黄嘴白鹭初雏性比与窝卵数之间的变化规律
雷 威,林清贤,陈小麟
(厦门大学 环境与生态学院,滨海湿地生态系统教育部重点实验室,福建厦门361102)
黄嘴白鹭初雏性比与窝卵数之间的变化规律
雷威,林清贤,陈小麟*
(厦门大学 环境与生态学院,滨海湿地生态系统教育部重点实验室,福建厦门361102)
摘要:采用性别鉴定分子技术研究易危物种黄嘴白鹭(Egretta eulophotes)的初雏性别比例(性比),探讨晚成鸟初雏性比与窝卵数之间的变化规律.在种群水平上,黄嘴白鹭初雏性比没有显著的雌雄偏向(47.37%雄性,p=0.37);平均窝卵数为3.71±0.09(n=109),其中,拥有4枚卵的巢最常见(57.80%).在巢水平上,初雏性比随窝卵数而变化(p=0.003);拥有3枚卵的巢的初雏性比具有显著的雄性偏向(69.23%雄性,p=0.02),而拥有4枚卵的巢无显著的性别偏向(44.23%雄性,p=0.17),拥有5枚卵的巢则有显著的雌性偏向(31.43%雄性,p=0.04);拥有3枚卵的巢的初雏性比显著大于拥有4枚卵的巢(p=0.005)及5枚卵的巢(p=0.001),而初雏性比在拥有4枚卵的巢与拥有5枚卵的巢之间无显著差异(p=0.17).综上可见,黄嘴白鹭具有晚成鸟特有的性别分配机制,即窝卵数的变化能调整初雏性比.
关键词:初雏性比;窝卵数;黄嘴白鹭;性双态;鹭科鸟类
性别比例(性比)是影响种群数量动态变化的重要参数[1-3].鸟类后代性比可分为初雏性比(hatching sex ratio)和离巢雏鸟性比(fledging sex ratio)[4].Fisher假说认为,理论上亲代对子女的抚育投入是相同的,且绝大部分有性繁殖物种性比接近1∶1;然而,在自然选择作用下,后代性比总是偏向有较高繁殖成效的性别[5-6].越来越多的鸟类学研究证实,雌亲能够调整后代性比以适应社会因素或生态条件的变化,这些因素包括雄性对雌性的吸引力[7-10]、雄性婚外配行为[9,11]、不同性别配偶的扩散能力[12]、不同性别配偶的食物资源多寡[9,13-15]等.Øigarden 和 Lifjeld提出的性别分配机制认为,晚成鸟类的雌亲能根据窝卵数的多寡适应性地调整后代性别组成[16].
黄嘴白鹭(Egrettaeulophotes,Chinese egret)隶属鹳形目(Ciconiiformes)鹭科(Ardeidae),其雏鸟为晚成性.黄嘴白鹭在1860年由英国人Swinhoe发现于厦门,目前全球种群数量只有2 600~3 400只,世界自然保护联盟(IUCN)将其列入易危物种[17-18].近年来,随着人们对濒危动物保护的关注,关于黄嘴白鹭的研究报道逐渐增多.Wei等[19]发现黄嘴白鹭雄鸟在繁殖季节有较高频率的婚外配行为;Fang等[20]分析了黄嘴白鹭雏鸟的食性;微卫星位点(SSR)、线粒体DNA(mtDNA)及主要组织相容性复合体(MHC)基因等分子标记逐渐应用于黄嘴白鹭种群遗传学研究[21-25];黄嘴白鹭性别及物种鉴定的分子方法也已成功建立[1-2,26].然而,对黄嘴白鹭乃至鹭科鸟类的性比研究至今却未见报道.
本研究主要内容:1) 调查黄嘴白鹭种群初雏性比;2) 探讨黄嘴白鹭初雏性比与窝卵数之间的变化规律;3) 通过比较卵体积及成鸟体尺的雌雄差异,评估雌亲对雌雄后代抚育投入的差异,探讨晚成鸟基于窝卵数变化的性别分配机制是否适用于黄嘴白鹭.
