Distribution of Mineral Elements and Influencing Factors in Scalp Hair of Childbearing Age Women in the County of Xinghe, Inner Mongolia of China
ZHOU Shan-shan, LIU Ying*
1. College of Life and Environmental Sciences, Minzu University of China, Beijing 100081, China
2. Beijing Engineering Research Center of Food Environment and Public Health, Minzu University of China, Beijing 100081, China
Distribution of Mineral Elements and Influencing Factors in Scalp Hair of Childbearing Age Women in the County of Xinghe, Inner Mongolia of China
ZHOU Shan-shan1,2, LIU Ying1,2*
1. College of Life and Environmental Sciences, Minzu University of China, Beijing 100081, China
2. Beijing Engineering Research Center of Food Environment and Public Health, Minzu University of China, Beijing 100081, China
The distribution and influencing factors on age, reproductive history and dietary habits in scalp hair of rural childbearing female in the county of XingHe, Inner Mongolia of China were studied. 21 mineral elements including essential and toxic elements (Al, B, Ba, Ca, Cd, Co, Cr, Cu, Fe, Mg, Mn, Mo, Ni, Pb, Se, Si, Sn, Sr, Ti, V and Zn ) in scalp hair samples from 180 females of childbearing age were measured with inductively coupled plasma-atomic emission spectrometry (ICP-AES) and atomic fluorescence spectrometry (AFS), respectively. The results demonstrated that the content of the most mineral elements (B, Cd, Co, Cr, Cu, Fe, Mg, Mn, Mo, Ni, Pb, Se, Si, Sn, Sr, Ti, V and Zn) existed in a descending order in the hair of young age group (18~29 years) to the elder age group (40~45 years), while elements of Ca, Mg, Mn and Pb were found to have the lowest content in the middle age group (30~39 years). Women having two children were more likely to have the lowest content of Ca, Mg, Mn, Mo, Ni, Pb, Sn, Sr and Zn due to the increased numbers of pregnancies, the statistically correlations had been proved among Ca, Pb and Sn. In addition, dietary habits also can affect the level of the mineral elements in hair, for example, consumingsuancaifood frequently can cause lower level of Zn, Fe, Cu, Mn, Sr, Mo and Pb, but higher Se, the frequent intake of meat resulted in increased content of Zn and Se. The individuals that declared intaking vegetables regularly had more Si in their hair. Also, daily intake of fruit leads to higher level of Mn, Ni and Ti. This study will provide basic and useful information when addressing reproductive and women health challenges in the rural areas where poor dietary habits are prevailing.
Distribution of mineral elements; Influencing factor; Scalp hair; Rural female at childbearing age; The county of Xinghe, Inner Mongolia of China
Introduction
Recently, increasing role of various biomonitoring techniques in estimation of the nutritional status of individuals has been underlined. As compared to other human biological matrices (e.g. blood, urine and organ), human hair is stable and therefore useful as a biomarker. Elements found in hair originate from blood, which provide nutrition to hair root. The advantage of using hair as a biomarker include easiness and painlessness of sampling, storage, the possibility of monitoring past exposure.
So far, many research literatures on the application of hair mineral analysis (HMA) in forensic and clinical toxicology, occupational medicine, bio-archeological studies and health assessments have been published. The statistically significant differences between exposed and non-exposed populations and the higher levels of toxic elements were confirmed, respectively. Also, HMA is used as the tool to diagnose mineral status of an organism and to prescribe supplementation programs[1]. Especially, the prognosis of increased probability of various diseases, e.g. autism, cancer using biomarker was carried out[2].
HMA is a very useful test in investigation of mineral status of human organism, which will give information on the diet, environmental exposure, health problems, medicines or supplements taken. Usually, the influence of some factors including age, sex, health condition, occupation, etc. can obviously cause the change in element levels, whereas the influence of other factors (color of hair, Body Mass Index (BMI) of the subject, etc.) is obscure. Studies had reported the different level and influence factors of mineral elements on sub-population, such as children, centenarians and so on. The distribution and influence factors of mineral elements in hair of women in reproductive period are scarce to some extent.
Women of childbearing age are at risk of nutrient deficiencies due to poor diets and higher requirements for micronutrients, such as Ca and Fe especially in pre -pregnancy period and during pregnancy. Dietary intake resulting in suboptimal Se status has been associated with increased risk of pre-eclampsia[3]. The effects of age and BMI on the essential elements have been studied in Korean and Taiwan female adult[4-5]. Five elements (Ca, Mg, Fe, Cu and Zn) have been discussed according to age and dietary intakes in the hair of Poland women of reproductive age[6].
