猪带绦虫重组Bb-TSO45W-4B-TSOL18疫苗诱导仔猪免疫应答的动态观察
王灵军,刘美辰,周 泠,杨凤娇,贾 启,江 楠,周必英
猪带绦虫重组Bb-TSO45W-4B-TSOL18疫苗诱导仔猪免疫应答的动态观察
王灵军1,刘美辰1,周泠2,杨凤娇1,贾启1,江楠1,周必英1
1.遵义医学院寄生虫学教研室,遵义563000;2.遵义医学院附属医院中医肛肠科,遵义563000
摘要:目的研究猪带绦虫重组Bb-TSO45W-4B-TSOL18疫苗免疫仔猪后诱导免疫应答的动态变化。方法将16头40 d龄健康仔猪均分为4组:重组Bb-TSO45W-4B-TSOL18疫苗组、重组Bb-TSO45W-4B疫苗组、重组Bb-TSOL18疫苗组和双歧杆菌液体培养基(MRS)对照组。各疫苗组均以1011CFU灌胃免疫,间隔2周免疫1次,共免疫2次。在免疫后0、2、4、6、8周采集仔猪前腔静脉血,分离血清和外周血淋巴细胞(PBMC)。采用ELISA法检测仔猪血清IgG、IgG1及IgG2a水平和PBMC培养上清液IL-2、IFN-γ、IL-4及IL-10水平;MTT比色法检测PBMC增殖水平;FCM检测CD4+和CD8+T细胞亚群。结果各疫苗组血清IgG、IgG1、IgG2a水平均在免疫后2~8周升高,均在免疫后4周、6周、4周达较高水平;PBMC增殖水平均在免疫后2~8周升高,均在免疫后6周达较高水平;CD4+和CD8+T细胞水平均在免疫后2~8周升高,均在免疫后8周和6周达较高水平;PBMC培养上清液IL-2、IFN-γ、IL-4、IL-10水平分别在免疫后2~6周、2~8周、4~6周和2~8周升高,分别在免疫后4周、6周、4周和8周达较高水平。各疫苗组上述指标与MRS对照组比较差异均有统计学意义(P<0.05);重组Bb-TSO45W-4B-TSOL18疫苗组与重组Bb-TSO45W-4B疫苗组和重组Bb-TSOL18疫苗组比较差异均有统计学意义(P<0.05)。结论猪带绦虫重组Bb-TSO45W-4B-TSOL18疫苗免疫仔猪可诱导产生特异性的免疫应答,且TSO45W-4B-TSOL18融合抗原疫苗组优于TSO45W-4B或TSOL18单一抗原疫苗组。
关键词:猪带绦虫;重组Bb-TSO45W-4B-TSOL18疫苗;免疫应答;仔猪
Dynamic observation of immune responses induced in piglets
Supported by the National Natural Science Foundation of China (No. 81160206)
猪囊尾蚴病(Cysticercosis)俗称囊虫病,是由猪带绦虫(Taeniasolium)幼虫-囊尾蚴引起的一种危害严重的人兽共患寄生虫病。药物及手术治疗都有其局限性,有保护作用的疫苗是控制该病流行的有效手段[1]。TSO45W-4B和TSOL18是猪带绦虫六钩蚴阶段的重要候选疫苗抗原[2]。双歧杆菌(Bifidobacterium,Bb)作为载体传递系统,已在细菌、病毒、肿瘤、寄生虫等领域得到了广泛应用[3]。本研究拟在成功构建猪带绦虫重组Bb-TSO45W-4B-TSOL18疫苗[4-5]的基础上,以该疫苗免疫仔猪,动态观察其诱导的免疫应答。
1材料与方法
1.1疫苗来源猪带绦虫重组Bb-TSO45W-4B-TSOL18疫苗、重组Bb-TSO45W-4B疫苗、重组Bb-TSOL18疫苗由本室构建保存[4,6-7]。
1.2实验动物40 d龄健康仔猪16头,每头体重约15 kg,购自遵义县三盆镇养殖场,由遵义医学院实验动物中心饲养管理。1.3主要试剂与仪器纯化的猪带绦虫TSO45W-4B-TSOL18/TSO45W-4B/TSOL18重组抗原由本室制备保存[8-10];IgG、IgG1和IgG2a试剂盒(美国Southern Biotech公司);IL-2、IFN-γ、IL-4和IL-10试剂盒[美国rndsystem(RD)北京永辉生物技术有限公司];异硫氰酸荧光素(FITC)标记CD4+单抗和藻红蛋白(PE)标记CD8+单抗(美国BD Biosciences公司);ConA(美国Sigma公司);RPMI1640培养基、FBS、MTT、DMSO(上海生工生物技术有限公司);猪PBMC分离液(大连宝生物公司)。酶标仪(山东高密彩虹分析仪器有限公司);流式细胞仪由遵义医学院免疫学教研室提供;37 ℃恒温水浴箱(上海市跃进农场医疗器械厂)。
1.4动物分组与免疫将16头健康仔猪均分为4组:重组Bb-TSO45W-4B-TSOL18疫苗组、重组Bb-TSO45W-4B疫苗组、重组Bb-TSOL18疫苗组和MRS对照组。疫苗均溶于50 mL MRS,均以1011CFU灌胃免疫,间隔2周免疫1次,共免疫2次。1.5血清收集及抗体检测在免疫后0、2、4、6、8周取前腔静脉血,4 ℃静置12 h,2 000 r/min离心10 min,收集上清,-20 ℃冻存,采用ELISA法检测血清IgG、IgG1和IgG2a水平。
