一个新的嘧啶酮-肉桂酸杂合体的设计与合成* 1
路东亮,邢艳玲,高晓培
(赣南师范学院 化学化工学院,江西 赣州 341000)
一个新的嘧啶酮-肉桂酸杂合体的设计与合成* 1
路东亮,邢艳玲,高晓培
(赣南师范学院 化学化工学院,江西 赣州341000)
摘要:由(1E, 4Z)-5-羟基-1-(3, 4, 5-三甲氧基苯)-1, 4-己二烯-3-酮 、3, 4, 5-三甲氧基苯甲醛、尿素三组分“一锅法”将3, 4-二氢嘧啶酮类化合物和肉桂酸骨架相结合,得到一个具有双重药效团的杂合分子骨架,并采用正交试验优化反应条件.通过单晶衍射、核磁共振以及红外光谱对产物进行结构表征,该方法操作简便、经济,是在环境友好、无毒、无污染的条件下完成的,符合绿色化学的要求.
关键词:Biginelli反应;一锅法;杂合体;新型的二氢嘧啶酮
近年来临床应用研究发现3,4-二氢嘧啶-2-酮衍生物(DHPMs)作为氮杂环化合物具有广泛的生理和药理活性.其在抗病毒、抗微生物、杀菌、消炎等药物领域有广泛的应用[1-2];还可用作钙拮抗剂,降压剂,α1a-拮抗物,止痛剂,抗人类免疫缺陷病毒和癌预防剂等[3-9],在医药领域中发挥着重要的作用.而且,一些从海洋来源分离得到的生物碱其核心结构也包含二氢嘧啶酮的结构,并表现一些有趣的生物性[10-11].因而DHPMs是有机杂环化合物研究的热点之一.Biginelli反应[12]是合成该类化合物常用、简便的方法.
随着对Biginelli反应研究的深入,人们对Biginelli产物结构的修饰和改造,以及结构和性能之间关系的研究越来越广泛.研究发现,通过对嘧啶酮进一步的结构修饰,即多官能化的二氢嘧啶酮衍生物具有更好的生物和药理活性,例如:吡啶并嘧啶类化合物具有高效抑制多种癌细胞的生物活性[13-17];N-甲酰胺,甲酯取代的二氢嘧啶酮可以用作口服的降压,钙拮抗和α1α拮抗药物[18-20].为了进一步提高嘧啶酮衍生物的生理和药理活性,我们以3,4-二氢嘧啶酮为先导化合物将肉桂酸骨架导入到嘧啶环的5位,在分子中引入活性基团,希望得到具有更优的生物和药理活性的杂合体药物分子.
本文报道了一个新型的3,4-二氢嘧啶酮化合物(如图1化合物2)即将3,4-二氢嘧啶酮和天然抗氧化剂肉桂酸结合,得到了一个具有双重药效团的杂合分子[21-23].在以往DHPMs的合成中一般只在C(5)位引入饱和酰基或酯基,如甲酰胺[24],芳甲酰基[25],二茂铁基的 DHPMs 化合物[26],乙酰基[27],乙氧酰基[28]等都是饱和的酰基和酯基.本文合成的杂合体药物分子打破了以往只在嘧啶酮骨架5位引入饱和酯基和酰基的先例,而是在嘧啶酮的5位引入一个苯环取代的α, β-不饱和羰基化合物,从而在嘧啶酮结构单元中引入了一个Michael加成受体.有研究证明Michael 加成受体单元在药物癌预防和治疗中有很重要的作用[29].细胞的氧化还原状态往往可通过含有Michael 加成受体单元的分子改变[30].因此,这种化合物有很大的发展前景.
本文在适宜温度下以ZnCl2为催化剂,无溶剂条件下进行反应,并采用正交实验优化反应条件.通过单晶衍射、核磁共振以及红外光谱确证了产物结构.
1实验部分
1.1仪器与试剂
红外光谱仪(360FT)美国Nicolet公司;X射线衍射仪(SMART APEX)德国布鲁克AXS公司;金叶牌旋转蒸发仪(RE-3000)上海亚荣生化仪器厂;核磁共振仪(AM-400)德国布鲁克AXS公司;金叶牌真空泵(SHZ-Ⅲ)上海亚荣生化仪器厂;熔点测定仪(WRS-3)上海精科.
3,4,5-三甲氧基苯甲醛,乙酰丙酮,正丁胺,硼酸三正丁酯,三氧化二硼,尿素均购买于阿拉丁公司,其余试剂均为市售分析纯试剂.