1材料与方法
1.1材料
黄嘴白鹭样品采自辽宁省大连市无居民海岛形人坨(39.52°N,123.05°E)及福建省厦门大屿岛白鹭自然保护区(24.45°N,118.03°E).组织或羽毛样品均采自形人坨:背部羽毛采自5~10日龄的雏鸟,组织样品采自野外死亡个体或未成功孵化的胚胎,并置于95%(体积分数)乙醇中-20 ℃保存;大屿岛成鸟血样使用肱骨下静脉抽取方法采集,并置于1%(质量分数) 乙二胺四乙酸(EDTA)中-20 ℃保存.基因组DNA提取试剂盒购自宝生物工程(大连)有限公司.
1.2方法
1.2.1野外调查
在2012年5—7月,每日搜寻并记录形人坨黄嘴白鹭巢、卵及雏鸟,标记新发现的巢及卵,环志新出壳的雏鸟.随机选取70枚容易获得的卵(取自70个不同的巢),用精确度为0.02 mm的游标卡尺测量卵的长、短径.为比较黄嘴白鹭雌雄成鸟体尺的差异,用精度为0.1 g的电子秤对大屿岛上随机选取的40只成鸟称质量,并用精确度为0.1 mm的皮尺测量其体长、嘴峰长、翅长及跗蹠长.野外调查时间均限于每天早晨的6:00—8:00内,完成调查取样后,将卵、雏鸟及成鸟及时放归原处,以减少人为干扰的伤害.
1.2.2物种与性别鉴定
使用基因组DNA提取试剂盒提取羽毛、血液及组织样品的基因组DNA(方法参见说明书).由于黄嘴白鹭经常与白鹭(E.garzetta)混群营巢[25-26],本研究首先利用Huang等[26]的方法对样本进行物种鉴定,以排除白鹭对实验结果可能造成的干扰,再利用Wang等[2]的方法对样本进行性别鉴定.
1.2.3数据分析
依据计算公式V=LW2π/6估算卵体积大小,其中V为卵体积,L为卵长径,W为卵短径[27].性比指雄性个体所占的比例.数据采用SPSS 17.0软件进行分析:利用二项式检验检测性比是否显著偏离1∶1,利用柯尔莫可洛夫-斯米洛夫检验检测数据的正态分布,利用独立样本t-检验比较各组平均值之间的差异,利用卡方检验比较各组比率值之间的差异.数据表示为平均值±标准差,显著水平设定为p<0.05.
2结果与分析
2.1种群初雏性比
在形人坨黄嘴白鹭繁殖种群,总共发现109个巢,404枚卵,窝卵数为3.71±0.09(n=109),其中拥有4枚卵的巢最为常见,占总巢数的57.80%(图1).在404枚卵中,最终能提供有效基因组DNA以供性别鉴定的卵数为323枚(包括出壳雏鸟及未成功孵化的胚胎),包括153枚雄性及170枚雌性,初雏性比没有显著的性别偏向(47.37%雄性,p=0.37).

图1 黄嘴白鹭繁殖种群窝卵数的分布频率Fig.1The distribution of clutch sizes in the Chinese egret
种群初雏性比观测值与取样样本量大小的变化关系如图2所示.当样本量达到170只(48个巢)时,种群初雏性比观测值趋于稳定,表明本研究基于323只雏鸟(109个巢)的种群初雏性比具有代表性.
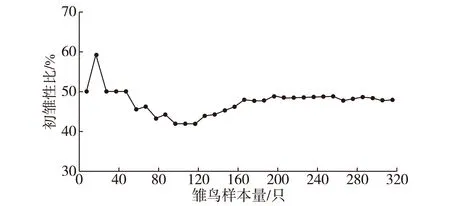
图2 种群初雏性比观测值随样本量的变化趋势Fig.2Hatching sex ratios of the Chinese egret plotted against numbers of hatchlings analyzed
在109个巢中,有59个巢共230枚卵或雏鸟获得完整取样,而另外50个巢则由于部分卵或雏鸟丢失,或无法提供有效基因组DNA导致最终取样不全.基于这59个巢共230枚卵或雏鸟得到的窝卵数(3.90±0.08;t=-1.38,df=166,p=0.17),拥有4枚卵的巢占比(66.10%;χ2=0.04,df=1,p=0.84)及初雏性比(46.52%雄性;χ2=1.11,df=1,p=0.29)与基于109个巢得到的数值均无显著差异.