1.6PBMC制备在免疫后0、2、4、6、8周取前腔静脉血1~2 mL于无菌EDTA抗凝管,用10% FBS的RPMI1640培养液稀释2倍。按2∶1缓慢加入到PBMC分离液上,2 500 r/min离心20 min,吸取上下液体交界处的白膜层。用RPMI1640培养液稀释5倍,洗涤2次,即为淋巴细胞,细胞沉淀用RPMI1640培养液悬浮后,用台盼蓝检测细胞活力和计数,计数后,用RPMI1640培养液调至为5×106/mL。
1.7PBMC增殖反应取出细胞培养板,分别加入1 mL原液(即5×106/mL PBMC)、1 mL原液+10 μL抗原(1 μg/μL)、1 mL原液+10 μL ConA(1 μg/μL)。于37 ℃ 5%CO2培养44 h,加入10 μL MTT(5 mg/mL),继续培养4 h,控干,加入100 μL DMSO,吹打溶解,立即用酶标仪测定OD630nm值。
1.8T细胞亚群检测吸取500 μL 5×106/mL PBMC于1.5 mL EP管,分别加入4 μL CD4+单抗和10 μL CD8+单抗,室温避光15~30 min,加入1 mL PBS缓冲液,1 200 r/min离心5 min,重复1次,弃上清,PBS悬浮,用流式细胞仪检测PBMC中CD4+和CD8+T细胞亚群百分率。
2结果
2.1血清IgG、IgG1、IgG2a水平重组Bb-TSO45W-4B-TSOL18疫苗组、重组Bb-TSO45W-4B疫苗组和重组Bb-TSOL18疫苗组血清IgG、IgG1、IgG2a水平均在免疫后2~8周升高,分别在免疫后4、6、4周达较高水平,与MRS对照组比较,差异均具有统计学意义(P<0.05)。重组Bb-TSO45W-4B-TSOL18疫苗组高于重组Bb-TSO45W-4B疫苗组和重组Bb-TSOL18疫苗组(P<0.05)。重组Bb-TSO45W-4B疫苗组与重组Bb-TSOL18疫苗组比较,差异无统计学意义(P>0.05),表1-3。



GroupWeek02468rBb-TSO45W-4B-TSOL18vac-cinegroup207.32±0.79209.26±2.71ab272.16±3.36ab252.85±3.76ab237.67±0.54abrBb-TSO45W-4Bvaccinegroup203.74±1.63208.15±2.01a263.58±2.42a245.05±3.66a232.14±1.60arBb-TSOL18vaccinegroup202.85±1.10207.43±2.06a254.44±3.32a243.98±1.36a225.46±3.37aMRScontrolgroup206.26±1.79207.21±3.12209.70±4.18206.58±1.97207.74±2.24
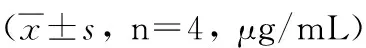

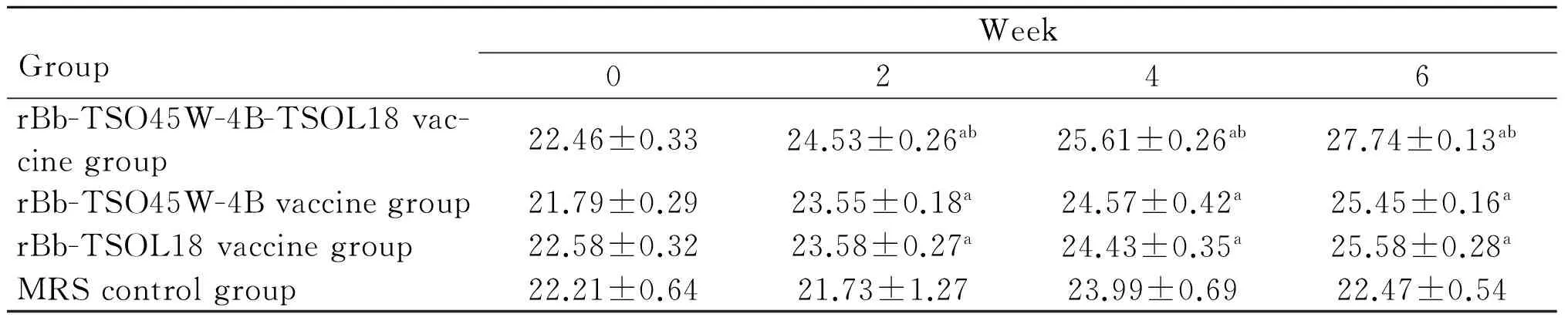
GroupWeek02468rBb-TSO45W-4B-TSOL18vac-cinegroup22.46±0.3324.53±0.26ab25.61±0.26ab27.74±0.13ab24.73±0.06abrBb-TSO45W-4Bvaccinegroup21.79±0.2923.55±0.18a24.57±0.42a25.45±0.16a23.44±0.11arBb-TSOL18vaccinegroup22.58±0.3223.58±0.27a24.43±0.35a25.58±0.28a22.76±1.62aMRScontrolgroup22.21±0.6421.73±1.2723.99±0.6922.47±0.5422.00±0.78
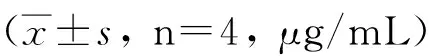
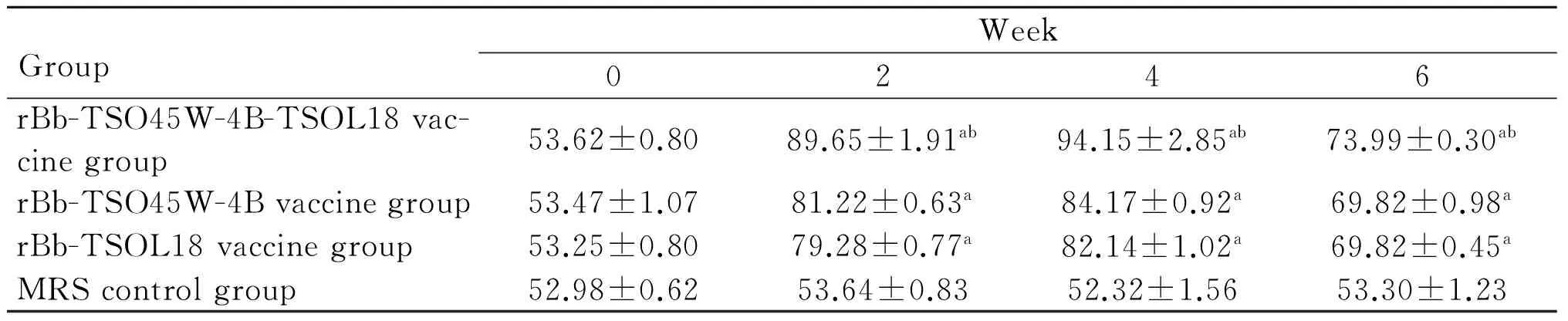
GroupWeek02468rBb-TSO45W-4B-TSOL18vac-cinegroup53.62±0.8089.65±1.91ab94.15±2.85ab73.99±0.30ab65.71±1.33abrBb-TSO45W-4Bvaccinegroup53.47±1.0781.22±0.63a84.17±0.92a69.82±0.98a62.55±0.15arBb-TSOL18vaccinegroup53.25±0.8079.28±0.77a82.14±1.02a69.82±0.45a62.43±0.83aMRScontrolgroup52.98±0.6253.64±0.8352.32±1.5653.30±1.2352.86±0.66
注:与MRS对照组比较,aP<0.05;与重组Bb-TSO45W-4B疫苗组和重组Bb-TSOL18疫苗组比较,bP<0.05。
Note: Compared with the MRS control group,aP<0.05; Compared with the rBb-TSO45W-4B vaccine group and rBb-TSOL18 vaccine group,bP<0.05.
