纳米氧化铜对硝基苯酚的电化学氧化及降解作用
徐白露, 张 唤, 杨周生
(安徽师范大学 环境科学与工程学院,安徽 芜湖 241000)
纳米氧化铜对硝基苯酚的电化学氧化及降解作用
徐白露,张唤,杨周生
(安徽师范大学 环境科学与工程学院,安徽 芜湖241000)
摘要:本文通过电化学方法探讨了纳米氧化铜修饰电极对硝基苯酚的电化学氧化及降解作用.考察了在不同pH值和修饰材料存在不同量度条件下,对硝基苯酚的电化学行为影响.结果显示:纳米氧化铜材料能明显地提高硝基苯酚在电极上氧化还原反应的可逆性,ΔE由在裸电极的0.862V降低为0.291V,氧化还原峰电流显著增加.使用钛基纳米氧化铜材料构建的电极,进行对硝基苯酚的电化学氧化,通过紫外可见光谱、高效液相色谱对电解后的溶液检测,结果表明:纳米氧化铜对硝基苯酚具有很好的催化降解作用.
关键词:纳米氧化铜;对硝基苯酚;催化降解
硝基苯酚类化合物是难降解的环境污染物质之一.研究其电化学反应机理不仅对硝基苯酚类化合物的降解具有意义,而且对于其在人体中的生物转化亦具有参考价值.目前,对于硝基苯酚类化合物通过探讨电化学氧化还原反应性质,研究其在环境中的转化规律和寻求解决方法[1].
金属氧化物纳米材料因其应用广泛更是受到人们的关注.具有尖晶石结构的四氧化三铁在磁制冷、磁流体和存储信息等方面有很大的价值[2].氧化铜是一种p型的半导体材料,频带间隙为1.2电子伏特[3].纳米氧化铜是一种很好的光敏材料,其被广泛的应用于光、电、半导体及电极材料等方面[4].纳米级的氧化铜在催化剂方面也有很大的应用前景[5].本文探讨了对硝基苯酚在纳米氧化铜材料修饰电极上的电化学行为,优化了实验条件,使用该材料对硝基苯酚实施电化学降解进行了探讨,取得了较好的降解效果.
1实验部分
1.1仪器及试剂
CHI660D电化学工作站,pHS-3C型酸度计,DJS-292B恒电位仪,78-1磁力加热搅拌器,水热反应釜,DHG-9053型电热恒温鼓风干燥箱,HC-2062型高速离心机,玻碳电极及修饰电极为工作电极,铂丝为对电极,Ag/AgCl电极为参比电极,LC20AT型高效液相色谱仪,UV757CRT型紫外分光光度计.氯化铜,硫堇,磷酸二氢钾,对硝基苯酚均为分析纯.
1.2修饰材料及电极的制备
纳米氧化铜是根据文献[6]的制备方法获得.修饰电极的制备过程为:将裸玻碳电极在粒径为0.05um氧化铝悬浮液中抛光至镜面,在二次水和无水乙醇中超声清洗各5分钟;将玻碳电极置于含5mM硫堇的0.1M磷酸缓冲溶液(pH=6)中,在电位为-0.5~0.5V范围内进行循环伏安扫图,扫速为50mV/s,扫描40圈,电聚合完成后,将电极用蒸馏水冲洗干净,室温晾干,得到聚硫堇修饰电极(PTH/GCE);将5mg纳米氧化铜分散于1.0ml的蒸馏水中,超声分散10分钟,然后取10uL分散液滴涂在硫堇/玻碳修饰电极上,室温晾干,即得纳米氧化铜/硫堇/玻碳修饰电极(CuO/PTH/GCE).
1.3对硝基苯酚电化学行为及降解实验
配制浓度为1.0×10-4mol/L对硝基苯酚溶液,缓冲溶液为0.1M的磷酸缓冲溶液,以铂丝电极为对电极、Ag/AgCl电极为参比电极,纳米氧化铜/硫堇/玻碳电极为工作电极的三电极体系中进行循环伏安实验.使用钛基纳米氧化铜电极作阳极,恒电流电解浓度为10mg/mL含有0.1M硫酸钠的对硝基苯酚溶液,在电解经过不同时间,取电解液进行紫外可见光谱、高效液相色谱检测.
2结果与讨论
2.1纳米材料表征分析
获得的氧化铜纳米材料粒子尺寸可用SEM来表征(图1a).由图可得,氧化铜纳米材料是形状均一的梭形结构.聚硫堇电聚合在玻碳电极表面,形成一个网状的聚合物结构(图1b),在这些网状空隙中,氧化铜纳米粒子能够被固定其中.梭形氧化铜被滴涂到PTH/GCE表面,被固定在网状结构中,同时聚硫堇分子中的巯基能够和氧化铜之间可以形成Cu-S化学键[7-8],从而使氧化铜被固定在PTH的网状中效果更好.
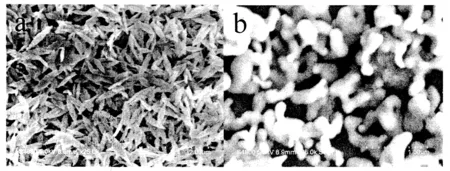