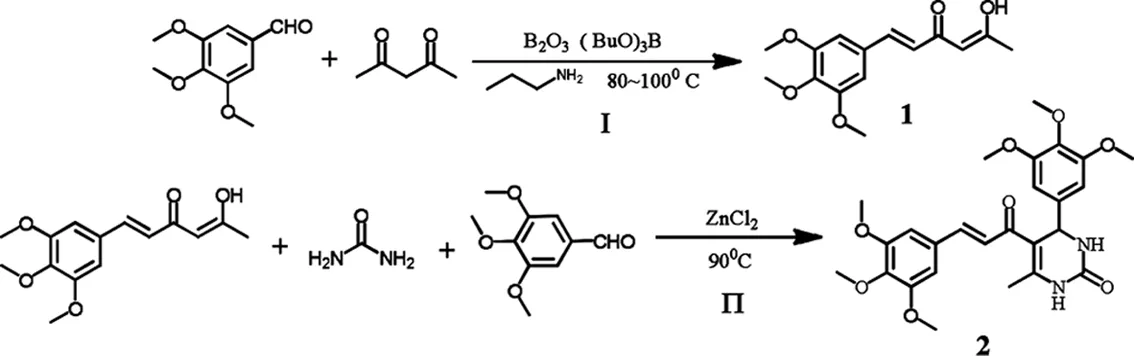
图1 “一锅法”合成嘧啶酮-肉桂酸杂合体的路线图
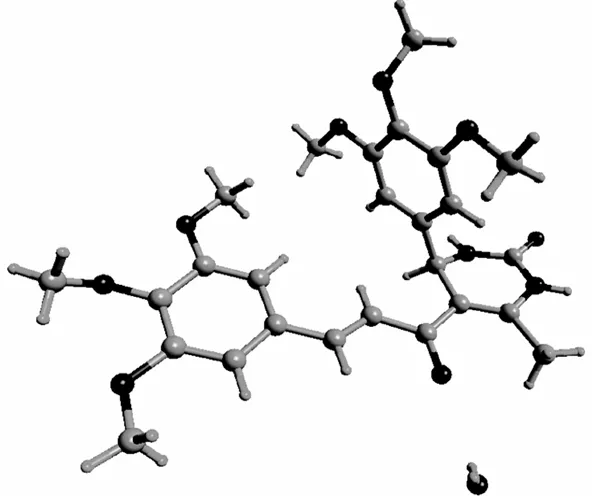
图2 化合物2的晶体结构图
1.2合成方法
标题化合物的合成路线见图1.
标题化合物的合成步骤: 乙酰丙酮1.62 mL(15.84 mmol),B2O31.00 g(14.36 mmol)和乙酸乙酯5 mL在80 ℃下回流30 min,然后加入溶于乙酸乙酯(5mL)的3,4,5-三甲氧基苯甲醛(7.2 mmol)和硼酸三正丁酯0.8 mL(2.96 mmol),同温度下反应30 min后,将正丁胺0.28 mL(2.86 mmol)逐滴加入,滴毕,反应体系在100 ℃下反应1 h,TLC跟踪反应.将反应温度降到50 ℃,加入HCl(15 mL, 1.5 N)继续搅拌30 min.反应结束后,冷却至室温,乙酸乙酯萃取,饱和食盐水洗涤,Na2SO4干燥,柱层析分离(乙酸乙酯∶石油醚=3∶1),用乙醇和水重结晶得到黄色针状固体合物1,产率75%.
将化合物1 0.066 g(0.24 mmol),3,4,5-三甲氧基苯甲醛0.039 g(0.2 mmol),和尿素0.018 g(0.3 mmol)加于20 mL圆底烧瓶中,90 ℃下反应3 h,TLC跟踪反应.反应结束后,冷却至室温,用甲醇溶解,过滤除去不溶物,滤液减压旋蒸至少量液体,柱层析分离(CH2Cl2∶CH2OH = 20∶1),用乙醇和水重结晶得黄色锥状固体化合物2,产率71.9%,即为嘧啶酮-肉桂酸杂合体.