2.2PBMC增殖水平不加刺激物时,重组Bb-TSO45W-4B-TSOL18疫苗组、重组Bb-TSO45W-4B疫苗组和重组Bb-TSOL18疫苗组PBMC增殖水平均在免疫后2~8周升高,均在免疫后6周达较高水平,与MRS对照组比较,差异均具有统计学意义(P<0.05)。重组Bb-TSO45W-4B-TSOL18疫苗组高于重组Bb-TSO45W-4B疫苗组和重组Bb-TSOL18疫苗组(P<0.05)。重组Bb-TSO45W-4B疫苗组与重组Bb-TSOL18疫苗组比较,差异无统计学意义(P>0.05)。用Ag或ConA刺激时,PBMC增殖水平类似于不加刺激物结果,见表4。



GroupStimulantionWeek02468rBb-TSO45W-4B-TSOL18Supernatant0.134±0.0320.366±0.044ab0.535±0.030ab1.367±0.009ab0.742±0.021abvaccinegroupAntigen0.218±0.0070.629±0.016ab0.829±0.039ab1.648±0.021ab0.680±0.018abConA0.567±0.0120.866±0.022ab1.361±0.029ab1.838±0.026ab1.151±0.036abrBb-TSO45W-4BSupernatant0.137±0.0400.332±0.033a0.567±0.025a0.857±0.037a0.550±0.036avaccinegroupAntigen0.241±0.0430.449±0.029a0.661±0.024a0.957±0.038a0.754±0.039aConA0.553±0.0370.645±0.035a0.937±0.012a1.145±0.039a0.824±0.030arBb-TSOL18vaccinegroupSupernatant0.161±0.0330.357±0.022a0.521±0.013a0.871±0.024a0.541±0.040aAntigen0.247±0.0400.444±0.012a0.663±0.026a0.943±0.029a0.737±0.034aConA0.537±0.0200.673±0.010a0.972±0.035a1.130±0.029a0.840±0.035aMRScontrolgroupSupernatant0.114±0.0370.233±0.0240.264±0.0400.239±0.0320.137±0.017Antigen0.274±0.0150.245±0.0330.356±0.0350.254±0.0210.357±0.029ConA0.468±0.0440.439±0.0320.462±0.0170.357±0.0230.452±0.035
注:与MRS对照组比较,aP<0.05;与重组Bb-TSO45W-4B疫苗组和重组Bb-TSOL18疫苗组比较,bP<0.05。
Note: Compared with MRS control group,aP<0.05; Compared with rBb-TSO45W-4B vaccine group and rBb-TSOL18 vaccine group,bP<0.05.
2.3CD4+和CD8+T细胞亚群重组Bb-TSO45W-4B-TSOL18疫苗组、重组Bb-TSO45W-4B疫苗组和重组Bb-TSOL18疫苗组CD4+、CD8+T细胞水平均在免疫后2~8周升高,均在免疫后8周、6周达较高水平,与MRS对照组比较,差异均具有统计学意义(P<0.05)。重组Bb-TSO45W-4B-TSOL18疫苗组高于重组Bb-TSO45W-4B疫苗组和重组Bb-TSOL18疫苗组(P<0.05)。重组Bb-TSO45W-4B疫苗组与重组Bb-TSOL18疫苗组比较,差异无统计学意义(P>0.05),见表5、6。
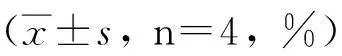

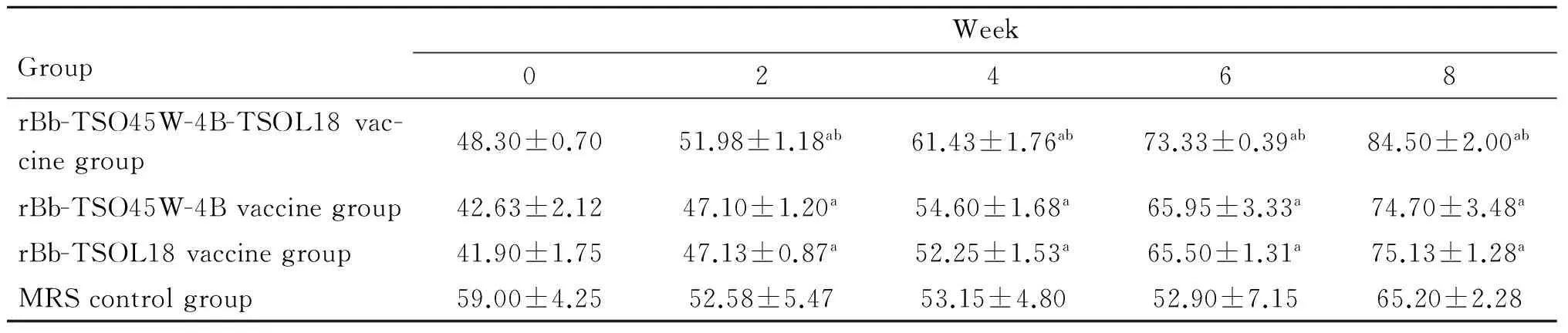