Recognition of mineral elements deficiencies in women of reproductive age is important not only because nutritional status affects women’s health and wellbeing, but also because deficiencies are associated with adverse pregnancy outcomes. Also, deficiencies in micronutrients can have adverse consequences on postmenopausal morbidity. There is no biochemical data on the mineral elements status of women at childbearing age in Inner Mongolia of China. The aims of the present study are to determine (1) the content distribution of selected 21 mineral elements in women of childbearing age; (2) the effect of age and reproductive history on the level of mineral elements; and (3) the relationships between dietary habits and elements contents that may exist in this population.
1 Materials and methods
1.1 Sample collection and analysis
The hair samples were provided by 180 healthy female volunteers (aged 18~45 on average 32.63±5.85 years age groups), who previously filled a questionnaire and declared having no adverse pregnancy experiences, lived in the county of Xinghe (situated at the central of Inner Mongolia, China with 314 800 residents). The hair (about 1 cm long from the nape of the neck) was collected with stainless steel scissors, coded and stored in clean polyethylene bags, transported to the laboratory and stored until analysis for chemical elements content.
The analyses of hair were finished by accredited diagnostics laboratories (China national analytical center, Guangzhou, China) by using inductively coupled plasma-atomic emission spectrometry (ICP-AES) and atomic fluorescence spectrometry (AFS).
1.2 Statistical analysis
Statistical analysis was comprised of the basic statistical parameters including mean, standard deviation, and the Kolgomorov-Smirnov test and non-parametric test (Mann-Whitney’s U test and Kruskal-Wallis test). The results were elaborated statistically by Statistical Packages for Social Sciences (SPSS19.0). Results were considered significantly different whenp<0.1.
2 Results and discussion
2.1 Distribution of mineral elements according to age and reproductive history
The distribution of 21 mineral elements according to age (a) and reproductive history (b) in the hair was shown in Fig.1. From Fig.1(a), it shows in the following order: the most metal content (B, Cd, Co, Cr, Cu, Fe, Mg, Mn, Mo, Ni, Pb, Se, Si, Sn, Sr, Ti, V and Zn) decrease with age, while the level of Cu and Fe increase with age. The lowest content of elements Ba, Ca, Mn, Ni, Pb, Si and V were found in age 30~39. Moreover, Ca and Ni of young age group (18~29 years) were markedly higher in comparison with that in the senior age group (40~45 years), and lower levels of Mn, Pb and Si were found in young aged group. Furthmore, as for the effect of reproductive history, mineral elements presented different tendencies. According to Fig. 1b, it showed that content of Ca, Mg, Mn, Mo, Ni, Pb, Sn, Sr and Zn decrease with the increase of number of pregnancies. The level of B, Cd, Co, Cr, Cu and Se increase with the first pregnancy but decreased with the second pregnancy. The essential element Fe is the only one that decreases with first pregnancy then increases with the second pregnancy.
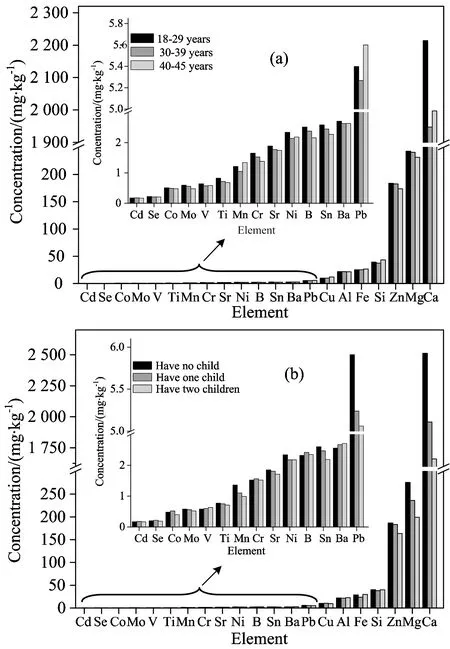
Fig.1 The mean concentration of mineral elements according to age (a) and reproductive history (b)
2.2 The effect of age on the level of mineral elements
The tendency that mineral elements (B, Cd, Co, Cr, Cu, Fe, Mg, Mn, Mo, Ni, Pb, Se, Si, Sn, Sr, Ti, V and Zn) decrease with age is suggested that young people might absorb higher doses of metals via inhalation and ingestion. The relationships between age and mineral elements are presented in Table 1.