图1 修饰电极表面的扫描电镜表征图Fig.1 SEM images of the as-prepared sample CuO(a), PTH(b)
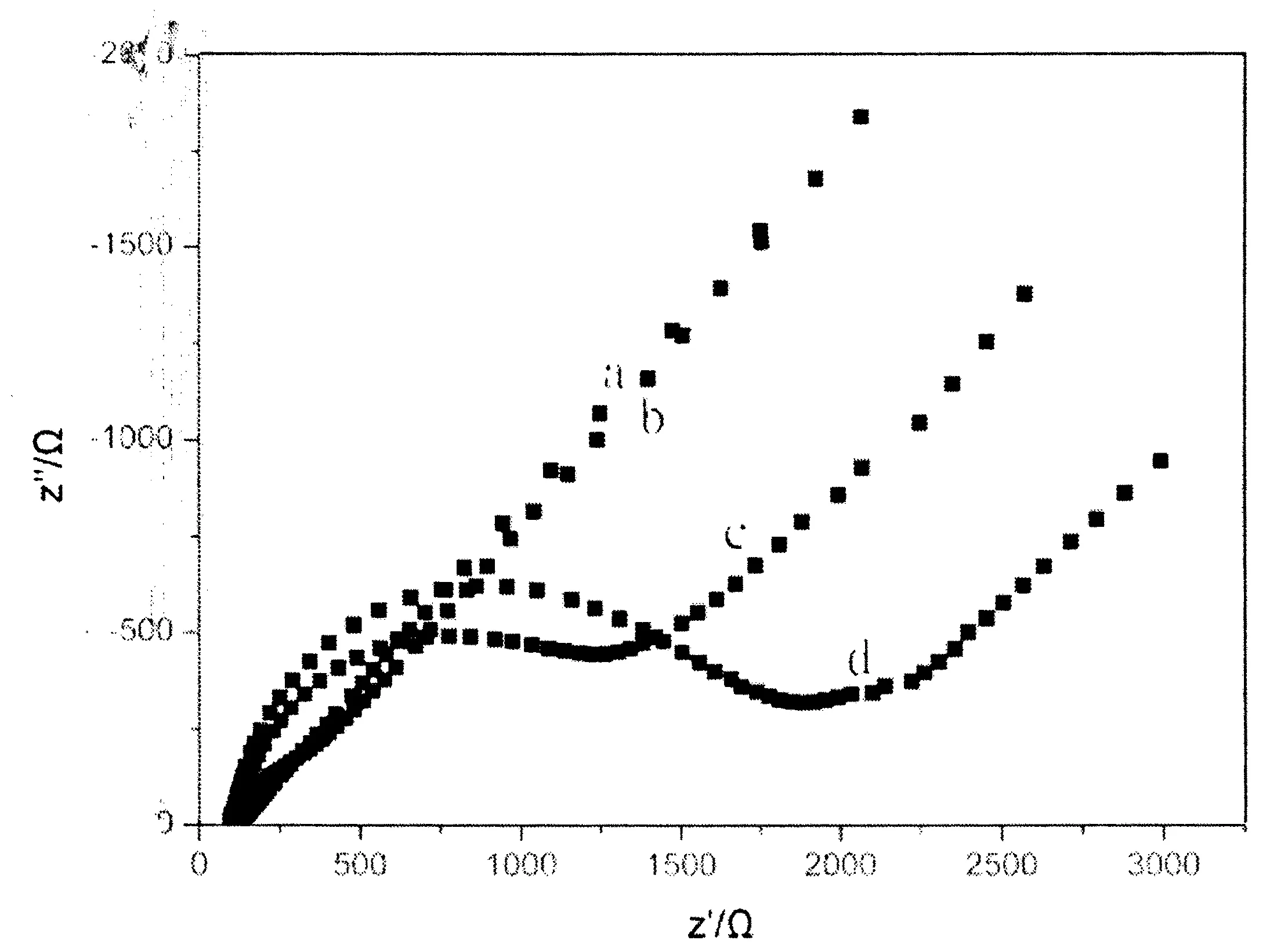
图2 不同修饰电极的电化学阻抗图Fig.2 EIS of different electrodes in 0.1 M KCl solution containing5.0mM Fe(CN)6]3-/[Fe(CN)6]4-:GCE(a),PTH/GCE(b),CuO/GCE(c), CuO/PTH/GCE(d)
为了考察不同电极的修饰情况,电化学阻抗曲线被用来表征这种变化.图2 为不同修饰电极的电化学阻抗图.由图2可知,在含0.1M KCl的[Fe(CN)6]3-/[Fe(CN)6]4-溶液中,GCE(曲线a)表现出的等效电阻值为300±10Ω,PTH/GCE(曲线b)修饰电极的电阻为310±10Ω,CuO/GCE(曲线c)为1250±10Ω,CuO/ PTH/GCE(曲线d)为1720±10Ω.硫堇/玻碳修饰电极与裸电极的电阻数据变化不大,这是由于聚硫堇具有很好的导电性所致.而在纳米CuO/GCE,由于CuO导电能力比GCE要弱得多,其修饰电极的阻抗变化大.这些变化表明:PTH、CuO被成功修饰到了玻碳电极表面.
2.2对硝基苯酚的电化学行为
为了考察对硝基苯酚在纳米氧化铜上的电化学性能,本工作分别以不同电极作为工作电极进行循环伏安实验.图3为对硝基苯酚在不同的工作电极上的循环伏安曲线.以GCE作为工作电极时,一对较弱的氧化还原峰电位出现在0.239V和-0.623V,在氧化峰电流是2.25uA,在还原峰电流是8.86uA(曲线a);以氧化铜/硫堇/玻碳修饰电极为工作电极时,在-0.083V出现氧化峰,在-0.214V出现还原峰,氧化峰电流是237.49uA,还原峰的峰电流是597.63uA(曲线c).通过比较发现,氧化还原峰电流明显增大,电位差值由原来的0.862V缩小为0.291V,反应的可逆性增加.这说明纳米氧化铜对对硝基苯酚电化学氧化还原具有明显的催化效果.而0.083V处的氧化峰和-0.214V处的还原峰对应的是酚羟基的氧化还原反应[9],-0.579V处的还原峰可能是硝基的还原反应.
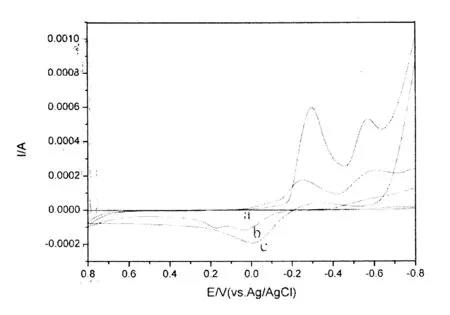
图3 不同电极对对硝基苯酚电化学反应催化效果图Fig.3 The catalytic efficiency of electrochemical response of p-nitrophenol on ent modified electrode: bare GCE(a),CuO/GCE(b),CuO/PTH/GCE(c)
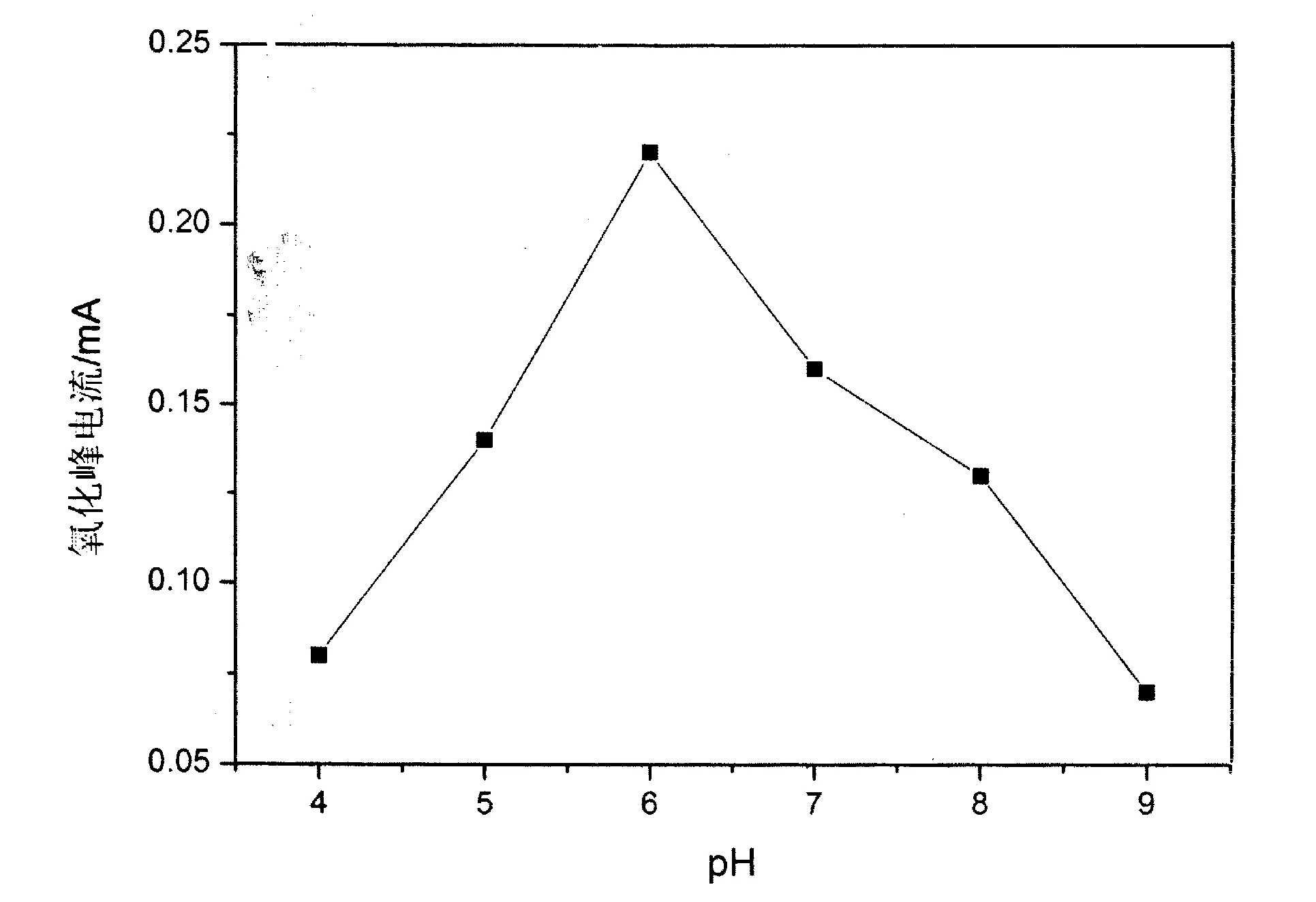
图4 硝基苯酚在CuO/PTH/GCE的电化学响应对pH值变化Fig.4 The effects of solution pH on at electrochemical differ response of p-nitrophenol on CuO/PTH/GCE
2.3影响对硝基苯酚电化学行为的因素
2.3.1pH值的影响:配制浓度为1×10-4mol/L对硝基苯酚溶液,缓冲溶液为0.1M的磷酸缓冲溶液,设置pH梯度为:4、5、6、7、8、9,以纳米氧化铜/硫堇/玻碳电极为工作电极,获得不同pH条件下的循环伏安曲线.如图4所示,取氧化峰的峰电流进行比较发现:在pH=6时,氧化峰电流达到最大.在酸性条件下,体系中质子浓度较大,阻碍了酚羟基氧化反应中的失电子过程[10].随着pH值的增大,氧化峰的峰电流持续减小,这是由于电极在碱性溶液中不稳定[8],造成了纳米氧化铜催化能力的降低.