GroupWeek02468rBb-TSO45W-4B-TSOL18vac-cinegroup48.30±0.7051.98±1.18ab61.43±1.76ab73.33±0.39ab84.50±2.00abrBb-TSO45W-4Bvaccinegroup42.63±2.1247.10±1.20a54.60±1.68a65.95±3.33a74.70±3.48arBb-TSOL18vaccinegroup41.90±1.7547.13±0.87a52.25±1.53a65.50±1.31a75.13±1.28aMRScontrolgroup59.00±4.2552.58±5.4753.15±4.8052.90±7.1565.20±2.28
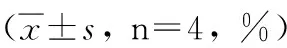

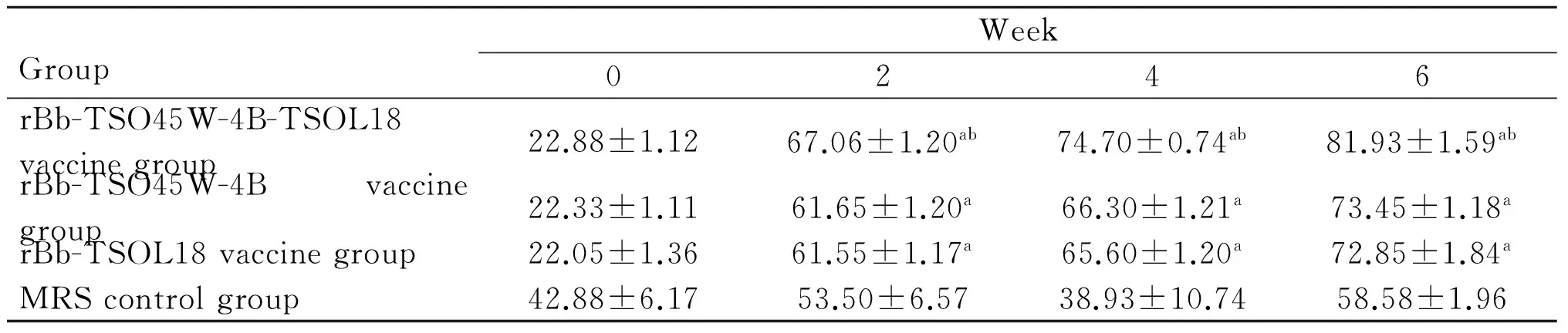
GroupWeek02468rBb-TSO45W-4B-TSOL18vaccinegroup22.88±1.1267.06±1.20ab74.70±0.74ab81.93±1.59ab78.33±1.20abrBb-TSO45W-4Bvaccinegroup22.33±1.1161.65±1.20a66.30±1.21a73.45±1.18a71.63±1.37arBb-TSOL18vaccinegroup22.05±1.3661.55±1.17a65.60±1.20a72.85±1.84a72.10±1.64aMRScontrolgroup42.88±6.1753.50±6.5738.93±10.7458.58±1.9661.73±5.90
注:与MRS对照组比较,aP<0.05;与重组Bb-TSO45W-4B疫苗组和重组Bb-TSOL18疫苗组比较,bP<0.05。
Note: Compared with MRS control group,aP<0.05; Compared with rBb-TSO45W-4B vaccine group and rBb-TSOL18 vaccine group,bP<0.05.
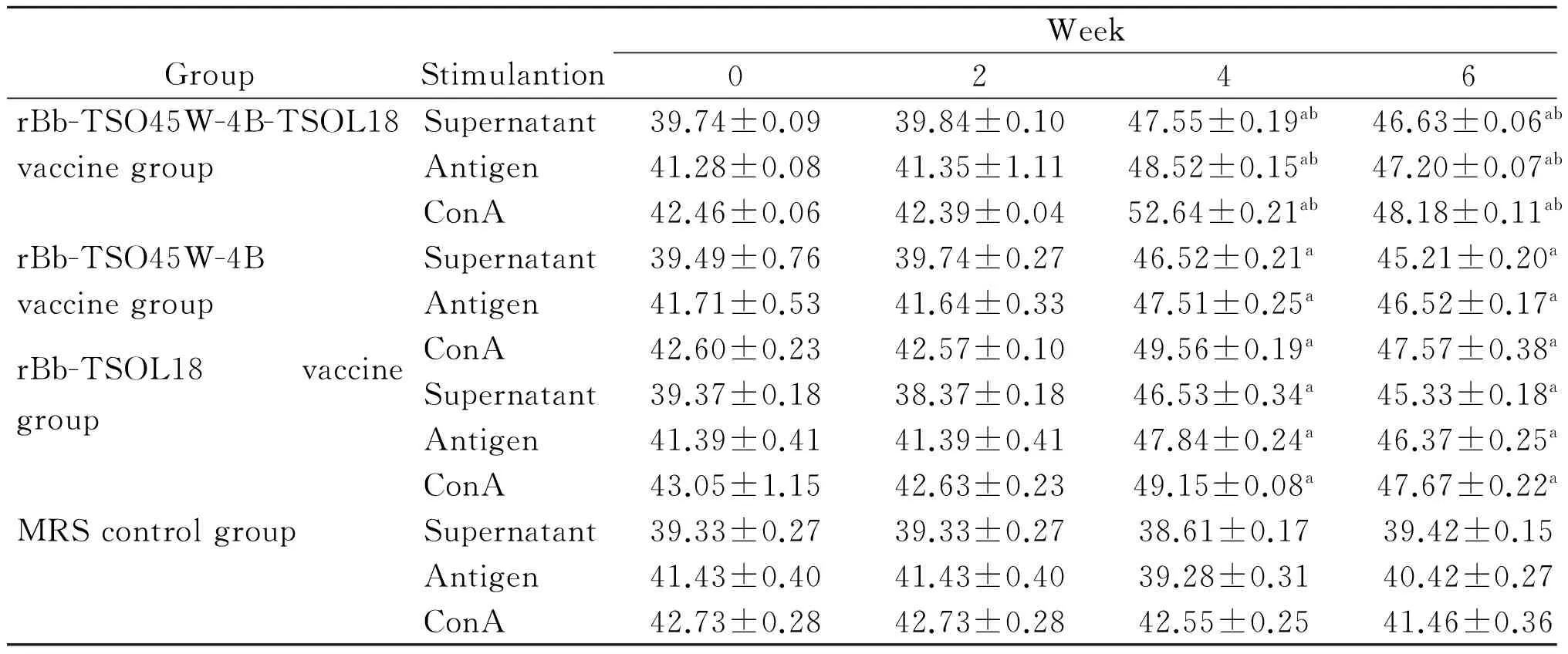
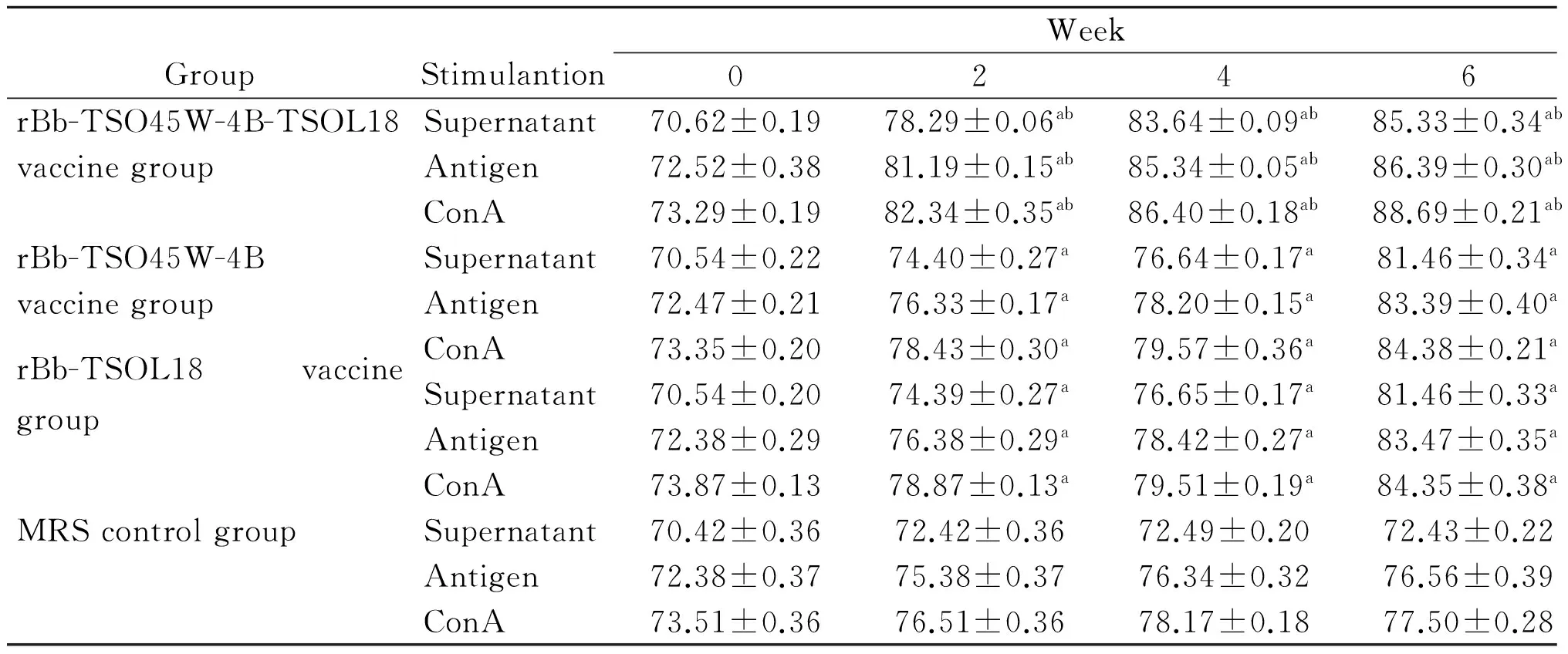
2.4IL-2、IFN-γ、IL-4及IL-10水平不加刺激物时,重组Bb-TSO45W-4B-TSOL18疫苗组、重组Bb-TSO45W-4B疫苗组和重组Bb-TSOL18疫苗组PBMC培养上清液IL-2、IFN-γ、IL-4及IL-10水平分别在免疫后2~6周、2~8周、4~6周和2~8周升高,分别在免疫后4周、6周、4周和8周达到较高水平,与MRS对照组比较,差异均具有统计学意义(P<0.05)。重组Bb-TSO45W-4B-TSOL18疫苗组高于重组Bb-TSO45W-4B疫苗组和重组Bb-TSOL18疫苗组(P<0.05)。重组Bb-TSO45W-4B疫苗组与重组Bb-TSOL18疫苗组比较,差异无统计学意义(P>0.05)。用Ag或ConA刺激时,PBMC培养上清液IL-2、IFN-γ、IL-4及IL-10水平类似于不加刺激物结果,见表7-10。
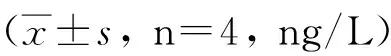


GroupStimulantionWeek02468rBb-TSO45W-4B-TSOL18Supernatant152.44±0.87183.69±1.07ab203.53±0.05ab177.54±0.87ab163.05±0.23vaccinegroupAntigen162.51±2.23190.44±1.94ab207.79±1.00ab182.37±0.72ab162.55±0.09ConA165.02±0.82204.16±2.63ab214.74±1.65ab184.51±0.29ab164.67±0.22rBb-TSO45W-4BSupernatant152.93±1.11173.18±1.84