Table 1 Spearman’s correlation coefficients for age, productive history and elements in hair of women at childbearing age
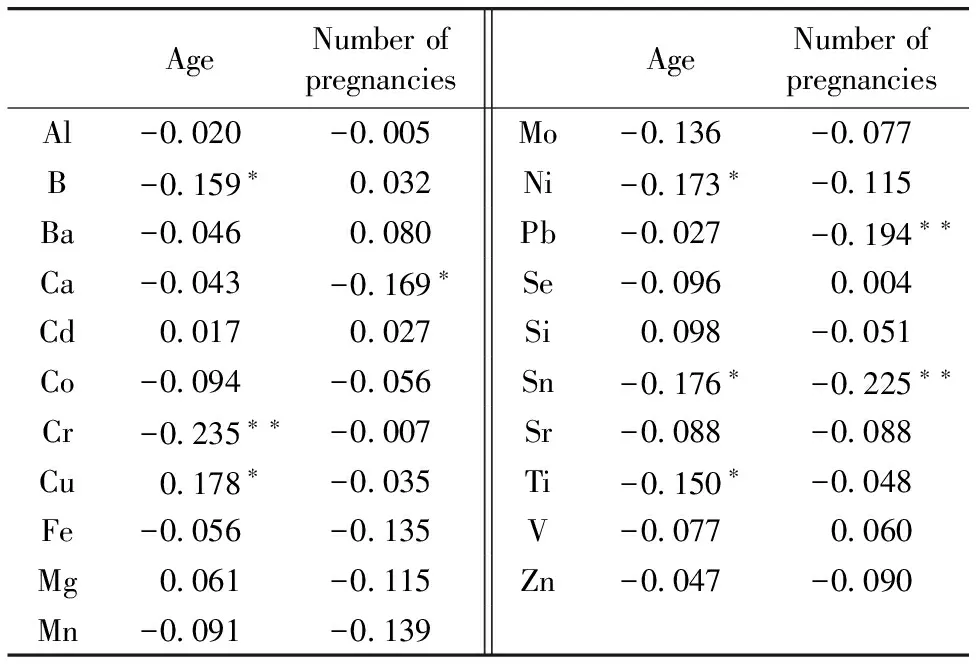
AgeNumberofpregnanciesAgeNumberofpregnanciesAl-0 020-0 005Mo-0 136-0 077B-0 159∗0 032Ni-0 173∗-0 115Ba-0 0460 080Pb-0 027-0 194∗∗Ca-0 043-0 169∗Se-0 0960 004Cd0 0170 027Si0 098-0 051Co-0 094-0 056Sn-0 176∗-0 225∗∗Cr-0 235∗∗-0 007Sr-0 088-0 088Cu0 178∗-0 035Ti-0 150∗-0 048Fe-0 056-0 135V-0 0770 060Mg0 061-0 115Zn-0 047-0 090Mn-0 091-0 139
Based on the Krusal-Wallis test, the differences of B, Cr, Fe, Mo, Sn and Ti content in the three agegroups are significant (p<0.05), which is supposed to be a reason why metabolic rate decreases with age[7]. This finding differs from a study conducted in a sample of Korean adult female[4], which showed that age-dependent increases in hair levels of Cr (r=0.280,p<0.05). The difference among age groups for Cu (r=0.178,p<0.05) was statistically observable in opposite tendency with above elements. There are differences with Poland premenopausal women[6], that the lowest content of Cu and Zn were recorded in the middle age group. The possible reason was that the women in Poland most become pregnant in that decade of their lives. The correlation coefficients (r) (Table 1) between Al, Ba, Ca, Co, Fe, Mn, Mo, Pb, Se, Sr, V, Zn and ages are negative but not statistically significant. This may be due to that the studied women from whom the hair samples were collected were limited in childbearing ages and different from literatures[7]whose sample sources contain postmenopausal women.
From literature[8], women have higher levels toxic element Cd in body, which is a result of bioaccumulatiog, and has a biological half-life of 10~30 years because of depleted Fe stores or deficiency among women of childbearing age. In our study, the coefficient between hair Cd content and age was positive but not statistically significant. The tiny accumulation of Cd in studied objects may due to lower content of Cd exposure. Pb, which was reported may affect adult cognitive function, was associated with hypertension, peripheral vascular disease, increased adult mortality[8], and cognitive decline in older people with higher Pb values. The changes of Pb were the same with Mn and Si, but unlike those of Ca, Si, V and Ni in hair, which was recorded higher content in the young age group than the senior aged group. It may be a consequence of women becoming pregnant in that decade of their lives.