2.3.2纳米材料厚度的影响:在最佳的pH条件下,考察了纳米材料厚度对硝基苯酚电化学行为的影响,获得的实验结果如图5所示.实验选择纳米氧化铜材料的浓度为:1mg/ml、2.5mg/ml、5mg/ml、7.5mg/ml、10mg/ml,分别取各浓度的材料10uL滴涂在3mm直径的玻碳电极上,修饰成不同厚度的电极进行试验比较.为了探讨材料厚度与对硝基苯酚电化学行为之间的关系,取氧化峰的电流进行比较,由图5可知:该处的峰电流值的随着材料厚度的增加而增大,在修饰厚度为0.708mg/cm2时,电流值达到最大,随着厚度进一步增加,峰电流随之减小.这是因为随着材料厚度的增加,酚羟基在电极表面催化反应的活性位点增加[11-12],纳米氧化铜对硝基苯酚的催化能力增强,从而使得相应的电流值增大;而当材料厚度继续增加时,阻碍了电子在电极表面的传递,使得氧化峰电流减小[13].
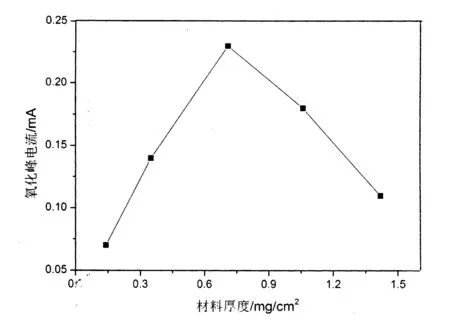
图5 纳米氧化铜材料厚度对硝基苯酚氧化峰电流的影响Fig.5 The effects of thickness of material on peak current at electrochemical degradation in different time
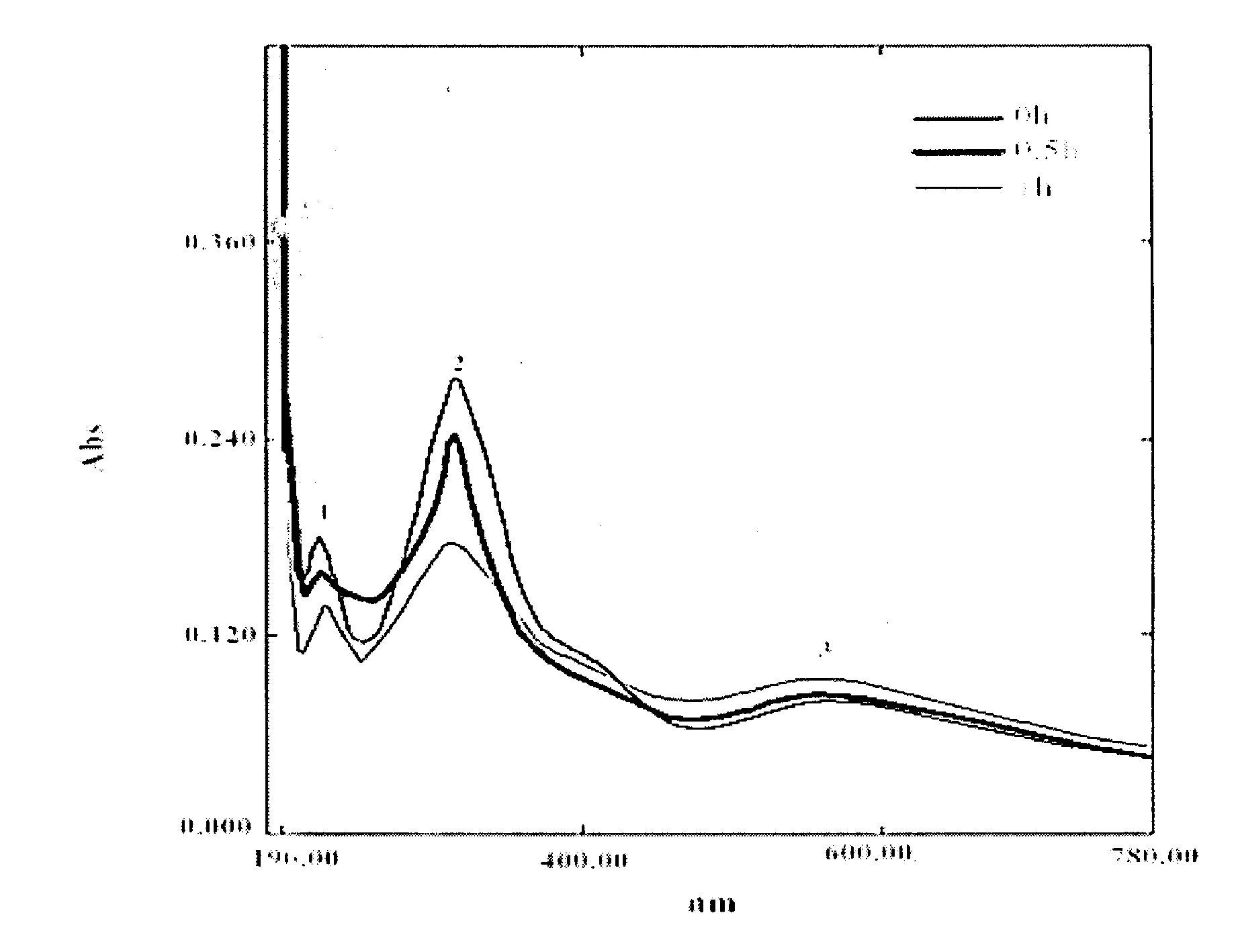
图6 电解不同时间后溶液的紫外吸收光谱变化Fig.6 Change of spectrum of p-nitrophenol after electrochemical reaction of p-nitrophenol by CuO/PTH/GCE
2.4纳米氧化铜对对硝基苯酚催化降解
以钛板(3.0×1.5×0.1cm)为基电极,根据文献[14]制备方法制得CuO/Ti电极.本研究采用二电极隔膜电解槽,以CuO/Ti电极为阳极,Pt电极为阴极,对浓度为10mg/L含有0.1M硫酸钠的对硝基苯酚溶液(pH=6)进行电化学降解.实验在DJS-292B恒电位仪上进行,对溶液持续通过恒电流30mA来进行降解,实验中分别取不同时间段的样品进行紫外光谱和液相色谱的测定.
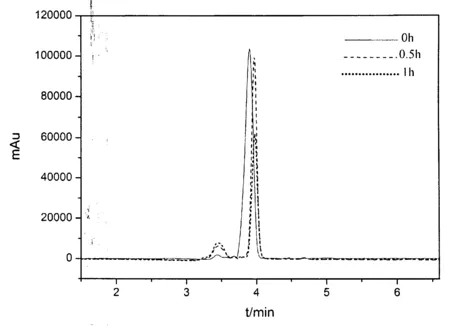
图7 电解不同时间后对硝基苯酚的高效液相色谱图的变化Fig.7 Change of HPLC of p-nitrophenol after electrochemicaldegradation in different time
如图6所示,从UV图谱中可以观察到,电解之前的对硝基苯酚存在明显的3个吸收峰,分别是258nm处的吸收峰(1)、359nm处的吸收峰(2)和560nm处的吸收峰(3),在降解一段时间后,峰1和峰2的吸光度减小,而峰3吸光度增加.这一结果表明随着电化学降解的进行,对硝基苯酚的浓度逐渐降低,同时可以观察到电解溶液由开始的微黄色逐渐转变为金黄色,这可能是由于醌类物质的产生所致[15-16].由此说明在降解实验中,对硝基苯酚的分子结构发生变化,在溶液中的含量逐渐降低,能够被成功降解.
从HPLC图谱中可以观察到(如图7所示),未降解的对硝基苯酚在3.42min和3.92min出现两个色谱峰,随着电化学降解的持续进行,保留时间为3.42min处的色谱峰逐渐增大,而保留时间为3.92min 处的色谱峰逐渐减小.降解前后色谱图上的这些变化与紫外吸收光谱的变化相一致,进一步表明:在电解过程中,对硝基苯酚的分子结构发生变化,在溶液中的含量逐渐降低,能够被成功降解.而降解后生成的物质有待进一步研究确认.
结论
本论文通过使用循环伏安法研究了纳米氧化铜对硝基苯酚的电化学氧化还原的催化作用,同时提高了对硝基苯酚氧化还原反应的可逆性;纳米氧化铜能够使得对硝基苯酚被有效的催化降解.
参考文献:
[1]刘相伟.工业含酚废水处理技术的现状与发展[J].工业水处理,1998,18(2):4-7.
[2]张立德,牟季美.纳米材料和纳米结构.科学出版社,2001,490-523.
[3]REITZ J B, SOLOMON E I. Propylene oxidation on copper oxide surfaces: electronic and geometric contributions. Journal of the American Chemical Society,1998,120,11467-11478.
