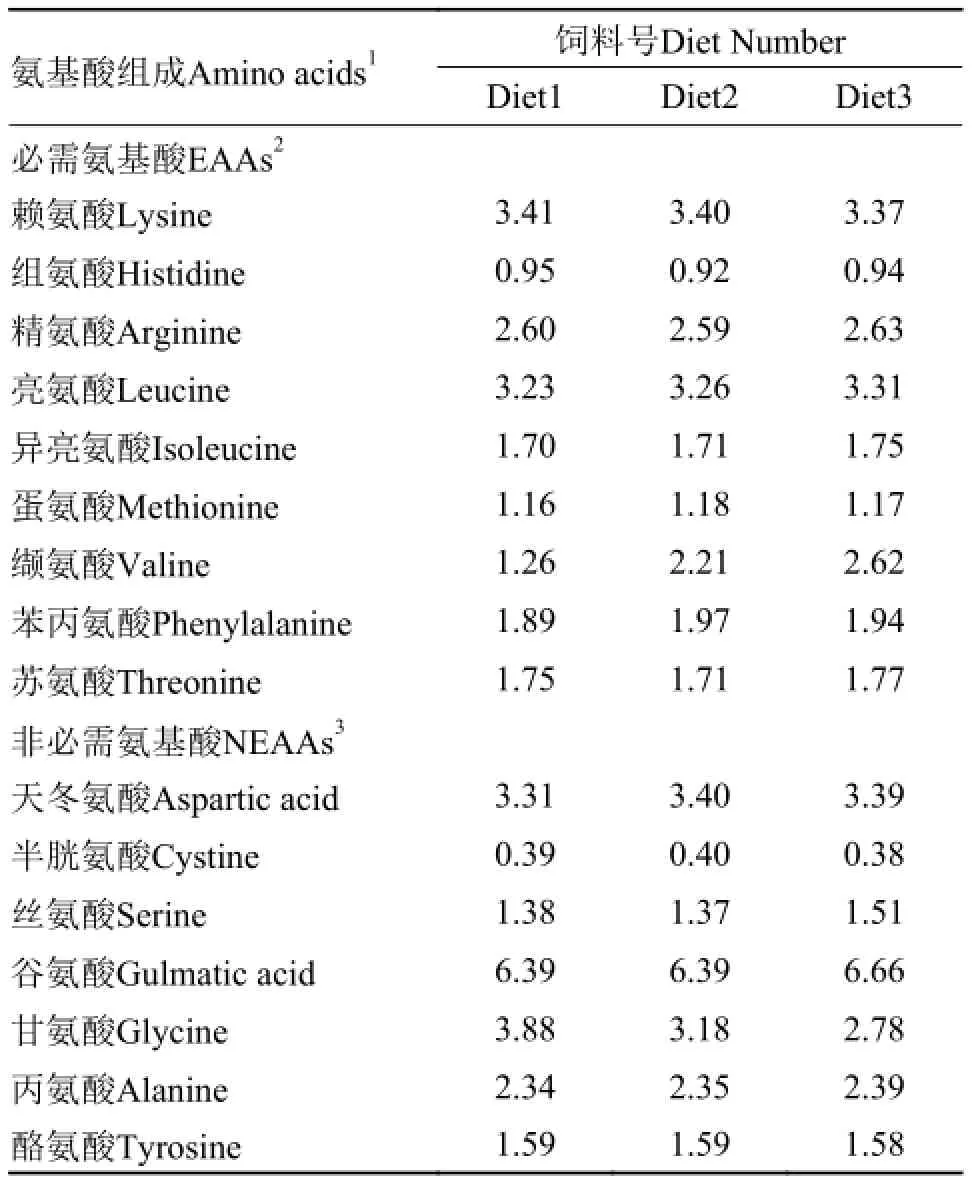
表 2 实验饲料的氨基酸组成(%干物质)Tab. 2 Amino acid (AA) composition of diets (dry matter%)
1.4 RNA提取和实时定量PCR
肝脏组织在研钵中用液氮研磨后, 用Trizol试剂盒(Invitrogen, USA)提取RNA。用琼脂糖凝胶电泳检测总RNA的质量。随后, 用无RNAase Dnase (TaKaRa, Japan)去除DNA污染物, 并用Prime ScriptTMRT试剂盒(TaKaRa, Japan)将RNA反转录为cDNA。cDNA用超纯水稀释至80 ng/μL。定量PCR反应体系为25 μL: 1 μL引物(10 μmol/L), 1 μL cDNA (80 ng/μL), 12.5 μL 2×SYBR Premix Ex TaqTMⅡ(TaKaRa, Japan)和9.5 μL 无RNase水。定量PCR使用的仪器是实时定量PCR仪(Eppendorf,Germany), 其反应条件为: 95°C 2min, 1循环;95°C变性10s, 退火10s, 59°C, 72°C延伸20s, 共计40个循环。表 3为脂肪酸合成酶(Fatty acid synthetase,FAS); 固醇调节元件结合蛋白-1 (Sterol regulatory element binding protein-1, SREBP-1); 过氧化物酶体增殖物激活受体γ (Peroxisome proliferator activated receptor, PPARγ); 肉碱脂酰转移酶(Carnitine acyl transferase-Ⅰ, CPT-1); 乙酰辅酶A羧化酶1 (ACC-1, acetyl-coenzyme A carboxylase-1); 硬脂酰辅酶A去饱和酶(Stearoyl-CoA desaturase-1, SCD-1); 葡萄糖-6-磷酸脱氢酶(Glucose-6-phosphate dehydrogenase,G6PD); PPARα; 脂蛋白脂酶(Lipase lipoprotein lipase, LPL)的特异性引物。每个PCR反应之后, 进行溶解曲线以检验定量PCR产物的单一性。通过2倍梯度稀释得到6个浓度cDNA模板, 以每个浓度cDNA为模板, 通过定量PCR得出每对引物每个浓度cDNA的Ct值, 拟合得到一条随拷贝数变化而Ct值变化的直线, 根据拟合得到直线斜率和E= 10(-1/Slope)-1, 并得出每对引物扩增效率(E)。本实验β-actin、FAS、SERBP-1、PPARα、PPARγ、CPT-1、ACC1、G6PD、SCD-1和LPL的扩增效率为0.92—1.05。ΔCt绝对值[目的基因—内参基因(βactin)]均小于0.100, 说明目的基因和内参基因扩增效率一致, 可以使用2-ΔΔCt方法进行定量目的基因表达量。然后, 每个处理cDNA均进行定量PCR反应, 并按计算公式ΔCt(内参基因)=Ct(样品内参基因)-Ct(对照内参基因)计算ΔCt及2-ΔΔCt[19]。