2.3 The effect of number of pregnancies on the level of mineral elements
Based on the Krusal-Wallis test that is used to Fig.1(b), the differences of Ca, Fe, Pb and Sn content are striking (p<0.05). It indicated that the mineral elements in women hair at reproductive age were likely to be affected by number of pregnancy. In our research, there is a significant negative correlation between number of pregnancy and hair Ca, Pb and Sn (p<0.05), that is, the content of some elements decrease with the number of pregnancies increase. As we all known, during gestation, elements circulating in the blood of pregnant women can reach the fetus, the mother-to-child transmission of these substances is modulated by the placenta, where these substances including essential elements (Ca, Cu, Zn, Se and Mn) during fetus growth and development can cross easily. Sometimes maternal under-nutrition or deficiency, fetus even can rob these nutrition substances from gravida to meet their development need. In addition, toxic elements Cd and Pb are associated with alterations in fetus development and are not needed for fetus. The placental barrier, which protected the fetus from Pb exposure to a limited degree, could work most strongly against Cd[8]. The different changes of hair Cd and Pb with the times of pregnancies change may due to the mother-to-child transmission by the placenta.
In China, women usually improve the macro- and micro-nutrient intake in the first few months after delivery, and then followed dietary habits as pre-pregnancy. Regarding food intake, rural residents appeared to consume more grains and fewer animal-based foods, which is well known to be associated with a lower energy and macro- as well as micro-nutrient intake. According to the 2002 National Health and Nutrition Examination Survey (NHANES), the Chinese still consume a largely plant-based diet providing at least 50% of dietary energy and nutrients. The high level of phytates in these foods decreases the bioavailability of critical nutrients by forming insoluble and indigestible complexes.
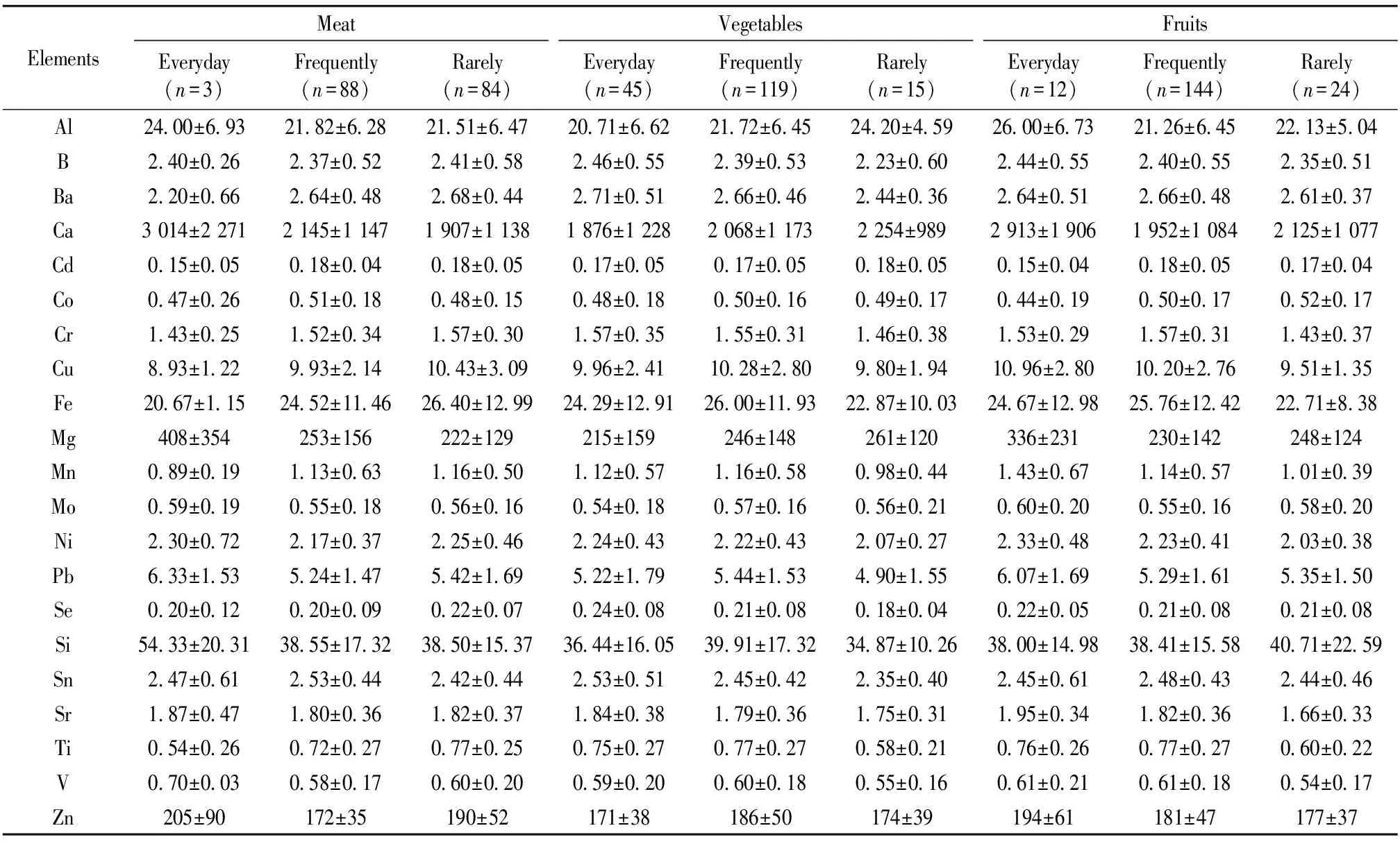
Table 2 The effect of meat, vegetables and fruit intake frequency on the contents of elements (mean±SD) in hair of reproductive age women in Xinghe County
2.4 The effect of meat, vegetable and fruit intake frequency on the level of mineral elements
In this paper, the effect of dietary habits on the 21 mineral elements in hair was studied. All participants were divided according to the frequent food consumption (rarely-about 1 to 3 days in one week, frequently-about 4 to 6 days in one week and everyday). The Krusal-Wallis test showed that the mean content of Se (p<0.05), Ti and Se (p<0.05) and Mn, Cr, Ni, Ti and Al (p<0.05) were significantly different among different meat intake frequency