[4]白春礼.纳米科技现在与未来[M].四川教育出版社,2001,31-33.
[5]董邵俊,车广礼,谢远武.化学修饰电极[M].北京:科学出版社,1995.
[6]CHEN D, SHEN G Z, TANG K B, QIAN Y T. Large-scale synthesis of CuO shuttle-like crystals via a convenient hydrothermal decomposition route. Journal of Crystal Growth (2003)254:225-228.
[7]朱俊武,张维光,等.纳米CuO的形貌控制合成及其性能研究[J].无机化学学报,2004,20(7):863-867.
[8]ZHANG Y G, WANG S T, LI X B, et al. CuO shuttle-like nanocrystals synthesized by oriented attachment[J]. Journal of Crystal Growth 291 (2006) 196-201.
[9]KARIM K H, GUPTA S K. Biotransformation of nitrophenols in up flow anaerobic sludge blanket reactors, Bioresource. Technology. 2001,80:179-186.
[10]ZAGGOUT F R, GHALWA N A. Removal of o-nitrophenol from water by electrochemical degradation using a lead oxide/titanium modified electrode[J]. Journal of environmental Management, 2008,86:291-296.
[11]PEDROSA V A, CODOGNOTO L, AVACA L A. Electroanalytical determination of 4-nitrophenol by square wave voltammetry on diamond electrodes[J]. Journal of the Brazilian Chemical Society,14(2003)530.
[12]BEN-MOSHE T, DROR I, BERKOWITZ B. Oxidation of organic pollutants in aqueous solutions by nanosized copper oxide catalysts. Applied Catalysis B Environmental . 2009.
[13]ZHANG D P, WU W L, LONG H Y, LIU Y C, YANG Z S. Voltammetric behavior of o-Nitrophenol and damage to DNA[J]. International Journal of Molecular Science, 2008,9:316-326.
[14]JI X X, WANG A H, ZHAO Q H. Direct growth of copper oxide films on Ti substrate for nonenzymatic glucose sensors. Biosensors and Bioelectronics, vol. 26, no. 8, pp. 3542-3548,2011.
[15]UBEROI V, BHATTACHARYA S K. Toxicity and degradability of nitrophenols in anaerobic systems, Water Environment Research, 1997,69:146.