1.3结构表征
1.3.1化合物2的核磁共振谱与红外光谱数据
Mp 99.7-100.2 ℃; Rf=0.56(CH2Cl2∶CH2OH=20∶1);1H NMR (400 MHz,CDCl3)δ 8.20 (s,1H), 7.81-7.49(m,2H), 7.38(d,J=15.5 Hz,1H), 6.72(d,J=15.6 Hz,1H), 6.60(d,3H), 5.92(s,1H), 5.54(s,1H), 3.83(dd,J=22.0,5.0 Hz,17H), 2.31(s,4H);13C NMR(100 MHz,DMSO)δ 188.38, 153.50, 153.29, 152.50, 147.36, 141.23, 140.66, 139.64, 130.98, 126.17, 109.88, 106.23, 104.31, 60.54, 60.40, 56.42, 56.23, 54.84, 19.13; IR(KBr)ν:3 345.3, 3 242.6, (-NH-); 2 934.6(-CH3), 1 693.1(C=O); 1 633.6, 971.0(反式C=C); 1 633.6, 1 585.8, 1 503.9, 1 459.6, (Ar-C-C); 1 414.7, 1 328.9, 1 281.9, (C-N); 1 237.6, 1 184.6, 1 125.8, (C-O-C).
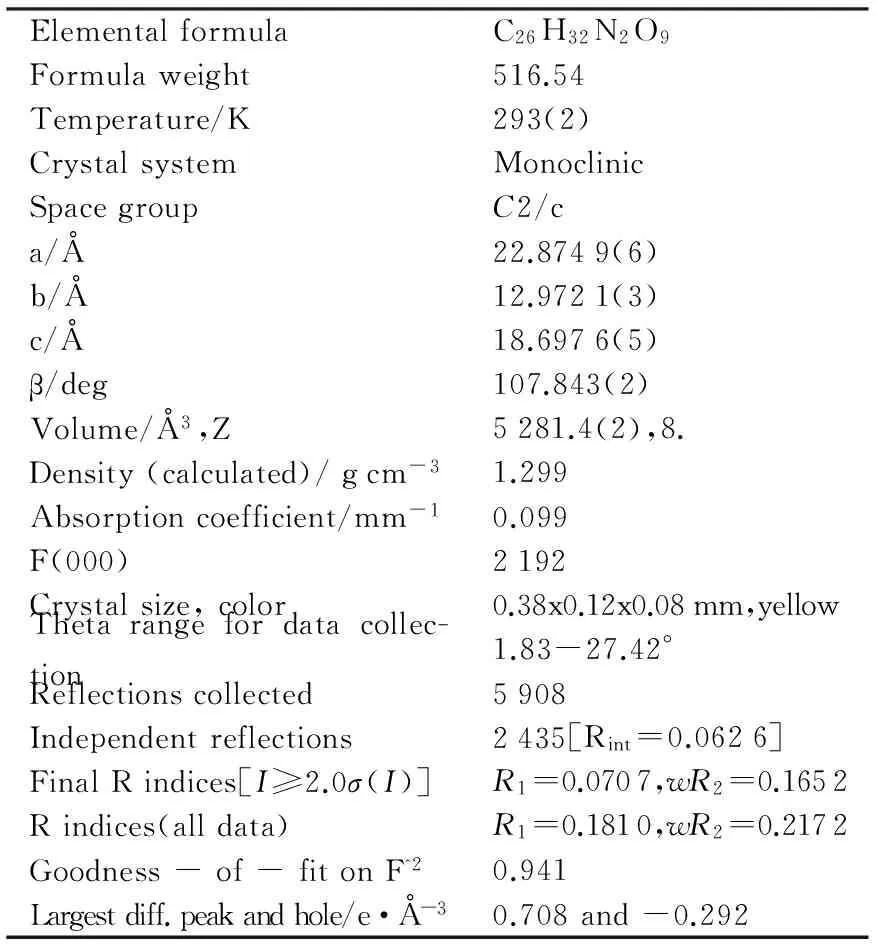
表1 化合物2的主要晶体结构数据
1.3.2化合物2的单晶结构测定
选择1颗尺寸大小为0.38×0.12×0.08 mm的化合物2,进行X-射线单晶结构测定,晶体结构见图2,结构衍射数据见表1.
2结果与讨论
2.1合成路线第Ⅱ步反应的正交试验设计与结果
根据正交试验表2,考察反应时间(A) ,反应温度(B),溶剂(C),和催化剂(D)四因素对化合物2产率的影响。实验结果见表2所示。由表2可知,各因素的影响次序为A>B>C=D ,优化方案为 A2B2C3D3,即反应时间4 h,反应温度90 ℃,无溶剂条件,催化剂为ZnCl2时化合物2的产率较高.