1.5 统计分析
实验数据采用平均值±标准误表示, 使用统计软件SPSS17.0对数据进行单因素方差分析(One-Way ANOVA), 差异时采用Tukey's进行多重比较,以P<0.05为差异显著性为标准。
2 结果
2.1 鱼体、肝脏、肌肉以及内脏团脂肪含量
缬氨酸缺乏组的军曹鱼鱼体脂肪含量显著低于缬氨酸适量组(2.21%)和过量组(2.62%) (P<0.05)。肝脏脂肪含量随着饲料缬氨酸含量从1.26%增加到2.21%而显著升高(P<0.05), 然后随之逐渐下降(P>0.05)。肌肉脂肪含量随缬氨酸水平变化趋势与鱼体粗脂肪含量变化趋势相似。军曹鱼内脏团脂肪含量各处理组间差异不显著(P>0.05) (表 4)。
2.2 血浆生化指标
军曹鱼血浆总蛋白(TP)和总胆固醇(TC)浓度在缬氨酸缺乏组显著低于其他各处理组(P<0.05),而其他各处理组间则差异不显著 (P>0.05)。饲料缬氨酸水平对血浆甘油三脂(TG)浓度影响不显著(P>0.05)。饲料缬氨酸水平对军曹鱼血浆葡萄糖、高密度脂蛋白、低密度脂蛋白浓度、谷草转氨酶和谷丙转氨酶活力均无显著影响(P>0.05) (表 5)。
2.3 军曹鱼肝脏脂肪代谢相关基因表达
军曹鱼肝脏固醇调节元件结合蛋白(SREBP-1)基因表达水平, 随着饲料缬氨酸水平增加而显著升高(P<0.05)。肝脏脂肪酸合成酶(FAS)表达量随饲料缬氨酸水平变化趋势与SREBP-1基因表达量变化趋势相似(P<0.05)。肝脏过氧化物酶体增殖物激活受体γ (PPAR γ), 乙酰辅酶羧化酶(ACC1), 葡萄糖-6-磷酸脱氢酶(G6PD)和硬脂酰辅酶A去饱和酶-1 (SCD-1)基因表达量, 则不受饲料缬氨酸水平调控(P>0.05) (图 1)。军曹鱼肝脏过氧化物酶体增殖物激活受体(PPARα)表达量在缬氨酸适量组显著低于缬氨酸过量处理组(P<0.05), 而与缺乏组差异不显著(P>0.05)。随着缬氨酸含量升高, 肉毒碱棕榈酰转移酶-1 (CPT-1)表达量逐渐下降(P<0.05)。肝脏脂蛋白脂酶(LPL) mRNA表达量在各处理组间差异不显著(P>0.05) (图 2)。
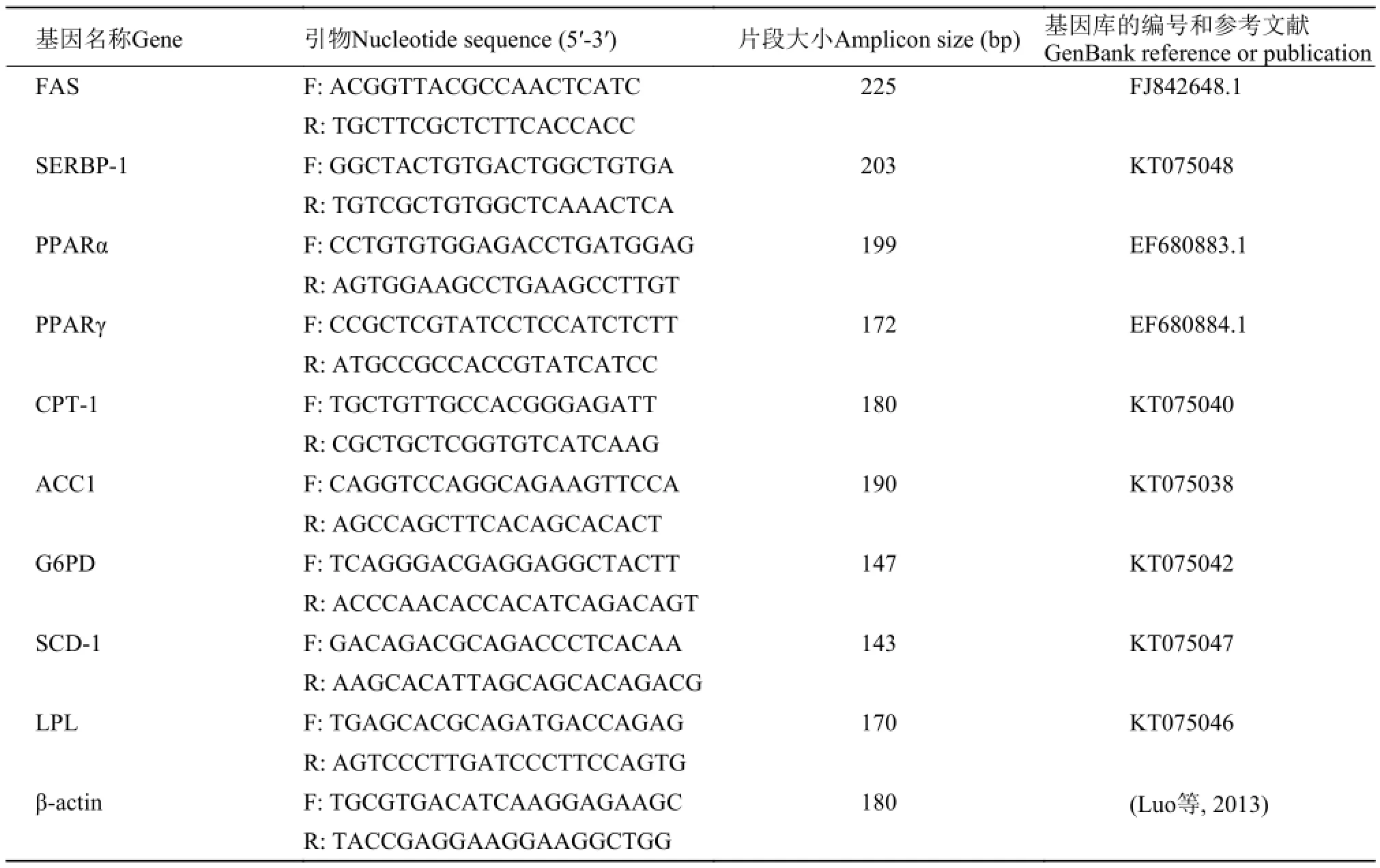
表 3 定量PCR的特异性引物以及扩增片段长度Tab. 3 Nucleotide sequence of primers for real-time quantitative PCR amplification

表 4 饲料缬氨酸水平对军曹鱼鱼体、肝脏、肌肉和内脏团的脂肪含量的影响(湿重%)Tab. 4 Lipid content of the whole body, liver, muscle and Visceral in cobia fed diets with graded levels of valine (% wet weight)
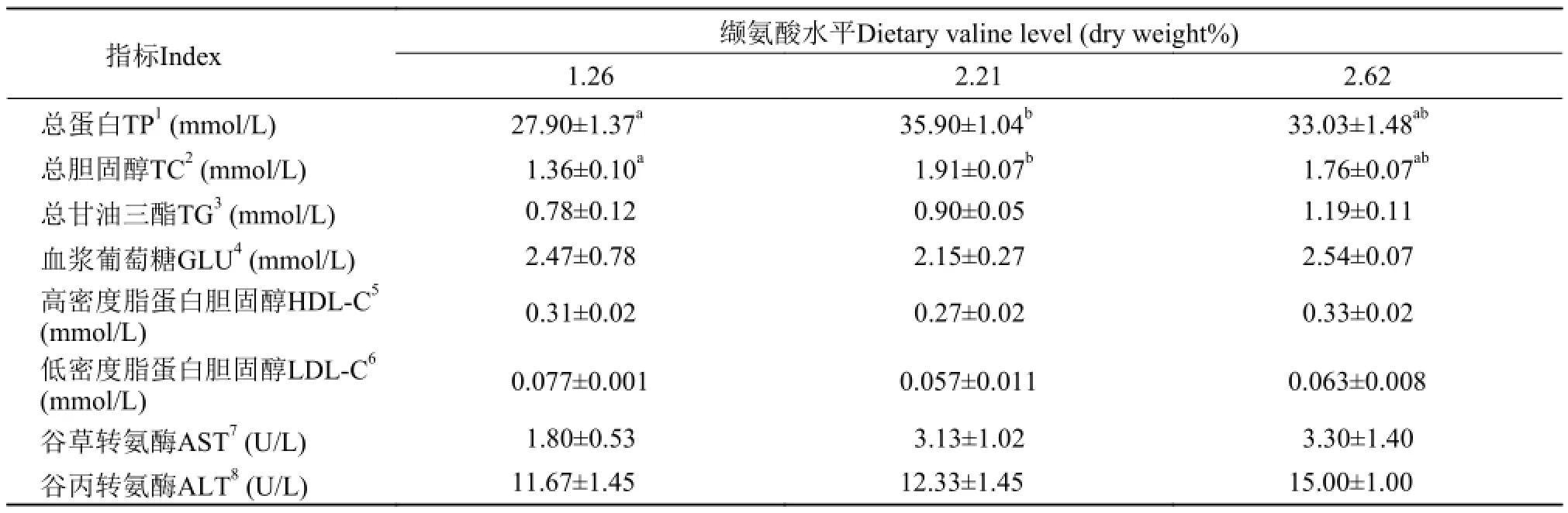
表 5 饲料缬氨酸水平对军曹鱼血浆生化指标的影响Tab. 5 Effects of dietary valine on the plasma biochemical index in cobia