[16]GUXTONG V G, GREESTOCKC R L, et al. Critical review of rate constants for reactions of hydrated electrons,hydrogen atoms and hydroxyl radicals (.OH/.O-)in aqueous solution. The Journal of Physical Chemistry C (ACS Publications), 1988.
Abstract: The composition of volatiles emitted from Newhall nucellar navel oranges were investigated during laboratory-controlled anaerobic storage for a period of 90 days, using preconcentrator coupled with gas chromatography-mass spectrum (GC-MS). Major relative changes occurred for oxygenated volatiles and monoterpene hydrocarbons, while no observable changes were found for sulfides. Before storage, terpenoid hydrocarbons and oxygenated volatiles dominated, with the most abundance of limonene, ethanol, β-myrcene, sabinene, α-pinene, acetaldehyde and Δ-3-carene. During the early 6 days of storage, terpenoid hydrocarbons decreased sharply as much loss of limonene, β-myrcene, sabinene, α-pinene and Δ-3-carene, while oxygenated volatiles increased abruptly and became the first predominant class, with observable growing of methanol, ethanol and ethyl acetate. After 6 days of storage, terpenoid hydrocarbons rose up progressively with storage time, whereas oxygenated volatiles dropped down gradually until the end of the experiment. It is worth noting that twenty-four oxygenated volatiles as artifacts were formed, with predominance of 2-butanol and methyl acetate.
Key words: volatiles; compositions; newhall nucellar navel oranges; anaerobic storage
Clssification No: TS207.3Document Code: APaper No:1001-2443(2016)04-0364-07
Newhall nucellar navel oranges (Citrus sinensis (L.) Osbeck) originating as a limb sport of Washington navel oranges are native to USA and now widely planted in middle part of China. The fruit of Newhall is oval-shaped, 6.48-7.18cm in long and 6.65-7.71cm in diameter, and it weights approximately from 250 to 350 g for each orange, containing 15.5% for soluble solids, 85-105g L-1for sugars, 10-11g L-1for titratable acids and 0.47-0.64g L-1for vitamin C, respectively[1].
Newhall nucellar navel oranges are the most popular navel orange cultivar in China because of their bright color, rich nutrition, sweet flavor and pleasant smelling. The attractive and pleasant flavor of citrus fruit is attributed to a combination of alcohols, aldehydes, ketones, esters, hydrocarbon terpenes, sulfur volatiles and so on in specific proportions[2-4]. For Newhall nucellar navel oranges, forty-two volaitle organic compounds including twenty-seven oxygenated volatiles, thirteen terpene hydrocarbons and three sulfides were identified and quantified in the gases from their fresh fruit in previous studies[5].
During their commercial packing and storage, citrus fruit not excepting Newhall nucellar navel oranges are usually exposed under various anaerobic conditions such as storing in modified or controlled atmospheres, coating with waxes or films, packing in plastic liners and holding in containers or trailers[6,7,4]. These anaerobic or
Received date:2015-10-21
Author’s brief:YANG Geng(1968-),Female,born in Tongcheng,Anhui Province, senior experimentalist.
引用格式:杨耿,张玉洁,刘书路,等.纽荷尔脐橙(Citrus sinensis (L.) Osbeck)厌氧保存过程中挥发性风味物质组成变化[J].安徽师范大学学报:自然科学版,2016,39(4):364-370.
anoxia storage and handing process can inhibit deterioration development of fruit, but anaerobic metabolism is induced, leading to decreases in aroma-active volatiles and accumulation of off-flavor volatiles such as ethanol, acetaldehyde and ethyl acetate[8,9,6,4]. The composition changes of volatiles from citrus fruit could lead to an altered balance of orange aroma away from what is considered “fresh” or desirable and thus becoming “rotten”[2-4]. Thus, measurement of the composition changes of volatiles from citrus fruit based on relative percentage may serve reliable and convenient information for quality evaluation.
Fresh Newhall nucellar navel oranges maintain their external and internal quality in regular atmospheres for only 2-3 weeks after harvest, and thus anaerobic or anoxia storage is often needed when they are to be kept for longer than 3 weeks after harvest. Consequently, in this study Newhall nucellar navel oranges were incubated under N2 atmospheres to stimulate anaerobic respiration in laboratory, and the relative compositional changes of volatiles and the possible artifacts of Newhall nucellar navel oranges were investigated during storage under anaerobic conditions for a period of 90 days.
1Materials and Methods
1.1Materials and chemicals
Fresh ripe Newhall nucellar navel oranges (Citrus sinensis (L.) Osbeck) were obtained from a commercial orchard in the city of Ganzhou in China in November 2012. Fruits at ripe stage were classified as those possessing totally orange-yellow color. Fruits were carefully selected for uniformity size, color, and absence of physical damage, and were randomly divided into three groups for the anaerobic treatment.
All volatiles listed in Table 1 were purchased from Sigma-Aldrich Inc. (Saint Louis, MO, USA) and were of analytical grade.

Table 1 Compositional changes of volatiles emitted from Newhall nucellar navel oranges during the anaerobic storagea
续表1

ChemicalsCompositions(%)b0(fresh)c361020304050607080903-heptanone*ndtrtrtrtrtrtrtrtrtrtrtr2,3-butanedionetr0.20.30.60.20.1trtrtrtrtrtr3-hydroxy-2-butanone*ndndndnd0.1ndndndndndndndEstersmethylformate*ndtrtrtrtr0.10.10.10.10.10.1trethylformatetrtr0.1tr0.20.40.30.20.40.20.20.11-methylpropylformate*ndtrtrtrtrtrtrtrtrtrtrtr3-methylbutylformate*ndndndndndtrtrtrtrtrtrtrmethylacetatetr0.70.80.91.21.21.61.81.81.71.31.1ethylacetate0.59.010.810.07.26.45.24.23.94.23.83.4propylacetatetrtrtrtr0.20.20.40.40.50.40.50.4butylacetatetr0.10.1trtrtrtrtrtrtrtrtr1-methylpropylacetate*ndndndtr0.20.81.11.21.31.00.90.72-methylpropylacetate*ndtr0.10.10.10.10.10.10.10.10.10.12-methylbutylacetate*ndtr0.1trtrtrtr0.10.10.10.1tr3-methylbutylacetate*nd0.30.60.30.10.20.10.20.20.10.20.13-methyl-2-butenylacetate*ndndtrtrtrtrtrtrtrtrtrtrmethylpropionate*ndndndndnd0.10.10.20.20.20.20.1ethylpropionate*ndtrtrtr0.30.40.50.50.70.40.40.4propylpropionate*ndndndndndtrtrtrtrtrtrtr1-methylpropylpropoinate*ndndndndndtr0.10.10.20.10.10.1methylbutyratetrtrndndndndndndndtrtrtrethylbutyrate0.10.10.00.00.00.10.10.10.10.10.10.1methylisobutyrate*ndndndndndndndndndtrtrtrethylvalerate*ndndndndndtrtrtrtrtrtrtrmethylisovalerate*ndndndndndndtrndtrtrtrtrmethylhexanionatetrtrtrtrtrtrtrtrtrtrtrtrAcetals1,1’-diethoxy-ethane*ndtrtrtrtrtrtrtrtrtrtrtr2,4,6-trimethyl-1,3,5-trioxane0.2tr0.10.10.10.10.10.10.2trtrtrtotaloxygenatedvolatilesd18.174.580.277.372.371.272.071.270.859.459.649.0Terpenoidhydrocarbonsisoprenetrtrtrtrtrtrtrtrtrtrtrtrα-thujene0.1trtrtr0.10.10.10.10.10.10.20.1camphenetrtrtrtrtrtrtrtrtrtrtrtrβ-pinene0.60.10.10.10.30.30.30.30.30.40.50.4α-terpinenetrtrtr0.10.30.40.40.40.40.40.70.5l-phellandrene0.90.20.10.30.60.70.60.60.70.70.90.7terpinolene0.80.40.30.50.40.70.70.60.70.40.50.6
aTable includes all identified chemicals and percentages add to 100% for each sample.bMean percentage of individual volatile constituents from triplicate experiments.cDays of incubation.dSum of alcohols, aldehydes, ketones, esters and acetals.*Artifact volatiles. nd, not detected. tr, trace (<0.05%).