2.2化合物2合成过程讨论
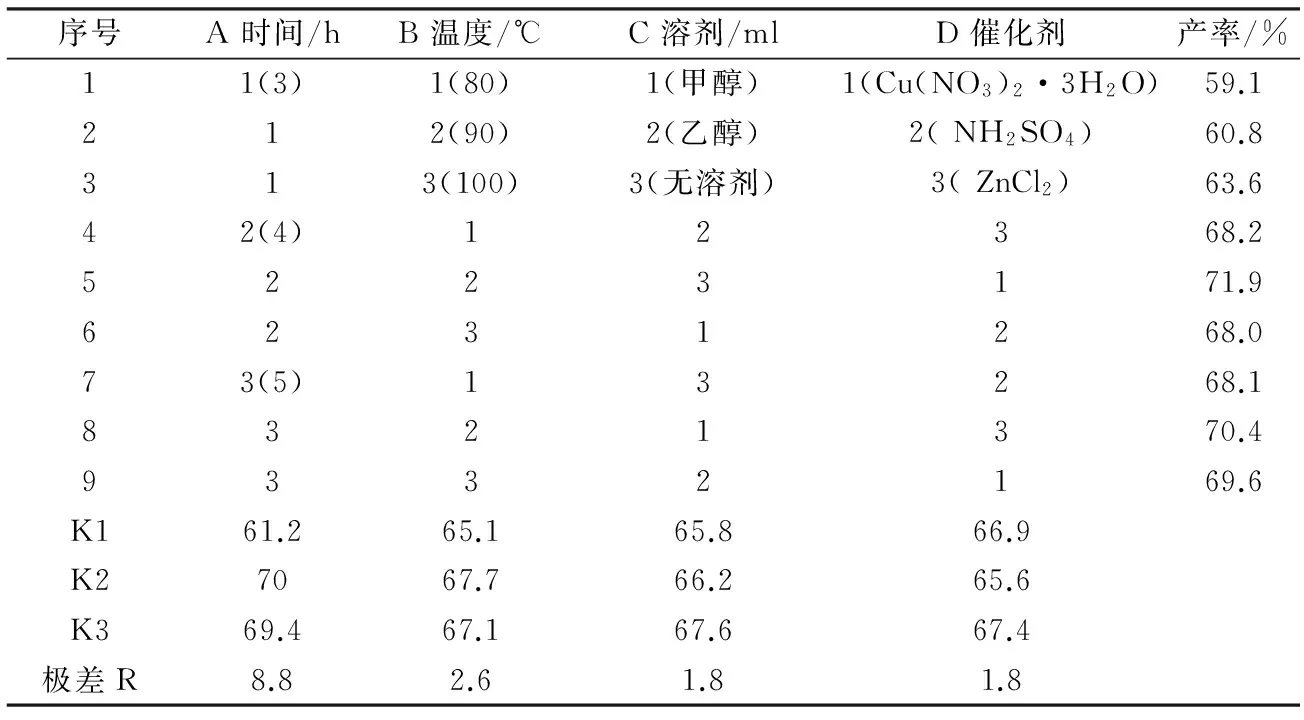
表2 化合物2合成的正交试验表
3结论
本文通过Biginelli-like反应合成了一种新型嘧啶酮-肉桂酸杂合体药物分子,并研究了反应催化剂,溶剂,时间和温度对合成杂合体药物分子产率的影响。通过正交试验得出:在ZnCl2为催化剂,无溶剂,反应温度为90 ℃,反应时间4 h的条件下,杂合体药物分子的产率最高;本文运用药物杂合理论(药物杂合是将作用机制不同的两类或多类药物分子或活性官能团有效拼合或连接的设计方法,能合成具有多靶点的药物),在嘧啶酮分子骨架的C5位引入天然抗氧化剂3,4,5-三甲氧基肉桂酸,它不仅实现了两个活性组分的结合,并且在嘧啶酮骨架上引入了Michael 加成受体,有望使二氢嘧啶酮的生理和药理活性显著提高,最重要的是为嘧啶酮衍生物的设计合成提供新的思路,有助于进一步研究其生理和药理活性
参考文献:
[1]Kappe C O, Fabian W M F, Semones M A. Conformational analysis of 4-aryldihydro pyrimidine calcium channel modulators. A comparison of Ab initio, semiempirical and x-ray crystallographic studies[J].Tetrahedron, 1997,53:2803-2816.