groups, various vegetable intake frequency groups and different fruit intake frequency groups, respectively (table 2). Meat is most important sources of dietary Fe, Zn and Se. But studies indicated that phytates inhibit the absorption of Fe, and Zn[6]. Low Fe stores occur with relatively high frequencies in fertile women and with lower frequencies in postmenopausal women. Young women are at high risk because of the effects of menstruation and pregnancy. On one hand, Fe deficiency does not only increase the efficiency of Fe absorption, it seems to affect absorption of chemically related elements such as Mn and possibly also toxic elements. On the other hand, Zn deficiency leads to mucosal dystrophy, which would reduce the absorption of the polyglutamine forms of folate and other nutrients.
It is well-known that Vitamin C is a strong promoter of Fe absorption from the diet and can counteract the inhibitory effects of dietary phytate. However, it has been suggested that long-term Vitamin C supplementation may impair the absorption of Cu and thereby counteract the positive effect on Fe absorption[9]. The strongest promoter of non-heme Fe absorption is Vitamin C from fresh fruits or vegetables. Only 8.3% of the participants rarely intake vegetable and 13% rarely intake fruit, and the mean levels of Fe were 22.87 and 22.71 mg·kg-1, respectively. All of them were lower than other two groups who were vegetable and fruit intake frequently and everyday. The hair Cu increased first with vegetable intake infrequency increase then decreased, but it kept increasing with fruit intake frequency increase. It suggested that Vitamin C in fruit did not hinder the absorption of Cu. Vitamin C appears to affect Se availability both positively and negatively depending on chemical form and dietary conditions, while Zn absorption seems to be unaffected. The interaction between Vitamin C and Fe takes place in the gastrointestinal tract; positive effects on non-heme Fe absorption are the greatest when promoters are consumed in the same meal[9]. Vegetables and fruit high in Vitamin C are good alternatives for improving Fe absorption. Small amounts of meat, complementing a largely vegetable-based dish, can boost the availability of non-heme Fe[10]. Unfortunately, the hair Fe in the various food intake frequency groups was not differed significantly. But subjects who intake meat and vegetable in meantime everyday had the highest level of Zn and the lowest level of Cu and Fe. That is, the intake of Zn affected Fe status. The negative correlation between Zn in hair and the intake of Fe, demonstrated in this study, is probably due to the interaction between the minerals. These interactions occur at the stage of their absorption and transport. It suggested that women in the area need some changes in food pattern.