a192.55±1.34a171.99±1.69a156.17±2.50vaccinegroupAntigen164.63±3.64184.29±1.60a196.92±1.17a176.35±1.38a165.21±3.54ConA164.60±0.99192.85±1.93a206.06±1.47a176.42±0.16a168.28±0.71rBb-TSOL18vaccinegroupSupernatant158.11±3.48178.36±1.39a193.91±1.32a173.82±1.57a154.62±1.99Antigen164.82±4.10184.82±1.53a196.89±1.45a175.87±2.05a165.17±3.50ConA165.62±3.77192.62±1.94a205.97±3.07a178.00±1.55a167.23±2.17MRScontrolgroupSupernatant152.60±1.61153.60±3.46152.16±0.87154.39±1.12156.28±1.17Antigen161.32±2.16160.32±2.98160.90±2.49160.92±1.62161.83±3.18ConA163.67±2.26163.67±2.37164.70±2.44165.74±0.80164.57±1.52
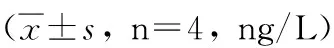
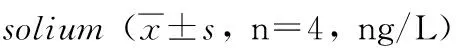

GroupStimulantionWeek02468rBb-TSO45W-4B-TSOL18Supernatant18.60±0.2721.45±0.07ab22.64±0.15ab24.72±0.21ab21.51±0.31abvaccinegroupAntigen19.44±0.0622.84±0.14ab23.65±0.37ab25.38±0.31ab22.76±0.22abConA20.58±0.1923.88±0.09ab24.54±0.29ab26.35±0.24ab23.78±0.23abrBb-TSO45W-4BSupernatant17.15±0.1219.80±0.14a21.60±0.23a23.53±0.28a21.73±0.29avaccinegroupAntigen18.70±0.1920.33±0.20a22.66±0.34a24.21±0.14a22.78±0.12aConA19.52±0.2521.73±0.29a23.36±0.28a25.81±0.20a23.44±0.35arBb-TSOL18vaccinegroupSupernatant17.47±0.2619.47±0.26a21.65±0.31a23.68±0.31a21.56±0.36aAntigen18.62±0.3020.62±0.30a22.34±0.22a24.61±0.17a22.53±0.42aConA19.59±0.3721.59±0.37a23.43±0.31a25.71±0.39a23.41±0.32aMRScontrolgroupSupernatant17.95±0.7618.20±0.8919.13±0.4120.29±0.4518.54±0.38Antigen19.43±0.9219.93±0.5820.07±0.3621.12±0.1919.53±0.20ConA21.58±0.7522.08±0.7620.97±0.4322.47±0.3221.49±0.23
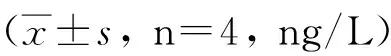