图 1 饲料中缬氨酸水平对于军曹鱼肝脏脂肪合成相关基因(SREBP-1、PPARγ、FAS、ACC-1、FAS、SCD-1和G6PD)表达的影响Fig. 1 Effect of cobia (Rachycentron canadum) fed diets containing deficient (1.26%), moderate (2.21%) and excess (2.62%) levels of valine on relative expression of hepatic lipogenesis related genes: SREBP-1, PPARγ, FAS, ACC-1, SCD-1 and G6PD

图 2 饲料中缬氨酸水平对于军曹鱼肝脏脂肪酸氧化相关基因(PPARα、CPT-1和LPL)Fig. 2 Effect of cobia (Rachycentron canadum) fed diets containing deficient (1.26%), moderate (2.21%) and excess (2.62%)levels of valine on fatty acid oxidation-related genes: peroxisome proliferator activated receptorα (PPARα), carnitine acyl transferase-Ⅰ (CPT-1) and lipoprotein lipase (LPL) in liver for 10 weeks
3 讨论
本研究发现, 军曹鱼摄食缬氨酸缺乏饲料, 显著降低了鱼体、肝脏和肌肉脂肪含量。印度鲤鱼(Cirrhinus mrigala)[5]、建鲤(Cyprinus carpio var. Jian)[6]和卡特拉魮(Catla catla)[9]的研究结果与本实验的结果相同, 发现鱼体脂肪含量随着饲料缬氨酸含量增加而显著升高。哺乳动物上研究表明, 氨基酸代谢能够显著影响体内脂肪沉积[11]。饲料缺乏或过量支链氨基酸均能够减少小鼠体内脂肪沉积[13, 14, 20, 21]。这些研究结果表明, 饲料缺乏缬氨酸能够抑制机体脂肪积累。缬氨酸过量所导致的肝脏脂肪含量减少现象, 可能是由于氨基酸不平衡导致过度的能量消耗引起。
肝脏作为鱼类营养物质(脂肪、蛋白和糖类等)代谢中心, 其健康状况对于鱼类正常代谢有重要影响[22, 23]。血清生化指标通常反映动物体内代谢情况[24—26]。谷草转氨酶和谷丙转氨酶是肝细胞最重要氨基转移酶, 通常参与机体内蛋白质和氨基酸代谢, 其活性代表体内氨基酸代谢强弱[27, 28]。肝脏处于健康状态转氨酶主要存在肝脏细胞中, 而机体肝脏发生病变会引起细胞通透性增加, 进而使细胞转氨酶转移到血液中, 因此血浆谷草转氨酶和谷丙转氨酶活力可以反映肝脏健康状况[22, 23]。本实验发现, 饲料缬氨酸含量对血浆谷草转氨酶和谷丙转氨酶活力影响不显著, 表明缬氨酸过量和缺乏对于军曹鱼肝脏没有明显损害, 肝脏能够进行正常的营养代谢。