2.2初雏性比与窝卵数之间的变化规律
59个完整取样的巢包括13个拥有3枚卵的巢、39个拥有4枚卵的巢及7个拥有5枚卵的巢.在巢水平上,初雏性比随窝卵数的不同而变化(χ2= 11.62,df=2,p=0.003).拥有3枚卵的巢的初雏性比有显著的雄性偏向(69.23%雄性,p=0.02),而拥有4枚卵的巢无明显的性别偏向(44.23%雄性,p=0.17),拥有5枚卵的巢则有显著的雌性偏向(31.43%雄性,p=0.04).拥有3枚卵的巢的初雏性比显著大于拥有4枚卵的巢(χ2= 7.80,df=1,p=0.005)及5枚卵的巢(χ2= 10.55,df=1,p=0.001),而初雏性比在拥有4枚卵的巢与5枚卵的巢之间则无显著差别(χ2= 1.93,df=1,p=0.17)(图3).

U表示无明显性别偏向,没有相同字母标注的 表示差异显著(p<0.05).图3 拥有不同窝卵数的巢的初雏性比差异比较Fig.3Hatching sex ratios varied with clutch sizes in the Chinese egret
表1 不同性别卵体积大小及成鸟体尺差异比较
Tab.1 Size differences of egg volume or adult body between different sexes in the Chinese egret
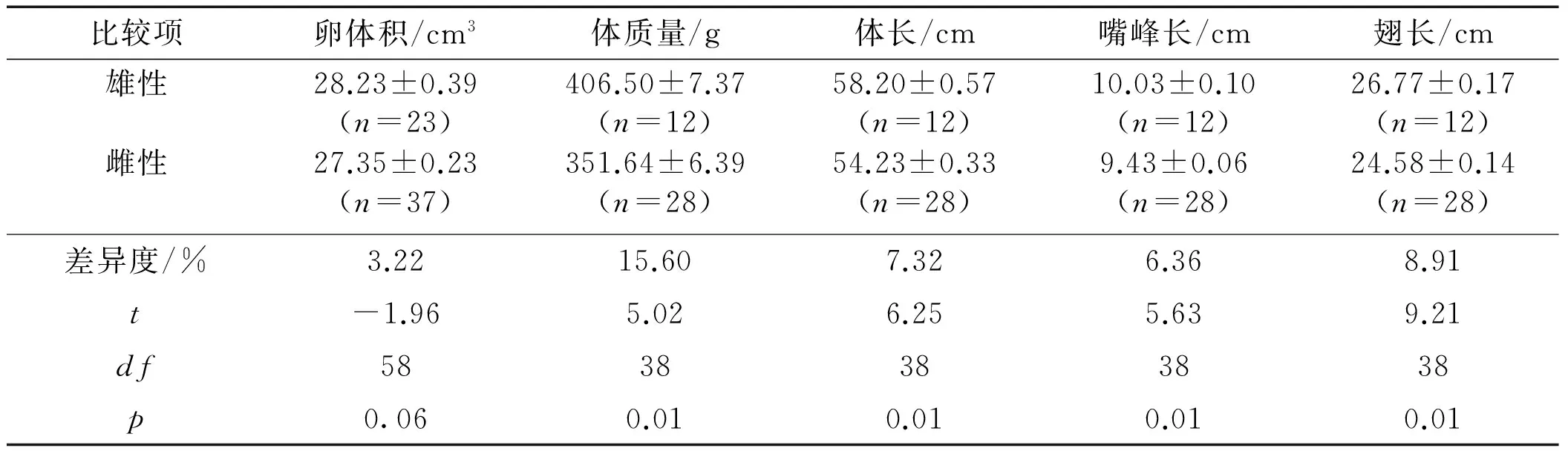
比较项卵体积/cm3体质量/g体长/cm嘴峰长/cm翅长/cm跗蹠长/cm雄性28.23±0.39(n=23)406.50±7.37(n=12)58.20±0.57(n=12)10.03±0.10(n=12)26.77±0.17(n=12)5.67±0.07(n=12)雌性27.35±0.23(n=37)351.64±6.39(n=28)54.23±0.33(n=28)9.43±0.06(n=28)24.58±0.14(n=28)5.26±0.04(n=28)差异度/%3.2215.607.326.368.917.79t-1.965.026.255.639.215.46df583838383838p0.060.010.010.010.010.01
注:差异度指雌雄之间测量值的差值占雌性测量值的百分比[16];t,df,p基于独立样本t-检验.