GroupStimulantionWeek02468rBb-TSO45W-4B-TSOL18Supernatant39.74±0.0939.84±0.1047.55±0.19ab46.63±0.06ab39.53±0.47vaccinegroupAntigen41.28±0.0841.35±1.1148.52±0.15ab47.20±0.07ab41.26±0.09ConA42.46±0.0642.39±0.0452.64±0.21ab48.18±0.11ab42.49±0.04rBb-TSO45W-4BSupernatant39.49±0.7639.74±0.2746.52±0.21a45.21±0.20a39.43±0.29vaccinegroupAntigen41.71±0.5341.64±0.3347.51±0.25a46.52±0.17a41.19±0.40ConA42.60±0.2342.57±0.1049.56±0.19a47.57±0.38a41.96±0.54rBb-TSOL18vaccinegroupSupernatant39.37±0.1838.37±0.1846.53±0.34a45.33±0.18a39.39±0.26Antigen41.39±0.4141.39±0.4147.84±0.24a46.37±0.25a41.03±0.66ConA43.05±1.1542.63±0.2349.15±0.08a47.67±0.22a41.86±0.78MRScontrolgroupSupernatant39.33±0.2739.33±0.2738.61±0.1739.42±0.1539.32±0.15Antigen41.43±0.4041.43±0.4039.28±0.3140.42±0.2740.64±0.23ConA42.73±0.2842.73±0.2842.55±0.2541.46±0.3641.31±0.30
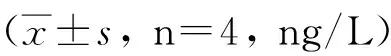

GroupStimulantionWeek02468rBb-TSO45W-4B-TSOL18Supernatant70.62±0.1978.29±0.06ab83.64±0.09ab85.33±0.34ab92.45±0.27abvaccinegroupAntigen72.52±0.3881.19±0.15ab85.34±0.05ab86.39±0.30ab93.55±0.28abConA73.29±0.1982.34±0.35ab86.40±0.18ab88.69±0.21ab95.64±0.14abrBb-TSO45W-4BSupernatant70.54±0.2274.40±0.27a76.64±0.17a81.46±0.34a85.66±0.20avaccinegroupAntigen72.47±0.2176.33±0.17a78.20±0.15a83.39±0.40a86.28±0.15aConA73.35±0.2078.43±0.30a79.57±0.36a84.38±0.21a88.43±0.41arBb-TSOL18vaccinegroupSupernatant70.54±0.2074.39±0.27a76.65±0.17a81.46±0.33a85.67±0.19aAntigen72.38±0.2976.38±0.29a78.42±0.27a83.47±0.35a86.64±0.28aConA73.87±0.1378.87±0.13a79.51±0.19a84.35±0.38a88.42±0.36aMRScontrolgroupSupernatant70.42±0.3672.42±0.3672.49±0.2072.43±0.2272.79±0.80Antigen72.38±0.3775.38±0.3776.34±0.3276.56±0.3975.44±0.03ConA73.51±0.3676.51±0.3678.17±0.1877.50±0.2876.94±0.60
注:与MRS对照组比较,aP<0.05;与重组Bb-TSO45W-4B疫苗组和重组Bb-TSOL18疫苗组比较,bP<0.05。
Note: Compared with MRS control group,aP<0.05; Compared with rBb-TSO45W-4B vaccine group and rBb-TSOL18 vaccine group,bP<0.05.