鱼体脂肪沉积主要是受体内脂肪合成和降解代谢过程调控[18, 29—31]。首先, 脂质合成主要是依赖于脂肪合成酶表达和活性高低, 脂肪合成酶基因表达则是主要是由SREBP-1调控[31, 32], FAS是脂肪酸从头合成的限速酶, 对体内脂肪从头合成发挥主导作用[11]。本实验发现, 缬氨酸缺乏显著降低了FAS 和SREBP-1转录水平, 这表明缬氨酸缺乏抑制了军曹鱼脂肪合成能力。这与哺乳动物上研究结果相似, 必需氨基酸缺乏(蛋氨酸、亮氨酸、异亮氨酸和缬氨酸)抑制了甘油三脂合成相关基因表达[11, 15]。机体脂肪沉积一般与SREBP-1和FAS表达呈正相关关系[15]。研究发现, 培养液添加亮氨酸显著提高了虹鳟肝脏细胞SREBP-1和FAS转录水平表达量[33]。GCN2 (General control nonrepressed 2)信号通路能够感知机体内必需氨基酸缺乏, 进而抑制体内脂肪酸从头合成能力[11, 15, 34—36]。因此, 缬氨酸缺乏所导致脂肪含量的减少, 可能是由于脂肪合成相关基因表达下降所导致, 更为直接的证据需要进一研究。本实验发现, 缬氨酸缺乏抑制了肝脏CPT-Ⅰ (β-氧化过程的关键基因)基因表达。这与哺乳动物研究结果不同, 小鼠摄食必需氨基酸缺乏饲料能够激活线粒体β-氧化[13, 15, 37]。投喂军曹鱼蛋氨酸缺乏饲料也能够显著上调军曹鱼β-氧化相关基因表达[38]。氨基酸对于β-氧化基因表达差异的影响, 可能是不同营养和物种对于β-氧化基因的调控方式不同, 其机制有待于进一步研究。
总之, 饲料中缺乏缬氨酸能够减少军曹鱼鱼体脂肪沉积。而饲料中缬氨酸水平影响军曹鱼鱼体的脂肪沉积, 可能是通过调控脂肪合成和β-氧化相关基因表达等途径实现的。
[1]Robaina L, Izquierdo M, Moyano F, et al. Soybean and lupin seed meals as protein sources in diets for gilthead seabream (Sparus aurata): nutritional and histological implications [J]. Aquaculture, 1995, 130(2): 219—233
[2]Aoyama T, Fukui K, Takamatsu K, et al. Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (yellow K K) [J]. Nutrition, 2000, 16(5): 349—354
[3]Dias J, Alvarez M, Arzel J, et al. Dietary protein source affects lipid metabolism in the European seabass (Dicentrarchus labrax) [J].Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology,2005, 142(1): 19—31