[2]Bahekar S S, Shinde D B. Synthesis and anti-inflammatory activity of some[4,6-(4-substituted aryl)-2-thioxo-1,2, 3,4-tetrahydro-pyrimidin-5-yl]-acetic acid derivatives[J].Bioorg. Med. Chem. Lett., 2004,14:1733-1736.
[3]Amr A E-G E, Sabry N M, Abdulla M M. Synthesis, Reactions, and Anti-inflammatory activity of heterocyclic systems fused to a thiophene moiety using citrazinic acid as synthon[J].Monatsh. Chem., 2007,138:699-707.
[4]Sakata K-I, Someya M, Matsumoto Y, et al. Gimeracil,an inhibitor of dihydropyrimidine dehydrogenase, inhibits the early step in homologous recombination[J].Cancer Sci., 2011,102:1712-1716.
[5]Atwal K S, Swanson B N, Unger S E, et al. Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1,2,3,4-tetrahydro-6-methyl-5-pyrimid-inecarboxylic acid esters as orally effective antihypertensive agents[J].J. Med. Chem., 1991,34:806-811.
[6]Nagarathnam D, Miao S W, Lagu B, et al. Design and Synthesis of Novel α1aAdrenoceptor-Selective Antagonists.1.Structure-Activity Relationship in Dihydropyrimidinones[J].J. Med. Chem., 1999,42:4764-4777.
[7]Barrow J C, Nantermet P G, Selnick H G, et al. In Vitro and in Vivo Evaluation of Dihydropyrimidinone C-5 Amides as Potent and Selective α-1A Receptor Antagonists for the Treatment of Benign Prostatic Hyperplasia[J].J. Med. Chem., 2000,43:2703-2718.
[8]Deres K, Schroder C H, Paessens A, et al. Inhibition of Hepatitis B Virus Replication by Drug-Induced Depletion of Nucleocapsids[J].Science, 2003:299,893.
[9]Ramesh B, Bhalgat C M, Novel dihydropyrimidines and its pyrazole derivations: Synthesis and pharmacological screening[J].Eur. J. Med. Chem., 2011,46:1882-1891.
[10]Franklin A S, Ly S K, Mackin G H, et al. Application of the Tethered Biginelli Reaction for Enantioselective Synthesis of Batzelladine Alkaloids. Absolute Configuration of the Tricyclic Guanidine Portion of Batzelladine B[J].J. Org. Chem., 1999, 64:1512-1519.
[11]Faulkner D J. Marine natural products[J].J. Nat. Prod. Rep., 1999,16:155-198.
[12]Biginelli P. Aldehyde-urea derivatives of aceto-and oxaloacetic acids[J].Gazz. Chim. Ital, 1893,23:360-413.
[13]Perez-Rebolledo A, Ayala J D, de Lima G M, et al. Structural studies and cytotoxic activity of N (4)-phenyl-2-benzoylpyridine thiosemicarbazone Sn (IV) complexes[J].Eur. J. Med. Chem., 2005,40:467-472.
[14]Bazgir A, Khanaposhtani M M, Soorki A A. One-pot synthesis and antibacterial activities of pyrazolo [4′, 3′: 5, 6] pyrido [2, 3-d] pyrimidine-dione derivatives[J].Bioorg. Med. Chem. Lett., 2008,18:5800-5803.
[15]Nair V, Chi G, Shu Q, et al. A heterocyclic molecule with significant activity against dengue virus[J].Bioorg. Med. Chem. Lett., 2009,19:1425-1427.
[16]Han Z G, Miao C B, Shi F, et al. Diversity synthesis of N-substituted 2-amino-1, 6-naphthyridine derivatives under microwave irradiation[J].J. Comb. Chem., 2009,12:16-19.
[17]Kappe C O. For a review of the Biginelli reaction, see[J].Tetrahedron, 1993,49:6937.
[18]Holla B S, Rao B S, Sarojini B K, et al. One pot synthesis of thiazolodihydropyrimidinones and evaluation of their anticancer activity[J].Eur. J. Med. Chen., 2004,39:777-783.
[19]Kappe C O. Biologically active dihydropyrimidones of the Biginelli-type—a literature survey[J].Eur. J. Med. Chem., 2000,35:1043-1052.