2.5 The effect of suancai on the level of mineral elements
The effect ofsuancai(a kind of traditional Chinese salted and fermented vegetable) on the content of elements in hair is also studied (Table 3). The results indicated that the mean content of hair Zn (p=0.044), Fe (p=0.036), Cu (p=0.031), Mn (p=0.02), Sr (p=0.02) and Pb (p=0.08) were significantly different betweensuancaiintake frequency groups based on Krusal-Wallis test. As discussed above, phytate is known to be the major dietary inhibitor.Suancai’s preparation can enhances the Fe absorption by reducing the levels of phytates in plant foods by fermentation, germination and soaking[10]. In present study, the higher content of hair B, Co, Mo, Sr, Zn were founded in subjects who intakesuancairarely, the lowest content were founded in subjects who intakesuancaifrequently. The level of Ca, Cu, Fe, Mn, Pb and Sn decreased with the increasingsuancaiintake frequency. Although the phytates in vegetable was reduced, the high dietary salt may affect the mineral elements absorption. The high-sodium diet significantly reduced Ca retention[11]. The interactions between Fe, Zn and Cu appear to be especially important. In populations where the major reason for Fe deficiency is a poor availability of Fe from the diet, there is also a risk for marginal Zn status especially in growing individuals.
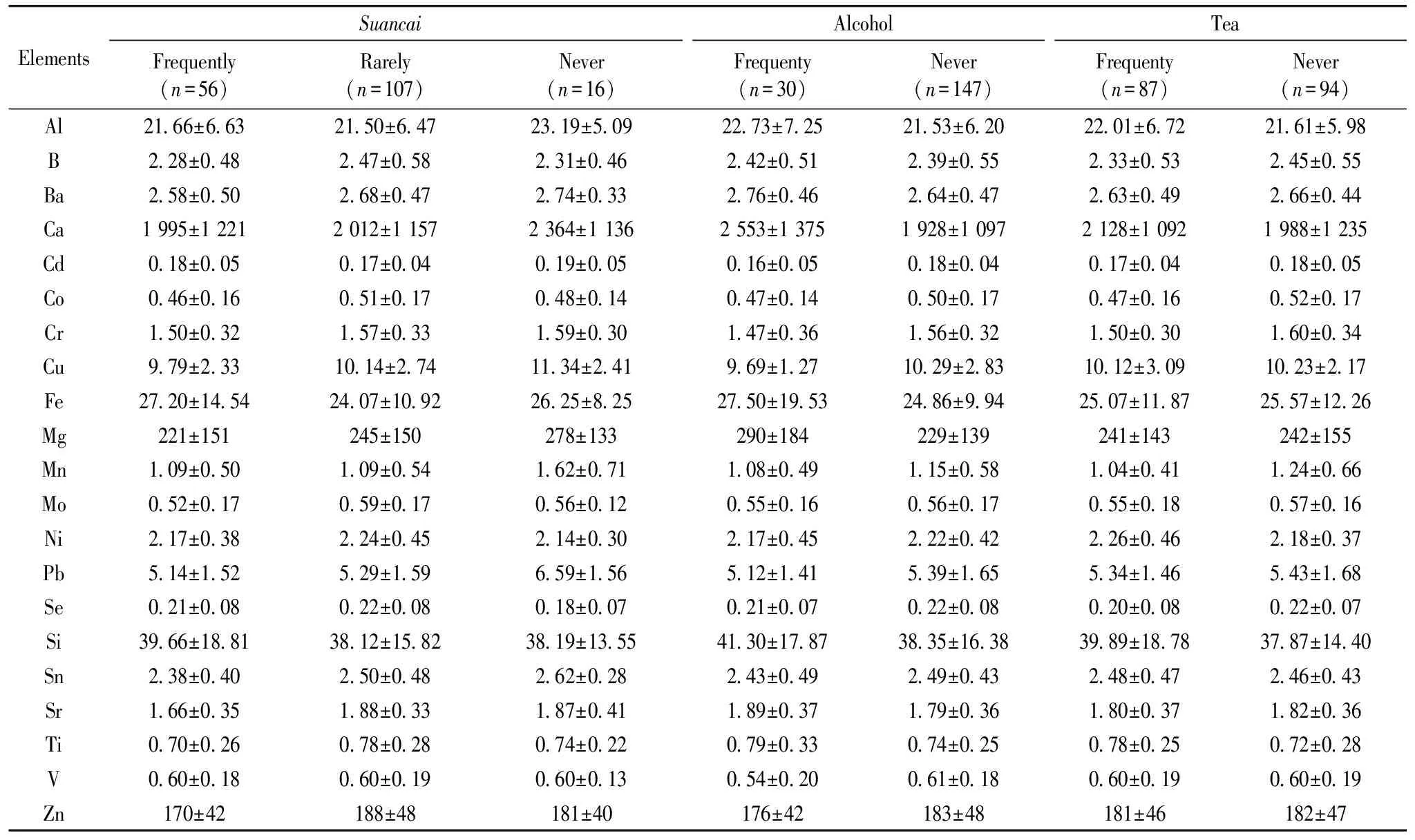
Table 3 The effect of suancai, alcohol and tea intaeke frequency on the contents of elements (mean±SD) in hair of reproductive women in Xinghe County
2.6 The effect of alcohol drink on the level of mineral elements
The relationships between drinking behavior and the content of twenty-one mineral elements (Table 3) showed that except for Zn, Cu, the mean content of Ca, Mg, Fe, Al and Si are higher in people who drank alcohol compared with those who never drink. The differences of Ca (p<0.05), Fe and Cd are statistically significant (p<0.1), while other metals fail to achieve statistical significance. These results revealed that drinking might be having some effects in metals metabolic process. Several studies indicate that Cd gains access to testicular cells by mimicking Zn at the site of Zn-transporters[12]. Same enzymes from which Cd could potentially displace Zn include lactic dehydrogenase, carboxypeptidase and alcohol dehydrogenase, which serve to break down alcohols that otherwise are toxic[12]. The only one who intake alcohol frequently has the lowest content of hair Zn, Fe, Cu, Mn, Mo, Co, V, Ti, Sn, Al, Si and Se, but the highest hair Cd. The result was inconsistent with other study[13], which reported higher content of Ni when compared with individuals who did not drink alcohol. The difference may due to that they studied male and female.