1.2Anaerobic treatment
For laboratory simulation study, about 2 kg fresh shredded Newhall nucellar navel oranges were weighed and placed in glass reactors with a volume of approximately 8 L. Treatments were tested in triplicate and incubated at room temperature (25±0.5℃) for 90 days. Pure N2gas was maintained between 40-60mL min-1per reactor, which was identified in preliminary work as sufficient to ensure that the O2containers were less than 0.5% for the anaerobic storage. When sampling, 1L Teflon sampling bags (SKC Inc., USA) was used to collect gas from the air outlet of each reactor. Volatile measurements were performed on days 0, 3, 6, 10, 20, 30, 40, 50, 60, 70, 80 and 90 during the incubation.
1.3Volatiles analysis
Volatiles were analyzed by an Entech Model 7100 Preconcentrator (Entech Instruments Inc., CA, USA) coupled with a gas chromatography/mass spectrum (GC/MS, Agilent 5973N). Detailed analysis steps were described elsewhere[10].
1.4Statistical Analysis
Statistical analysis was performed using SPSS 10.0 for Windows. A one-way ANOVA was performed to test the significant variance between the samples. A post hoc examination was conducted to test the significance using the LSD test. The significance level was set as p<0.05.
2Results and Discussion
2.1General

Fig.1 Typical chromatograms showing selected volatiles from fresh Newhall nucellar navel oranges at day 0 when fresh(A) and at day 50(B).
As presented in Fig.1, differences between the chromatogram of the fresh oranges and that of the oranges at day 50 were noticeable. Several new peaks of artifact compounds occurred at day 50. The peak identities and their relative percentages, and the artifacts identified, are listed in Table 1 according to functional classes. In total, sixty-seven volatiles were identified, among which twenty-four volatile chemicals were absent in gases of fresh Newhall nucellar navel oranges and occurred as artifacts in the following storage process, consisting of 3 alcohols(2-butanol, 2-pentanol and 2-methyl-3-buten-2-ol), 3 aldehydes(2-methypropanal, 2-methylbutanal and pentanal), 2 ketones(3-heptanone and 3-hydroxy-2-butanone), 15 esters(methyl formate, 1-methylpropyl formate, 3-methylbutyl formate, 1-methylpropyl acetate, 2-methylpropyl acetate, 2-methylbutyl acetate, 3-methylbutyl acetate, 3-methyl-2-butenyl acetate, methyl propionate, ethyl propionate, propyl propionate, 1-methylpropyl propionate, methyl isobutyrate, ethyl valerate and methyl isovalerate) and 1 acetals (1,1’-diethoxy-ethane) (Table 1 and Fig.1(B)). The concentration of total volatile chemicals gradually rose up to 2729.1μg L-1upon 90 days of storage, approximately 5 times higher than that at day 0(558.8 μg L-1)(Fig.2), and the percentage of total artifact volatiles increased with storage time and attained the peak at day 70, sharing 15.9% of total volatile chemicals released (Table 1). For volatile groups or individual volatile chemicals, their compositions changed significantly during the anaerobic storage of Newhall nucellar navel oranges.
The numbered peaks indicate compounds: 1 acetaldehyde; 2 methanol; 3 ethanol; 4 methyl acetate; 5 2-propanol; 6 2-methyl-propanal; 7 1-propanol; 8 2-butanone; 9 ethyl acetate; 10 2-butanol; 11 2-methyl-1-propanol; 12 2-methyl-butanal; 13 1-butanol; 14 ethyl propionate; 15 propyl acetate;16 3-methyl-1-butanol; 17 2-methyl-1-butanol; 18 1-methylpropyl acetate; 19 2-methylpropyl acetate; 20 2-methyl-3-buten-2-ol; 21 2,4,6-trimethyl-1,3,5-trioxane; 22 ethyl butyrate; 23 butyl acetate; 24 3-hexen-1-ol; 25 1-hexanol; 26 3-methylbutyl acetate; 27 2-methylbutyl acetate; 28 α-thujene; 29 α-pinene; 30 camphene; 31 sabinene; 32 β-pinene; 33 β-myrcene; 34 l-phellandrene; 35 Δ-3-carene; 36 α-terpinene; 37 limonene; 38 γ-terpinene; 39 terpinolene.

Fig.2 Concentrations of three volatile groups and total volatile compounds released from Newhall nucellar navel oranges during the anaerobic storage. Error bars represent the standard deviation
2.2Change in oxygenated volatiles
Fifty-one oxygenated volatiles were determined during the anaerobic storage of Newhall nucellar navel oranges(Table 1), and the concentration of total oxygenated volatiles increased rapidly to attain the maximum(1924.2μg L-1) at day 50, about 18 times higher than that at day 0(101.7 μg L-1)(Fig.2). Methanol, ethanol, 2-butanol, acetaldehyde, 2-butanone and ethyl acetate dominated and were the most important oxygenated volatiles. For the total oxygenated volatiles or major oxygenated compounds except acetaldehyde, their relative percentages showed a significant increasing trend during the anaerobic incubation. The relative percentage of total oxygenated volatiles increase from 18.1% to 49.0% upon 90 days of anaerobic storage, attaining the maximum (80.2%) at day 6. Also, oxygenated compounds were the most predominant function group after 3 days, although they were less than terpenes to some extent again after 90 days. The observably growing oxygenated volatiles were methanol (from 0.8% to 5.9%), ethanol (from 13.9% to 15.6%), 2-butanol (from 0.0% to 11.1%), 2-butanone (from>0.05% to 8.1%) and ethyl acetate (from 0.5% to 3.4%). Particular for ethanol, its ratio reached the peak (59.0%) at day 3 and it became the first abundant volatile chemicals. 2-Butanol and ethyl acetate were the third abundant volatile chemicals at day 40 and at day 3, respectively. For acetaldehyde, it decreased steadily to 0.1% at day 90, but its concentration actually increased and reached a peak at day 50 during the anaerobic storage.