[20]Vilar S, Quezada E, Santana L, et al. Design, synthesis, and vasorelaxant and platelet antiaggregatory activities of coumarin-resveratrol hybrids[J].Bioorg. Med. Chem. Lett. 2006,16:257-261.
[21]Vilar S, Quezada E, Alcaide C, et al. Quantitative Structure Vasodilatory Activity Relationship. Synthesis and "In Silico" and "In Vitro" Evaluation of Resveratrol-Coumarin Hybrids[J].Qsar Comb. Sci. 2007, 26:317-332.
[22]Meunier B. Hybrid molecules with a dual mode of action: Dream or reality[J].Acc. Chem. Res. 2007,41:69-77.
[23]Gross G A, Wurziger H, Schober A. Solid-Phase Synthesis of 4, 6-Diaryl-3, 4-dihydropyrimidine-2 (1 H)-one-5-carboxylic Acid Amide Derivatives: A Biginelli Three-Component Condensation Protocol Based on Immobilized β-Ketoamides[J].J. Comb. Chem, 2006,8:153-155.
[24]Pisani L, Prokopcova H, Kremsner J M, et al. 5-Aroyl-3, 4-dihydropyrimidin-2-one library generation via automated sequential and parallel microwave-assisted synthesis techniques[J].J. Comb. Chem., 2007,9:415-421.
[25]Wang R, Liu Z J, et al. Solvent-Free and Catalyst-Free Biginelli Reaction To Synthesize Ferrocenoyl Dihydropyrimidine and Kinetic Method To Express Radical-Scavenging Ability[J].J. Org. Chem., 2012,77:3952-3958.
[26]Wang D C, Guo H M, Qu G R, et al. Efficient, Green, Solvent-Free Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones via Biginelli Reaction Catalyzed by Cu(NO3)2·3H2O[J].Synth. Commun. 2010,40:1115-1122.
[27]Chen W Y, Qin S D, Jin J R. Efficient Biginelli Reaction Catalyzed by Sulfamic Acid or Silica Sulfuric Acid under Solvent-Free Conditions[J].Synth. Commun. 2007,37:47-52.
[28]Wondrak G T, et al. Redox-directed cancer therapeutics: molecular mechanisms and opportunities[J].Antioxid. Redox Sign. 2009,11:3013-3069.
[29]Avonto C, Taglialatela-Scafati O, Pollastro F, et al. An NMR Spectroscopic Method to Identify and Classify Thiol-Trapping Agents: Revival of Michael Acceptors for Drug Discovery[J].Angew. Chem. Int. Ed. 2011,50:467-471.
[30]Amslinger S, The Tunable Functionality of α, β-Unsaturated Carbonyl Compounds Enables Their Differential Application in Biological Systems[J].ChemMedChem 2010,5:351-356.
* 收稿日期:2016-01-13
DOI:10.13698/j.cnki.cn36-1037/c.2016.03.013
基金项目:国家自然科学基金(21461002);江西省自然科学基金(20151BAB213005);研究生创新专项资金项目(YCX14B005)
作者简介:路东亮(1985-),男,河南林州人,赣南师范学院化学化工学院讲师、博士,研究方向:光功能药物分子的设计合成与活性评价.
中图分类号:TQ463
文献标志码:A
文章编号:1004-8332(2016)03-0051-04
Design and Synthesis of a Novel Hybrid Containing Pyrimidone and Cinnamic Acid
LU Dongliang, XING Yanling, GAO Xiaopei
(ShoolofChemistryandChemicalEngineering,GannanNormalUniversitry,Ganzhou341000,China)
Abstract:3, 4-Dihydropyrimidin-2(1H)-one of cinnamic acid, which has dual-pharmacophore due to the backbone combined by 3,4-dihydropyrimidone and hydroxycinnamic acid compound was synthesized in good yield by a one-pot multi-component cyclocondensation using (1E, 4Z)-5-hydroxy-1-(3, 4, 5-trimethoxyphenyl)hexa-1,4-dien-3-one, 3, 4, 5-trimethoxybenzaldehyde and urea. Orthogonal test was used to explore the optimum reaction conditions. The structure of the compound was identified by XRD,1H-NMR,13C-NMR and IR. This method have the advantages of simple work-up procedure, economy and under environmentally friendly conditions, which are acceptable in the context of green synthesis.
Key words:biginelli reaction; one-pot; hybrids; new dihydropyrimidinone
网络出版地址:http://www.cnki.net/kcms/detail/36.1037.C.20160510.1210.024.html