[4]Abidi S, Khan M. Dietary valine requirement of Indian major carp, Labeo rohita (Hamilton) fry [J]. Journal of Applied Ichthyology, 2004, 20(2): 118—122
[5]Ahmed I, Khan M A. Dietary branched-chain amino acid valine, isoleucine and leucine requirements of fingerling Indian major carp, Cirrhinus mrigala (Hamilton) [J]. British Journal of Nutrition, 2006, 96(3): 450—460
[6]Dong M, Feng L, Kuang S Y, et al. Growth, body composition, intestinal enzyme activities and microflora of juvenile Jian carp (Cyprinus carpio var. Jian) fed graded levels of dietary valine [J]. Aquaculture Nutrition, 2013,19(1): 1—14
[7]Pohlenz C, Buentello A, Miller T, et al. Effects of dietary arginine on endocrine growth factors of channel catfish,Ictalurus punctatus [J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology,2013, 166(2): 215—221
[8]Rahimnejad S, Lee K J. Dietary valine requirement of juvenile red sea bream Pagrus major [J]. Aquaculture,2013, 416: 212—218
[9]Zehra S, Khan M A. Dietary Valine Requirement of fingerling Catla catla [J]. Journal of Applied Aquaculture,2014, 26(3): 232—251
[10]Zehra S, Khan M A. Dietary isoleucine requirement of fingerling catla, Catla catla (Hamilton), based on growth,protein productive value, isoleucine retention efficiency and carcass composition [J]. Aquaculture International,2013, 21(6):1243—1259