2.7 The effect of tea drink on the level of mineral elements
The relation between drinking tea and hair mineral content was presented in Table 3. The groups were formed according to the frequency of drinking: Never-never drinking tea, Rarely-about 1 to 3 days in a week. Based on Mann-Whitney’s U test, the differences statistically significant (atp<0.1) between the groups were found for: Cap=0.079, Mnp=0.053, Cop=0.093, Crp=0.078, Tip=0.089. Mn affects Fe absorption in a way that indicates that the intestine cannot differentiate between Mn and Fe. A high Mn intake, as could be the case in tea drinking populations, may therefore impose a risk of reduced Fe utilization[9].
Milk and dairy products, Tofu dishes or secondary tofu products are greatly enriched in Ca, but the content of hair Ca is not associated with different intake frequency. Diets that have adequate amounts of Ca will reduce Pb absorption and may provide additional protection against Pb toxicity by inhibiting the adverse effects of Pb on Ca -mediated cellular functions. There is considerable evidence that a diet low in Ca can enhance gastrointestinal Pb absorption and toxicity in humans and experimental animals[14]. The changes of content of hair Ca and Pb from the young age group to the senior age group may confirm the conclusion above.
3 Conclusions
Based on the hair mineral analyses in women of reproductive age, we studied the distribution of the 21 mineral elements and influence factors, such as age, reproductive history and dietary habits. It was found that the content of most mineral elements (B, Cd, Co, Cr, Cu, Fe, Mg, Mn, Mo, Ni, Pb, Se, Si, Sn, Sr, Ti, V and Zn) existed in a descending order in the hair of young age group to the elder aged group. However, elements of Ca, Mg, Mn and Pb were found to have the lowest content in the middle age group. Women having two children were more likely to have the lowest content of Ca, Mg, Mn, Mo, Ni, Pb, Sn, Sr and Zn due to the increase of number of pregnancy, but this effect was not tested statistically in most of the 21 elements. Women who frequently intake meats have increased level of Zn and Se in hair. The average of content of Ti and Se were significantly different between various vegetable intake frequency groups. Mn, Cr, Ni, Ti and Al were significantly different between different fruit intake frequency groups. The individuals, who declared the intake ofsuancaifrequently, had lower level of Zn, Fe, Cu, Mn, Sr, Mo and Pb in their hair. Drinking alcohol contributes to the higher level of Cd in hair. Drinking tea reduced the level of Mn and Cr. HMA can be used to assess the risk with regards to intake of alcohol or supplements. Diet diversity is suggested as the key intervention to improve the macro- and micro-nutrient intake of women at childbearing age living in rural areas of Inner Mongolia. Based on the study public health interventions involved pregnant and non-pregnant women who needed to address nutrient deficiencies in this population can be developed effectively.
[1] Chojnacka K, Zielin′ ska A, Michalak I, et al. Environ. Toxicol. Pharmacol.,2010, 30: 188.
[2] Wolowiec P, Michalak I, Chojnacka K, et al. Clinica Chimica Acta, 2013, 419: 139.
[3] Rayman M P, Searle E, Kelly L, et al. Br. J. Nutr., 2014, 112(1): 99.
[4] Hong S R, Lee S M, Lim N R, et al. Nutr. Res. Pract., 2009, 3(3): 212.