The relative changes of the oxygenated compounds from Newhall nucellar navel oranges on the anaerobic storage were in accordance with reports on commercial packing and storage of navel oranges[7]. The considerable enhancements of the oxygenated compounds could be related to secondary metabolites of orange substrates from biochemical reaction caused by enzymes[11]or microorganisms[12,13]. The production of alcohols, aldehydes, ketones and esters from fruit under anoxic or anaerobic conditions had been reported[9,6]. For example, methanol, ethanol, acetaldehyde and ethyl acetate as anaerobic metabolites were reported to be strongly accumulated in fruit such as mandarin[14], grapefruit[14]and pear[15]on storage under conditions favoring anaerobiosis. As well known, ethanol as major component of wines is produced from anaerobic fermentation of substrates, and its biosynthesis in fruit such as apples enhanced at greater rate under hypoxic or anoxic storage conditions[9]. 2-Butanol as undirable constituent was found in spirits of grape pomace, which fermented under anaerobic conditions[16]. 2-Butanone had been reported in gases purged and trapped from cherry fruit homogenates after storage under controlled atmosphere (anoxic condition)[8]. The production of esters in fruit could be attributed to esterification of various alcohol moieties and acetyl CoA[9]. Actually, a good correlation were between total alcohols and total esters (r=0.77,p<0.01), particularly between ethanol and ethyl acetate (r=0.90,p<0.01). As also reported by[8], qualitative and quantitative changes in ester production, particularly ethyl acetate, were coincident with the accumulation of ethanol. Acetals were usually produced during the anaerobic fermentation of fruit and grain. For example, 1,1’-diethoxy-ethane was present in grape wine[17]and Chinese ‘Yanghe Daqu’ liquor, which was made from the anaerobic fermentation of grains[18].
2.3Change in isoprene and monoterpenes
As shown in Table 1, isoprene was merely a trivial constituent detected in emitted volatiles and was under 0.05% during the whole anaerobic storage. β-Myrcene, sabinene, α-pinene and Δ-3-carene were the major monoterpenes in addition to limonene during the anaerobic storage of Newhall nucellar navel oranges. During the early 6 days, the concentration of total monoterpenes decreased sharply from 457.1μg L-1to 288.6μg L-1(Fig.2). Also, the relative percentages of total monoterpenes decreased abruptly from 81.9% to 19.8%, as much loss of limonene (from 54.9% to 15.8%), β-myrcene (from 10.8% to 1.8%), sabinene (from 6.6% to 0.8%), α-pinene (from 5.0% to 0.4%) and Δ-3-carene (from 2.0% to 0.3%). Some slight dropping also occurred for α-thujene (from 0.1% to <0.05%), β-pinene (from 0.6% to 0.1%), l-phellandrene (from 0.9% to 0.1%) and terpinolene (from 0.8% to 0.3%). The decrease of monoterpene hydrocarbons from Newhall nucellar navel oranges during the early stage was in accordance with reports on storage of some citrus fruit oil such as Yuzu[19], and Daidai[20]. The monoterpene hydrocarbons can be lost through chemical process and/or physical process. Chemical degradation such as polymerization, oxidation and rearrangement of monoterpene was reported by[19]. For example, limonene could be oxidized to cis- and trans-limonene oxides as artifacts in the citrus fruit oil[12]and also be cyclized to camphene, α-pinene and β-pinene as previously noted[21]. Mycrene could be cyclized to γ-terpinene, α-terpinene, limonene and terpinolene[21]. This process would imply an increase of some monterpenes such as α-pinene and β-pinene and terpinolene, which was inconsistent with the data in this study. For this reason, we assume that this process can be neglible for the loss of monoterpenes. Physical evaporation of inherited constituents could be mainly responsible for the observed relative decrease of monnoterpnes. Oranges as a pool of monoterpenes were shredded before incubation, making these compounds not to be locked in clumps but volatilize rapidly due to increased surface area. For camphene, α-terpinene and γ-terpinene, their percentages were merely trivial and kept steady during the early 6 days of anaerobic incubation, but their concentrations had a decreasing trend.
After the early 6 days of incubation, the concentration of total terpenes increased progressively to reach the peak upon 90 days (1391.9μg L-1), about three times higher than that at day 0 (457.1μg L-1) (Fig.2). The relative percentages of total monoterpenes and all monoterpene hydrocarbons except camphene also grew steadily until the end of the experiment. Monoterpenes shared 51.0% of total volatiles released and became the prevailed class upon 90 day again. The preminently enhanced monoterpenes were limonene (from 15.8% to 36.0%), β-myrcene (from 1.8% to 6.6%), α-pinene (from 0.4% to 2.9%) and Δ-3-carene (from 0.3% to 1.4%). Some slight growing also occurred for α-thujene (from <0.05% to 0.1%), β-pinene (from 0.1% to 0.4%), l-phellandrene (from 0.1 to 0.7%), α-terpinene (from <0.05 to 0.5%), γ-terpinene (from 0.2% to 0.8%), terpinolene (from 0.3% to 0.6%) and sabinene (from 0.8% to 1.0%). For camphene, its percentage still kept trivial and steady. The results indicated that monoterpenes emitted after 6 days were mainly secondary production, most probably the microbial degradation of pectin, which had high contents in oranges and would emit a high rate of monoterpenes when biologically metabolized.
References:
[1]CHEN J. Newhall Navel Oranges[M]. Beijing: Jindun Press. (In Chinese). 2006:1-12.
[2]BRAT P, REGA B, ALTER P, REYNES M, BRILLOUET J-M. Distribution of volatile compounds in the pulp, cloud, and serum of freshly squeezed orange juice[J]. Journal of Agricultural & Food Chemistry, 2003,51:3442-3447.
[3]ROUSEFF R L, PEREZ-CACHO P R, JABALPURWALA F. Historical review of citrus flavor research during the past 100 years[J]. Journal of Agricultural & Food Chemistry, 2009,57:8115-8124.
[4]TIETEL Z, PLOTTO A, FALLIK E, LEWINSOHN E, PORAT R. Taste and aroma of fresh and stored mandarins[J]. Journal of the Science of Food and Agriculture, 2011,91:14-23.
[5]WANG X M, WU T. Chemical composition analysis of volatile components in Newhall navel oranges[J]. Food Science, 2013,34(06),160-163.(In Chinese)
[6]PESIS E. The role of the anaerobic metabolites, acetaldehyde and ethanol, in fruit ripening, enhancement of fruit quality and fruit deterioration[J]. Postharvest Biology and Technology, 2015,37:1-19.
[7]OBENLAND D, COLLIN S, SIEVERT J, et al. Commercial packing and storage of navel oranges alters aroma volatiles and reduces flavor quality[J]. Postharvest Biology and Technology, 2008,47:159-167.
[8]MATTHEIS J P, BUCHANAN D A, FELLMAN J K. Volatile constituents of Bing sweet cherry fruit following controlled atmosphere storage[J]. Journal of Agricultural & Food Chemistry, 1997,45:212-216.