[11]Guo F, Cavener D R. The GCN2 eIF2α kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid [J]. Cell Metabolism, 2007, 5(2):103—114
[12]Zhang Y, Guo K, LeBlanc R E, et al. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms [J]. Diabetes, 2007, 56(6): 1647—1654
[13]Cheng Y, Meng Q, Wang C, et al. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue [J]. Diabetes, 2010,59(1): 17—25
[14]Nishimura J, Masaki T, Arakawa M, et al. Isoleucine prevents the accumulation of tissue triglycerides and upregulates the expression of PPARα and uncoupling protein in diet-induced obese mice [J]. The Journal of Nutrition,2010, 140 (3): 496—500
[15]Du Y, Meng Q, Zhang Q, et al. Isoleucine or valine deprivation stimulates fat loss via increasing energy expenditure and regulating lipid metabolism in WAT [J]. Amino Acids, 2012, 43(2): 725—734
[16]AOAC W H. Official Methods of Analysis of the Associa tion of Official Analytical Chemists [M]. Association of Official Analytical Chemists, Arlington, VA, USA. 1990
[17]Herzig S, Hedrick S, Morantte I, et al. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPARγ [J]. Nature, 2003, 426 (6963): 190—193
[18]Peng M, Xu W, Mai K, et al. Growth performance, lipid deposition and hepatic lipid metabolism related gene expression in juvenile turbot (Scophthalmus maximus L.)fed diets with various fish oil substitution levels by soybean oil [J]. Aquaculture, 2014, 433: 442—449.