3讨论
Bueno等(2000)发现囊虫病患者脑脊液、血清和唾液中存在特异性IgG、IgA和IgE;方文等[11]发现囊虫病猪肝、脑、骨骼肌和心肌IL-6、IL-8、TNF-α和sIL-2R升高;叶红等[12]发现脑囊虫病患者外周血IL-4、IL-10升高,IL-2和IFN-γ降低,丙硫咪唑治疗后IL-4、IL-10降低,IL-2、IFN-γ升高。胡守锋等[13]发现囊虫病患者外周血CD4+升高,CD8+降低,CD4+/CD8+升高。上述资料表明宿主在防御囊虫病的过程中不仅有体液免疫反应参与,而且有Th1、Tp和细胞免疫反应参与,这就为我们研制猪带绦虫疫苗提供了理论依据。
TSO45W-4B和TSOL18具有较强的免疫原性和免疫保护性[14-16],我们研制的猪带绦虫重组Bb-TSO45W-4B-TSOL18疫苗,动物实验证实,以该疫苗1011CFU口服灌胃免疫仔猪,猪带绦虫卵攻击后,可诱导仔猪获得83.09%的减蚴率,具有一定的保护性[17]。本研究进一步将猪带绦虫重组Bb-TSO45W-4B-TSOL18疫苗免疫仔猪后,分别从体液免疫、细胞免疫和细胞因子的变化观察其免疫效果。结果表明,猪带绦虫重组Bb-TSO45W-4B-TSOL18疫苗免疫后,一方面刺激仔猪产生了相应的抗体,表现为血清IgG、IgG1、IgG2a水平明显升高,提示该疫苗在仔猪体内有效地诱导了体液免疫反应;另一方面刺激PBMC发生了显著增殖,且CD4+和CD8+T细胞亚群亦呈增高趋势,提示该疫苗在仔猪体内有效地诱导了细胞免疫反应;再者,刺激PBMC分泌了高水平的IL-2、IFN-γ、IL-4及IL-10。鉴于IgG1和IgG2a抗体亚类作为免疫反应类型的指征,其中Th1细胞分泌的IL-2、IFN-γ有利于补体结合IgG2a的合成,主要介导细胞免疫反应,而产生IL-4、IL-10的Tp细胞参与辅助B细胞刺激IgG1的生成,主要介导体液免疫反应[18]。因此我们推测猪带绦虫重组Bb-TSO45W-4B-TSOL18疫苗在仔猪体内诱导产生了Th1型和Tp型的免疫反应。
本实验还发现,TSO45W-4B-TSOL18融合抗原疫苗的免疫效果优于TSO45W-4B或TSOL18单一抗原疫苗,可能与2种抗原融合后,综合了这2种抗原分子的协同作用有关,提示多抗原、多表位、多基因融合的复合多价疫苗可能会提高免疫效果,或许是未来抗囊虫病疫苗研究的一个方向。
参考文献:
[1] Liu MC, He LF, Zhou BY. Research progress on genetic engineering vaccine ofTaeniasolium[J]. Chin J Endemiol, 2014, 33(2): 233-236. (in Chinese)
刘美辰,贺莉芳,周必英.猪带绦虫基因工程疫苗研究进展[J].中华地方病学杂志,2014,33(2):233-236.
[2] Zhou BY. Research progress on recombinant antigens ofTaeniasolium[J]. Chin J Zoonoses, 2014, 30(4): 418-422. (in Chinese)
周必英.猪带绦虫重组抗原研究进展[J].中国人兽共患病学报,2014,30(4):418-422.
[3] Liu MC, He LF, Zhou BY. Advances in the study of recombinantbifidobacterium[J]. Chin J Pathogen Biol, 2014, 9(5): 470-472. (in Chinese)
刘美辰,贺莉芳,周必英.重组双歧杆菌研究进展[J].中国病原生物学杂志,2014,9(5):470-472.
[4] Zhou BY, Liu MC, He LF. Construction and identification of a recombinant Bb (pGEX-TSO45W-4B-TSOL18) vaccine ofTaeniasolium[J]. Chin J Pathogen Biol, 2014, 9(4): 289-292, 298. (in Chinese)
周必英,刘美辰,贺利芳.猪带绦虫重组Bb(pGEX-TSO45W-4B-TSOL18)疫苗的构建及鉴定[J].中国病原生物学杂志,2014,9(4):289-292,298.
[5] Zhou BY, Liu MC, Yang FJ.TaeniasoliumTSO45W-4B-TSOL18 fusion gene expression inbifidobacteriumlongum[J]. Chin J Zoonoses, 2014, 30(9): 889-892. (in Chinese)
周必英,刘美辰,杨凤娇.猪带绦虫TSO45W-4B-TSOL18融合基因在长双歧杆菌中的表达[J].中国人兽共患病学报,2014,30(9):889-892.
[6] Zhou BY, Liu MC, He LF. Construction and identification of a recombinant Bifidobacteria(pGEX-TSO45W-4B)vaccine ofTaeniasolium[J]. Chin J Endemiol, 2014, 33(6): 591-595. (in Chinese)
周必英,刘美辰,贺利芳.猪带绦虫重组双歧杆菌(pGEX-TSO45W-4B)疫苗构建及鉴定[J].中华地方病学杂志,2014,33(6):591-595.
[7] Zhou BY, Liu MC, He LF. Construction and identification of the bifidobacterium expression system pGEX-TSOL18/B.longum ofTaeniasolium[J]. Chin J Parasitol Parasit Dis, 2014, 32(3): 239-241. (in Chinese)
周必英,刘美辰,贺莉芳.猪带绦虫双歧杆菌表达系统pGEX-TSOL18/B.longum的构建及鉴定[J].中国寄生虫学与寄生虫病杂志,2014,32(3):239-241.
[8] Zhou BY, Zhou L, Liu MC, et al. Expression and purification of a fusion gene TSO45W-4B-TSOL18 ofTaeniasoliuminEscherichiacoliArcticExpress(DE3) and preparation of rabbit antiserum[J]. Chin J Endemiol, 2013, 32(6): 619-624. (in Chinese)
周必英,周泠,刘美辰,等.猪带绦虫TSO45W-4B-TSOL18融合基因在大肠埃希菌ArcticExpress(DE3)中的表达、纯化和兔抗血清的制备[J].中国地方病学杂志,2013,32(6):619-624.
[9] Zhou BY, Zhou L, Liu MC, et al. Cloning, expression of TSO45W-4B gene fromTaeniasoliumand preparation of its polyclonal antibody[J]. Chin J Parasitol Parasit Dis, 2013, 31(5): 372-375. (in Chinese)
周必英, 周泠, 刘美辰, 等. 猪带绦虫TSO45W-4B基因的克隆、表达和兔抗血清的制备[J].中国寄生虫学与寄生虫病杂志, 2013, 31(5): 372-375.
[10] Zhou BY, Zhou L, Liu MC, et al. Expression, purification and preparation of rabbit antiserum of the gene TSOL18 ofTaeniasolium[J]. Chin J Zoonoses, 2013, 29(10): 977-980, 985. (in Chinese)
周必英,周泠,刘美辰,等.猪带绦虫TSOL18基因的表达、纯化和兔抗血清的制备[J].中国人兽共患病学报,2013,29(10):977-980,985.
[11] Fang W, Bao HA, Xiao JJ, et al. Investigation on the changes of contents of IL-6,IL-8,TNF-α and sIL-2 receptor in tissues of pigs infected with cysticercus cellulosae ofTaeniasolium[J]. Chin J Zoonoses, 2009, 25(6): 556-559. (in Chinese)
方文,包怀恩,肖靓靓,等.感染猪囊尾蚴的家猪组织中不同时间IL-6、IL-8、TNF-a和SIL-2R含量变化[J].中国人兽共患病学报,2009,25(6):556-559.