[9]RUDELL D R, MATTINSON D S, MATTHEIS J P, et al. Investigations of aroma volatile biosynthesis under anoxic conditions and in different tissues of “Redchief Delicious” apple fruit (Malus domestica Borkh.). Journal of Agricultural & Food Chemistry, 2002,50:2627-2632.
[10]WANG X M, WU T. Release of isoprene and monoterpenes during the aerobic decomposition of orange wastes from laboratory incubation experiments[J]. Environmental Science & Technology, 2008,42:3265-3270.
[11]PETERSON D, REINECCIUS G A. Biological Pathways for the Formation of Oxygen-Containg Aroma Compounds[M]//In: Reineccius G A, Reineccius T A. Eds., Heteroatomic Aroma Compounds Washington: American Chemical Society, 2002:227-242.
[12]PEREZ-CACHO P R, ROUSEFF R L. Fresh squeezed orange juice odor: A Review[J]. Critical Reviews in Food Science and Nutrition, 2008,48:681-695.
[13]ARREBOLA E, SIVAKUMAR D, KORSTEN L. Effect of volatile compounds produced by Bacillus strains on postharvest decay in citrus[J]. Biological Control, 2010,53:122-128.
[14]SHI J X, PORAT R, GOREN R, GOLDSCHMIDT E E. Physiological responses of ‘Murcott’mandarins and ‘Star Ruby’ grapefruit to anaerobic stress conditions and their relation to fruit taste, quality and emission of off-flavor volatiles[J]. Postharvest Biology and Technology, 2005,38:99-105.
[15]MATTHEIS J P, RUDELL D. Responses of ‘d’Anjou’ pear (Pyrus communis L.) fruit to storage at low oxygen setpoints determined by monitoring fruit chlorophyll fluorescence[J]. Postharvest Biology and Technology, 2011,60:125-129.
[16]SILVA M L, MALCATA F X. Relationships between storage conditions of grape pomace and volatile composition of spirits obtained therefrom[J]. American Journal of Enology and Viticulture, 1998,49:56-63.
[17]LEE S-J, NOBLE A C. Characterization of odor-active compounds in californian chardonnay wines using GC-olfactometry and GC-mass spectrometry[J]. Journal of Agricultural & Food Chemistry, 2003,51:8036-8044.
[18]FAN W L, QIAN M C. Identification of aroma compounds in Chinese ‘Yanghe Daqu’ liquor by normal phase chromatography fractionation followed by gas chromatography/olfactometry[J]. Flavour and Fragrance Journal, 2006,21:333-342.
[19]NJOROGE S M, UKEDA H, SAWAMURA M. Changes in the volatile composition of Yuzu (Citrus junos Tanaka) cold-pressed oil during storage[J]. Journal of Agricultural & Food Chemistry, 1996,44:550-556.
[20]NJOROGE S M, UKEDA H, SAWAMURA M. Changes of the volatile profile and artifact formation in Daidai (Citrus aurantium) cold-pressed peel oil on storage[J]. Journal of Agricultural & Food Chemistry, 2003,51:4029-4035.
[21]DIECKMANN R H, PALAMAND S R. Autoxidation of some constituents of hops. I. The monoterpene hydrocarbon, myrcene[J]. Journal of Agricultural & Food Chemistry, 1974,22:498-503.
纽荷尔脐橙(Citrus sinensis (L.) Osbeck)厌氧保存过程中挥发性风味物质组成变化
杨耿,张玉洁,刘书路,于越刚
(安徽师范大学 环境科学与工程学院,安徽 芜湖241003)
摘要:采用预浓缩系统与气相色谱质谱联用技术分析检测纽荷尔脐橙在实验室控制厌氧条件下保存90天过程中释放出来的挥发性风味物质组成变化.结果表明,纽荷尔脐橙厌氧保存过程中含氧化合物和萜烯化合物两类挥发性风味物质组成比例变化明显,含硫化合物变化不明显.在尚未保存前,纽荷尔脐橙释放出来的挥发性风味物质主要是含氧化合物和萜烯化合物两类化合物,其中柠檬烯、乙醇、β-月桂烯、桧烯、α-蒎烯,乙醛和蒈烯是最主要的成分.在保存的前6天,由于柠檬烯、β-月桂烯、桧烯、α-蒎烯和蒈烯5种化合物大量减少导致萜烯类化合物比例随时间急剧下降,同时由于甲醇、乙醇和乙酸乙酯3种化合物大量增加使得含氧化合物比例随时间急剧升高,成为最主要的挥发性风味物质.在保存6天以后到实验结束,萜烯类化合物比例随时间逐渐增高,而含氧化合物比列随时间逐渐降低.特别值得注意的是纽荷尔脐橙保存过程中有24种含氧化合物是新生成的,其中最主要是2-丁醇和乙酸甲酯.
关键词:挥发性风味物质;纽荷尔脐橙;厌氧保存
DOI:10.14182/J.cnki.1001-2443.2016.04.009 10.14182/J.cnki.1001-2443.2016.04.011
收稿日期:2015-11-20
基金项目:国家自然科学基金(20775002).
作者简介:徐白露(1991-),女,安徽繁昌县人,硕士生;通讯作者:杨周生(1963-),安徽桐城人,教授.
中图分类号:X132
文献标志码:A
文章编号:1001-2443(2016)04-355-04
Foundation item:Sponsored by National Natural Science Foundation of China(41273095 and 41103067).
Electrochemical Behavior of P-Nitrophenol on Nano Copper Oxide Electrode and Its Degradation
XU Bai-lu,ZHANG Huan,YANG Zhou-sheng
(College of Environmental Science and Engineering, Anhui Normal University, Wuhu 241000, China)
Abstract:Electrochemical behavior of p-nitrophenol on nano copper oxide electrode and its degradation were discussed. The effects of solution pH and thickness of material on electrochemical response of p-nitrophenol were examined. The results show that the difference (ΔE) of peak potential changed from 0.862V on bare GCE to 0.291V on CuO/PTH/GCE and the oxidation peak current increased obviously. Nano copper oxide could improve redox reversibility p-nitrophenol on electrode significantly. Electrochemical oxidation of p-nitrophenol was performed with nano CuO/Ti electrode and electrolyte solution was tested by UV-visible spectra and high performance liquid chromatography, respectively. It illustrated the nano copper oxide could catalyze degradation of p-nitrophenol.
Key words:nano copper oxide; p-nitrophenol; catalyses degradation
Changes in the Composition of Volatiles Emitted from Newhall Nucellar Navel Oranges (Citrus Sinensis (L.) Osbeck)During Anaerobic Storage
YANG Geng,ZHANG Yu-jie,LIU Shu-lu,YU Yue-gang
(College of Environmental Sciences and Engineering, Anhui Normal University, Wuhu 241003, China)
引用格式:徐白露,张唤,杨周生.纳米氧化铜对硝基苯酚的电化学氧化及降解作用[J].安徽师范大学学报:自然科学版,2016,39(4):355-358.