2.3卵体积及成鸟体尺的雌雄差异
随机选取的70枚卵的长、短径分别为(46.12±0.18),(33.85±0.09) mm,其中有60枚卵最终能提供有效基因组DNA并用于后续研究,而另外10枚卵丢失或无法提供有效基因组DNA.基于这60枚卵比较雌雄卵体积大小差异,雄性卵体积((28.23±0.39) cm3,n=23)比雌性((27.35±0.23) cm3,n=37)大3.22%(p=0.06);随机选取的40只黄嘴白鹭成鸟包括12只雄性及28只雌性,成鸟性比有显著的雌性偏向(30.00%雄性,p=0.02);雄鸟体质量、体长、嘴峰长、翅长及跗蹠长比雌鸟分别大15.60%,7.32%,6.36%,8.91%及7.79%(表1).
3讨论
黄嘴白鹭种群的初雏性比无明显性别偏向,其平均窝卵数(3.71枚)与最常见窝卵数(4枚)相近,且在拥有4枚卵的巢中,其初雏性比也无明显性别偏向.Lack窝卵数假说认为,鸟类在拥有最佳窝卵数时的繁殖成效达到最高,而最佳窝卵数近似于最常见窝卵数[28-29].可见,黄嘴白鹭窝卵数在4枚时,其后代性别比例达到了最优化,繁殖成效也最高.黄嘴白鹭种群的初雏性比观测值受样本量大小影响,当样本量达到170只雏鸟(48个巢)时,观测值能准确地代表种群初雏性比的真实值.这与Johnson等[11]的观点一致,即要对某个种群性别比例进行可信的估算,样本量应超过150只个体(30个巢).
黄嘴白鹭的窝卵数变化能够调整初雏性比,这种性别分配机制与雌雄个体需要的抚育投入差异有关,黄嘴白鹭雄性个体所需要的抚育投入大于雌性.尽管其雄性与雌性的卵体积之间无显著差异,但是前者略大于后者,提示雄性在胚胎发育过程中对营养及能量的需求可能大于雌性[30-31];而雄性成鸟体尺显著大于雌性成鸟,也说明亲代对雄性个体的抚育投入大于雌性[16,32].黄嘴白鹭初雏性比随窝卵数的不同而出现规律性的变化.本研究发现,拥有4枚卵的巢无明显性别偏向,拥有3枚卵的巢具有显著的雄性偏向,而拥有5枚卵的巢则有显著的雌性偏向.这一现象可由Trivers-Willard假说得到解释:在有利于繁殖成效的进化适应上,当食物资源充裕时,亲代优先繁育较强壮、生长快的雄性后代;反之,当食物比较匮乏时,则繁育更多的雌性后代.显然,雄性后代的抚育需要投入较大,食物资源充裕时才更有可能存活;食物匮乏时,繁育更多的雄性后代则导致竞争更激烈,这种进化适应有利于提高后代的繁殖成效[6,9,13-15,33].由于黄嘴白鹭雏鸟食物单一,主要来源于滨海湿地[20],窝卵数较大势必会造成同巢雏鸟的食物相对匮乏,竞争相对激烈.在拥有5枚卵的巢中,相比于拥有3枚卵或4枚卵的巢中,其后代食物相对比较匮乏,亲代适应性地繁育更多雌性后代,这种繁殖策略有利于保证后代对食物资源的需求;反之,在拥有3枚卵的巢中,由于后代食物相对比较充裕,亲代则繁育较多的雄性后代.近年来,在对其他晚成鸟,如河乌(Cincluscinclus)和红隼(Falcotinnunculus)进行性比研究时也发现,当食物资源比较匮乏时,在拥有较大窝卵数的巢中,后代性比会出现明显雌性偏向[16,34].
本研究分析了黄嘴白鹭初雏性比与窝卵数之间的变化规律,证实晚成鸟基于窝卵数变化的性别分配机制适用于黄嘴白鹭,该结果为今后深入阐明鹭科鸟类性别分配机制奠定了基础.本研究初步获得黄嘴白鹭成鸟性比具有显著的雌性偏向,然而,该结果仅基于40只个体,远低于黄嘴白鹭种群性比可信估算样本量(170只),因此,该雌性偏向是否具备种群的代表性仍有待于今后加大样本量的进一步检验.
参考文献:
[1]HUANG X,ZHOU X,LIN Q,et al.An efficient molecular sexing of the vulnerable Chinese egretEgrettaeulophotesfrom faeces samples[J].Conservation Genetics Resources,2012,4(2):391-393.