[19]Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCTmethod [J]. Methods, 2001, 25(4): 402—408
[20]Chen H, Simar D, Ting J, et al. Leucine improves glucose and lipid status in offspring from obese dams, dependent on diet type, but not caloric intake [J]. Journal of Neuroendocrinology, 2012, 24(10): 1356—1364
[21]Hasek B, Boudreau A, Shin J, et al. Remodeling the integration of lipid metabolism between liver and adipose tissue by dietary methionine restriction in rats [J], Diabetes, 2013, 62: 3362—3372
[22]Pianesso D, Neto J R, Da Silva L, et al. Determination of tryptophan requirements for juvenile silver catfish (Rhamdia quelen) and its effects on growth performance, plasma and hepatic metabolites and digestive enzymes activity [J]. Animal Feed Science and Technology, 2015, 210:172—183
[23]Qiang J, Yang H, Wang H, et al. Effects of different dietary protein on serum biochemical indices and expression of liver HSP70 mRNA in gift tilapia (Oreochromis Nioticus) under low temperature stress [J]. Acta Hydrobiologica Sinca, 2013, 37(3): 434—443 [强俊, 杨弘, 王辉, 等.饲料蛋白水平对低温应激下吉富罗非鱼血清生化指标和HSP70 mRNA表达的影响. 水生生物学报, 2013,37(3): 434—443]
[24]Zhang S, Ai Q H, Mai K S, et al. Effects of fish meal replacement with crystalline amino acid on digestive and metabolic enzymes of tongue sole (Cynoglossus Semilaevis Gunther, 1873) larvae [J]. Acta Hydrobiologica Sinca, 2014, 38(5): 801—808 [张珊, 艾庆辉, 麦康森, 等.晶体氨基酸替代鱼粉蛋白对半滑舌鳎稚鱼消化酶和代谢酶活力的影响. 水生生物学报, 2014, 38(5):801—808]
[25]Zhou Q C, Wu Z H, Chi S Y, et al. Dietary lysine requirement of juvenile cobia (Rachycentron canadum) [J]. Aquaculture, 2007, 273(4): 634—640
[26]Zhou X, Yang F, Zhou A, et al. The lysine requirement of juvenile soft shell turtle [J]. Journal of Fisheries of China,2001, 25(5): 454—459
[27]Wang J Y, Li P Y, Song Z D, et al. Effects of dietary Yucca Schidigera extract on the growth performance,blood physiological and biochemical indices of turbot (Scophihalmus Maximus) [J]. Acta Hydrobiologica Sinca,2014, 38(6): 1117—1126 [王际英, 李培玉, 宋志东,等.饲料中添加丝兰提取物对大菱鲆幼鱼生长和生理及水环境的影响. 水生生物学报, 2014, 38(6): 1117—1126]
[28]Zehra S, Khan M A. Dietary leucine requirement of fingerling Catla catla (Hamilton) based on growth, feed conversion ratio, RNA/DNA ratio, leucine gain, blood indices and carcass composition [J]. Aquaculture International, 2015, 23(2): 577—595
[29]Shi X, Luo Z, Huang C, et al. Effect of substituting chlorella SP for regular fishmeal on growth, body composition, hepatic lipid metabolism and histology crucian carp Carassius auratus [J]. Acta Hydrobiologica Sinca,2015, 39(3): 498—506 [石西, 罗智, 黄超,等. 小球藻替代鱼粉对鲫生长、体组成、肝脏脂肪代谢及其组织学的影响. 水生生物学报, 2015, 39(3): 498—506]
[30]Qin C J, Shao T, Yang J P, et al. The effect of starvation on lipid metabolism of darkbarbel catfish, Pelteobagrus vachelli [J]. Acta Hydrobiologica Sinca, 2015, 39(1):58—65 [覃川杰, 邵婷, 杨洁萍,等. 饥饿胁迫对瓦氏黄颡鱼脂肪代谢的影响. 水生生物学报, 2015, 39(1):58—65]
[31]Brown M S, Goldstein J L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor [J]. Cell, 1997, 89(3):331—340
[32]Shimano H. Sterol regulatory element-binding protein-1 as a dominant transcription factor for gene regulation of lipogenic enzymes in the liver [J]. Trends in Cardiovascular Medicine, 2000, 10(7): 275—278
[33]Lansard M, Panserat S, Plagnes J E, et al. L-leucine, L-methionine, and L-lysine are involved in the regulation of intermediary metabolism-related gene expression in rainbow trout hepatocytes [J]. The Journal of Nutrition, 2011,141(1): 75—80