[12] Ye H, Wang L. Detection of Thl/Tp cytokines before and after treatment in patients with cerebral cysticercosis[J]. Chin J Zoonoses, 2005, 21(4): 361-363. (in Chinese)
叶红,王丽.脑囊虫病患者治疗前后Thl/Tp细胞因子的检测[J]. 中国人兽共患病杂志, 2005,21(4):361-363.
[13] Hu SF, Wang XM, Sun X, et al. Study on the proportion of peripheval blood T cell subsets and the level of IFN-γ and IL-4 production in patients with cysticercosis[J]. J Bengbu Med Coll, 2009, 34(9): 756-758. (in Chinese)
胡守锋, 王雪梅, 孙新,等. 猪囊尾蚴病患者外周血T细胞亚群比例和产生IFN-γ、IL-4水平研究[J]. 蚌埠医学院学报, 2009, 34(9): 756-758.
[14] Wang FM, Luo XN, Jing ZZ, et al. Study on immunogenicity in pigs elicited by recombinant protein 45W-4B vaccine ofTaeniasoliumoncosphere[J]. Chin J Vet Parasitol, 2006, 14(3): 1-5. (in Chinese)
王福梅,骆学农,景志忠,等. 猪带绦虫六钩蚴45W-4B重组蛋白的免疫原性研究[J].中国兽医寄生虫病,2006,14(3):1-5.
[15] Luo XN, Zheng YD, Hou JL, et al. Protection against asiaticTaeniasoliuminduced by a recombinant 45W-4B protein[J]. Clin Vaccine Immunol, 2009, 16(2): 230-232.
[16] Ding J, Zheng Y, Wang Y, et al. Immune responses to a recombinant attenuatedSalmonellatyphimuriumstrain expressing aTaeniasoliumoncosphere antigen TSOL18[J]. Comp Immunol Microbiol Infect Dis, 2013, 36(1): 17-23.
[17] Jia Q, Liu MC, Zhou L, et al. Protective immune responses induced by recombinant Bifidobacterium-TSO45W-4B-TSOL18 vaccine ofTaeniasoliumin domestic pigs[J]. Chin J Endemiol, 2015, 34(10): 717-722. (in Chinese)
贾启, 刘美辰, 周泠, 等. 猪带绦虫重组Bb-TSO45W-4B-TSOL18疫苗诱导仔猪的保护性免疫应答[J].中华地方病学杂志, 2015, 34(10):717-722.
[18] Mosmann TR, Coffman RL. Th1 and Tp cells: different patterns of lymphokine secretion lead to different functional properties[J]. Annu Rev Immunol, 1989, 7: 145-173.
DOI:10.3969/j.issn.1002-2694.2016.04.008
通讯作者:周必英,Email:zbyzl01@126.com
中图分类号:R383.32
文献标识码:A
文章编号:1002-2694(2016)04-0356-07
Corresponding author:Zhou Bi-ying, Email: zbyzl01@126.com
收稿日期:2015-08-03修回日期:2015-11-21
by immunization with recombinant bifidobacterium(Bb)-TSO45W-4B-TSOL18 vaccine ofTaeniasolium
WANG Ling-jun1, LIU Mei-chen1, ZHOU Ling2, YANG Feng-jiao1,JIA Qi1, JIANG Nan1, ZHOU Bi-ying1
(1.DepartmentofParasitology,ZunyiMedicalCollege,Zunyi563000,China;2.DepartmentofTraditionalChineseMedicineandAnorectum,theAffiliatedHospital,ZunyiMedicalCollege,Zunyi563000,China)
Abstract:To study dynamical changes of immune responses induced in piglets by immunization with recombinant bifidobacterium (Bb)-TSO45W-4B-TSOL18 vaccine of Taenia solium, sixteen healthy piglets of 40 days old were randomly divided into four groups: rBb-TSO45W-4B-TSOL18 vaccine group, rBb-TSO45W-4B vaccine group, rBb-TSOL18 vaccine group, and Bb liquid medium (MRS) control group. All vaccine groups were given 1011CFU through intragastric immunization. A total of two times of immunization were conducted, once for every two weeks. On 0th, 2nd, 4th, 6th and 8th week after immunization, the blood from precaval vein were collected to separate serum and peripheral blood lymphocytes (PBMC). Levels of IgG, IgG1 and IgG2a was detected by enzyme linked immunosorbent assay(ELISA). Proliferation level of PBMC was detected by methyl thiazolyl tetrazolium (MTT) method. Levels of IL-2, IFN-γ, IL-4 and IL-10 in PBMC culture supernatant were determined by ELISA. Percentage of CD4+ and CD8+ T cell subsets were detected by flow cytometry (FCM). The study demonstrated that levels of IgG, IgG1 and IgG2a in all vaccine groups increased from 2 to 8 weeks after immunization, and reached the highest level by the 4th week, 6th week, and 4th week. Proliferation level of PBMC increased from 2 to 8 weeks, and reached the highest level by the 6th week. Percentage of CD4+ and CD8+ T cells increased from 2 to 8 weeks, and reached the highest level by the 8th week and 6th week. Levels of IL-2, IFN-γ, IL-4 and IL-10 in PBMC culture supernatant increased from 2 to 6 weeks, 2 to 8 weeks, 4 to 6 weeks and 2 to 8 weeks, and reached the highest level by the 4th week, 6th week, 4th week and 8th week. The differences of above indexes between all vaccine groups and MRS control group were statistically significant. The differences between rBb-TSO45W-4B-TSOL18 vaccine group and rBb-TSO45W-4B vaccine group or rBb-TSOL18 vaccine group were statistically significant. It was concluded that rBb-TSO45W-4B-TSOL18 vaccine of Taenia solium might induce piglets to produce specific immune responses, and the immune effect of TSO45W-4B-TSOL18 fusion antigen vaccine is better than that of TSO45W-4B or TSOL18 single antigen vaccine.
Keywords:Taenia solium; recombinant Bb-TSO45W-4B-TSOL18 vaccine; immune responses; piglets
国家自然科学基金项目(No.81160206)资助