[2]WANG Z,ZHOU X,LIN Q,et al.New primers for sex identification in the Chinese egret and other ardeid species[J].Molecular Ecology Resources,2011,11(1):176-179.
[3]WOOLAVER L G,NICHOLS R K,MORTON E,et al.Nestling sex ratio in a critically endangered dimorphic raptor,Ridgway′s HawkButeoridgwayi[J].Journal of Raptor Research,2013,47(2):117-126.
[4]SZÉKELY T,LIKER A,FRECKLETON R P,et al.Sex-biased survival predicts adult sex ratio variation in wild birds[J].Proceedings of the Royal Society B:Biological Sciences,2014,281(1788):20140342.
[5]FISHER R A.The genetical theory of natural selection[M].Oxford:Clarendon Press,1930:1-356.
[6]TRIVERS R L,WILLARD D E.Natural selection of parental ability to vary the sex ratio of offspring[J].Science,1973,179(4068):90-92.
[7]ADDISON B,KITAYSKY A S,HIPFNER J M.Sex allocation in a monomorphic seabird with a single-egg clutch:test of the environment,mate quality,and female condition hypotheses[J].Behavioral Ecology and Socio-biology,2008,63(1):135-141.
[8]DELMORE K E,KLEVEN O,LASKEMOEN T,et al.Sex allocation and parental quality in Tree Swallows[J].Behavioral Ecology,2008,19(6):1243-1249.
[9]DREISS A,RICHARD M,MOYEN F,et al.Sex ratio and male sexual characters in a population of blue titsParuscaeruleus[J].Behavioral Ecology,2006,17(1):13-19.
[10]PRYKE S R,GRIFFITH S C.Genetic incompatibility drives sex allocation and maternal investment in a polymorphic finch[J].Science,2009,323(5921):1605-1607.
[11]JOHNSON L S,THOMPSON C F,SAKALUK S K,et al.Extra-pair young in house wren broods are more likely to be male than female[J].Proceedings of the Royal Society B:Biological Sciences,2009,276(1665):2285-2289.
[12]ROMANO A,AMBROSINI R,CAPRIOLI M,et al.Secondary sex ratio covaries with demographic trends and ecological conditions in the barn swallow[J].Evolutionary Ecology,2012,26(4):1041-1053.
[13]BRADBURY R R,BLAKEY J K.Diet,maternal condition,and offspring sex ratio in the Zebra FinchPoephilaguttata[J].Proceedings of The Royal Society B:Biological Sciences,1998,265(1399):895-899.
[14]KILNER R.Primary and secondary sex ratio manipulation by Zebra Finches[J].Animal Behaviour,1998,56(1):155-164.
[15]PRYKE S R,ROLLINS L A.Mothers adjust offspring sex to match the quality of the rearing environment[J].Proceedings of the Royal Society B:Biological Sciences,2012,279(1744):4051-4057.
[16]ØIGARDEN T,LIFJELD J T.Primary sex ratios vary with clutch size in the size-dimorphic White-throated DipperCincluscinclus[J].Journal of Ornithology,2013,154(1):91-97.
[17]BirdLife International.Species factsheet:Egrettaeulophotes[EB/OL].[2015-09-01].http:∥www.birdlife.org/.
[18]IUCN.IUCN red list of threatened species[EB/OL].[2015-09-01].http:∥www.iucnredlist.org/.
[19]WEI G,YIN Z,LEI F.Copulations and mate guarding of the Chinese egret[J].Waterbirds,2005,28(4):527-530.
[20]FANG W,LIN Q,CHEN X,et al.Nestling diet of the vulnerable Chinese egret on offshore islands in southern China[J].Waterbirds,2011,34(2):246-251.
[21]DAI Y,ZHOU X,FANG W,et al.Development and cross-species transferability of 23 microsatellite markers from the vulnerable Chinese egret (Egrettaeulophotes) using 454 sequencing[J].Conservation Genetics Resources,2013,5(4):1035-1038.
[22]LEI W,FANG W,LIN Q,et al.Characterization of a non-classical MHC class Ⅱ gene in the vulnerable Chinese egret (Egrettaeulophotes)[J].Immunogenetics,2015,67(8):463-472.