[34]Plaisance E P, Van N, Orgeron M, et al. Role of general control nonderepressible 2 (GCN2) kinase in mediating responses to dietary methionine restriction [J]. The Faseb Journal, 2012, 26 (1_MeetingAbstracts): 255. 251
[35]Ana LDS C, Pedro F M, Diego H. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation [J]. Biochemical Journal, 2012, 443(1):165—171
[36]De Sousa-Coelho A L, Relat J, Hondares E, et al. FGF21 mediates the lipid metabolism response to amino acid starvation [J]. Journal of Lipid Research, 2013, 54(7):1786—1797
[37]Plaisance E P, Henagan T M, Echlin H, et al. Role of β-adrenergic receptors in the hyperphagic and hypermetabolic responses to dietary methionine restriction [J]. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2010, 299(3): R740-R750
[38]Wang Z, Mai K, Xu W, et al. Dietary methionine level influences growth and lipid metabolism via GCN2 pathway in cobia (Rachycentron canadum) [J]. Aquaculture, 2016,454: 148—156
THE EFFECTS OF VALINE LEVEL ON PLASMA BIOCHEMICAL INDEXES,LIPID CONTENT AND GENE EXPRESSION INVOLVED IN LIPID METABOLISM IN COBIA (RACHYCENTRON CANADUM)
WANG Zhen, XU Wei, MAI Kang-Sen, LU Kai, LIU Ying-Long and AI Qing-Hui
(The Key Laboratory of Aquaculture Nutrition and Feed (Ministry of Agriculture) and Key Laboratory of Mariculture (Ministry of Education), Ocean University of China, Qingdao 266003, China)
The present study was conducted to investigate the effects of dietary valine on plasma biochemical indexes,lipid content and gene expression involved in lipid metabolism in cobia (Rachycentron canadum). Fish [mean initial weight, (40.9±0.8) g] were fed with soybean meal based on diets with graded levels of valine (1.26%, 2.21% and 2.62%) for 10 weeks. Results showed that lipid content of the whole body and muscle of fish fed the diet with deficient valine (1.26%) was significantly lower than that fish fed the moderate (2.21%) and excess (3.23%) valine treatment groups (P<0.05). Plasma total protein (TP) fish increased significantly as dietary valine increased from 1.26% to 2.21% (P<0.05), and kept relatively constant when dietary valine level was above 2.21% (P>0.05). Plasma total cholesterol (TC) and the lipid content of liver increased with dietary valine increasing from 1.26 % to 2.21% (P<0.05), but decreased with higher levels of dietary valine (2.21% to 2.62%) (P>0.05). Hepatic mRNA levels of lipid synthesis related genes (SREBP-1, and FAS) were significantly up-regulated in fish fed the diet with moderate level of valine (2.21%)(P<0.05), while hepatic mRNA transcriptional levels PPARα were significantly elevated in fish fed the diet with high level of valine (P<0.05). Overall, results of this study suggested that valine deficiency could decrease lipid content and inhibit expressions of some lipid synthesis related genes of cobia. This may contribute to understanding the mechanisms related to the physiological effects of dietary valine in cobia.
Cobia; Valine; Lipid content; Plasma biochemical indexes; Lipid metabolism
S965.1
A
1000-3207(2016)04-0744-08
10.7541/2016.98
2016-03-31;
2016-04-30
国家公益性行业(农业)科研专项(201003020, 200903029)资助 [Supported by the Ministry of Agriculture of the People's Republic of China (No. 201003020, 200903029)]
王震(1988—), 男, 山东即墨人; 博士研究生; 研究方向为水产动物营养与饲料。E-mail: sunderw@163.com
艾庆辉(1972—), 教授, 博士生导师; E-mail: qhai@ouc.edu.cn