[5] Wang C T, Chang W T, Zeng W F, et al. Clin. Chem. Lab. Med., 2005, 43(4): 389.
[6] Suliburska J. Biol. Trace Elem. Res., 2011, 144: 77.
[7] Luo R, Zhuo X, Ma D, et al. Ecotoxicol. Environ. Saf., 2014, 104: 215.
[8] Esteban-Vasallo M D, Aragonés N, Pollan M. Environ. Health Perspect.,2012, 120(10): 1369.
[9] Sandström B. Br. J. Nutr., 2001, (Suppl. 2): S181.
[10] Gao H Y, Stiller C K, Scherbaum V. Nutrients, 2013, 5: 2933.
[11] Wigertz K, Palacios C, Jackman L A, et al. Am. J. Clin. Nutr., 2005, 81: 845.
[12] Thompson J, Bannigan J. Reprod. Toxicol., 2008, 25(3): 304.
[13] Michalaka I, Mikulewiczb M, Chojnacka K, et al. Environ. Toxicol. Pharmacol., 2012, 34(3): 727.
[14] Han S, Pfizenmaier D H, Garcia E, et al. Environ. Health Perspect., 2000, 108(6): 527.
*通讯联系人
O657.3
A
内蒙古兴和县育龄妇女头发中矿质元素分布及影响因素研究
周珊珊1,2, 刘 颖1,2*
1. 中央民族大学生命与环境科学学院,北京 100081
2. 中央民族大学北京市食品环境与健康工程技术研究中心,北京 100081
使用电感耦合等离子体原子发射光谱(ICP-AES)和原子荧光光谱法(AFS)分别对内蒙古自治区兴和县180名农村育龄妇女头发中21种矿物元素(铝,硼,钡,钙,镉,钴,铬,铜,铁,镁,锰,钼,镍,铅,硒,硅,锡,锶,钛,钒和锌)的含量及年龄、生育史及饮食习惯等对其产生的影响进行了研究。结果表明,大多数矿物元素(硼,镉,铬,钴,铜,铁,镁,锰,钼,镍,铅,硒,硅,锡,锶,钛,钒和锌)含量从青年组(18~29岁)到老年组(40~45岁)依次减少,而中年组(30~39岁)的钙,镁,锰、铅的含量最低。生育二胎的妇女钙,镁,锰,钼,镍,铅,锡,锶和锌含量最低,这可能与生育次数的增加有关,相关性分析证实钙,铅和锡与生育史有显著性相关。此外,饮食习惯也会影响头发中矿物质元素的含量。经常食用酸菜会导致锌,铁,铜,锰,锶含量水平较低,但钼,铅和硒含量水平较高。而且,锌和硒含量随肉类摄入频率增加而增加。规律性摄入蔬菜会增加硅的含量。同时,经常食用水果会增加锰,镍和钛的含量水平。因此,该研究为解决不均衡饮食习惯盛行的农村地区生育和妇女健康提供基础数据和有用的信息。
矿质元素分布;影响因素;头发;生育年龄的农村女性;内蒙古自治区兴和县
2015-09-12,
2015-12-08)
Foundation item:The National Natural Science Foundation of China (21177163), 111 Project B08044, First-class University First Class Academic Program of Minzu University of China (YLDX01013), Coordinate Development of First-class and First-class University Discipline Construction Funds(10301-0150200604), The Academic Team Construction Project of Minzu University of China (2015MDTD25C&13C), Special Guidance Fund of Building World First-Class Universities (Disciplines) and Characteristic Development of Minzu University of China (2016)
10.3964/j.issn.1000-0593(2016)10-3422-07
Received:2015-09-12; accepted:2015-12-08
Biography:ZHOU Shanshan, (1981—), Doctor of College of Life and Environmental Sciences, Minzu University of China e-mail: deleteshan@126.com *Corresponding author e-mail: liuying4300@163.com
- 光谱学与光谱分析的其它文章
- Gd靶激光等离子体光源离带辐射及其等离子体演化的研究
- Probing the Binding of Torasemide to Pepsin and Trypsin by Spectroscopic and Molecular Docking Methods
- Mn(Ⅱ)-5-Br-PADAP共沉淀-火焰原子吸收光谱法测定虾、贝样中的镉
- Near Infrared Spectroscopy Study on Nitrogen in Shortcut Nitrification and Denitrification Using Principal Component Analysis Combined with BP Neural Networks
- 内蒙古草原植被最大光能利用率取值优化研究
- 健康和糖尿病大鼠红细胞荧光光谱非线性程度差异