[23]LI L,ZHOU X,CHEN X.Characterization and evolution of MHC class Ⅱ B genes in ardeid birds[J].Journal of Molecular Evolution,2011,72(5/6):474-483.
[24]WANG Z,ZHOU X,LIN Q,et al.Characterization,polymorphism and selection of major histocompatibility complex (MHC)DABgenes in vulnerable Chinese egret (Egrettaeulophotes)[J].PLoS One,2013,8(9):e74185.
[25]ZHOU X,FANG W,CHEN X.Mitochondrial DNA diversity of the vulnerable Chinese egretEgrettaeulophotesfrom China[J].Journal of Ornithology,2010,151(2):409-414.
[26]HUANG X,ZHOU X,LIN Q,et al.PCR-RFLP technique for species identification of molted feathers in six species of co-occurring Ardeids[J].Conservation Genetics Resources,2013,5(3):817-819.
[27]HOYT D F.Practical methods of estimating volume and fresh weight of bird eggs[J].Auk,1979,96(1):73-77.
[28]CHARNOV E L,KREBS J R.On clutch-size and fitness[J].Ibis,1974,116(2):217-219.
[29]LACK D.The natural regulation of animal numbers[M].Oxford:Clarendon Press,1954:1:343.
[30]CORDERO P J,GRIFFITHS S C,APARICIO J M,et al.Sexual dimorphism in house sparrow eggs[J].Beha-vioral Ecology and Sociobiology,2000,48(5):353-357.
[31]MEAD P S,MORTON M L,FISH B E.Sexual dimorphism in egg size and implications regarding facultative manipulation of sex in mountain white-crowned sparrows[J].The Condor,1987,89(4):798-803.
[32]BORTOLOTTI G R.Influence of sibling competition on nestling sex ratios of sexually dimorphic birds[J].Ame-rican Naturalist,1986,127(4):495-507.
[33]CHARNOV E L.The theory of sex allocation[M].Princeton:Princeton University Press,1982:1-355.
[34]WU H,WANG H T,JIANG Y L,et al.Offspring sex ratio in Eurasian KestrelFalcotinnunculuswith reversed sexual size dimorphism[J].Chinese Birds,2010,1(1):36-44.
doi:10.6043/j.issn.0438-0479.201512020
收稿日期:2015-12-21录用日期:2016-02-16
基金项目:国家自然科学基金(41476113,31272333);科技部科技基础性工作专项(2015FY110200);福建省科技创新平台项目(2010Y2007)
*通信作者:xlchen@xmu.edu.cn
中图分类号:Q 958.1
文献标志码:A
文章编号:0438-0479(2016)04-0510-05
Hatching Sex Ratio Varied with Clutch Size in the Vulnerable Chinese Egret (Egretta eulophotes)
LEI Wei,LIN Qingxian,CHEN Xiaolin*
(Key Laboratory of the Coastal and Wetland Ecosystems,Ministry of Education,College of the Environment & Ecology,Xiamen University,Xiamen 361102,China)
Abstract:This study used a molecular sexing technique to investigate the hatching sex ratio in the vulnerable Chinese egret (Egretta eulophotes) for the first time,and examined the variation of hatching sex ratio in relation to clutch size in this altricial bird.The hatching sex ratio of the Chinese egret was unbiased in the whole population (47.37% males,p=0.37).In this egret,the mean clutch size was 3.71±0.09 (n=109),and four-egg occurred frequently in clutches (57.80%).However,there was a significant excess of males in three-egg clutches (69.23% males,p=0.02),whereas deficit in five-egg clutches (31.43% males,p=0.04),but no deviation from parity for four-egg clutches (44.23% males,p=0.17).The three-egg clutch category differed significantly from four-egg (p=0.005) and five-egg (p=0.001) clutch categories in hatching sex ratios,whereas the latter two clutch-size categories did not significantly differ from each other (p=0.17).These results confirm that the Chinese egret has the sex allocation mechanism specific in altricial birds,that is,the hatching sex ratio varies with clutch size.
Key words:hatching sex ratio;clutch size;Egretta eulophotes;sexually dimorphic;Ardeidae
Citation:LEI W,LIN Q X,CHEN X L.Hatching sex ratio varied with clutch size in the vulnerable Chinese egret (Egrettaeulophotes)[J].Journal of Xiamen University(Natural Science),2016,55(4):510-514.(in Chinese)

