Growth and Characteristics of ZnO Needles in the Surface of “Platanus Occidentalis”Made of Al2O3,Au and ZnO
ZHU Lin, CUI Hai-ning*, B. Marí, WANG Yi-xin, WANG Rong*, LI Xiao-xia
1. Department of Optical Information Science and Technology, College of Physics and College of Zhaoqing (526061), Key Lab of Ministry of Education for Coherent Light and Atomic and Mol. Spect., Jilin University,Changchun 130021, China
2. Departament de Física Aplicada-IDF. Universitat Politècnica de València, Camí de Vera s/n, 46022 València, Spain
Growth and Characteristics of ZnO Needles in the Surface of “Platanus Occidentalis”Made of Al2O3,Au and ZnO
ZHU Lin1, CUI Hai-ning1*, B. Marí2, WANG Yi-xin1, WANG Rong1*, LI Xiao-xia1
1. Department of Optical Information Science and Technology, College of Physics and College of Zhaoqing (526061), Key Lab of Ministry of Education for Coherent Light and Atomic and Mol. Spect., Jilin University,Changchun 130021, China
2. Departament de Física Aplicada-IDF. Universitat Politècnica de València, Camí de Vera s/n, 46022 València, Spain
Complex ZnO compound material has great potential applications for optoelectronic devices. In this article, we give report to a simulation growth of a kind of special“fruiting ball”——natural “Platanus Occidentalis”made of Al2O3, Au and ZnO. The surface of “Platanus Occidentalis ”has numerous tiny Au seeds on the surface of Al2O3ball. ZnO needles synthesized by the electrodeposition technique grow well on the Au seed layer. The obtained mono dispersive needles (or hexagonal columns) have different distribution density. The prepared sample looks like the assembled “Platanus Occidentalis”in photographs of scanning electron microscopy (SEM). Photoluminescence (PL) of samples at 6 K temperature and other characteristics are investigated. The appearance of sharp bound exciton (BE) emission line and the longitudinal optical (LO)-phonon replicas imply that the ZnO nanocolumns are of high optical quality.
Hexagonal columns of ZnO; Al2O3-Au-ZnO nanostructure; Photoluminescence; Electrodeposition
Introduction
ZnO films has attracted much attention of researchers as a sensor material, potential ultraviolet (UV),blue and other visible optical device materials due to its wide and direct band gap about 3.1~3.3 eV[1-3]. Especially, a nano/micro structure surface is well suited to be used as different kinds of sensors and optoelectronic devices such as light-emitting diode (LED) etc. It has shown that ZnO thin films can be deposited electrochemically[4], and the epitaxial growth of hexagonal ZnO has been obtained electrochemically on GaN[5]and gold single crystals[6]for getting the column structures. Now the most previous results have shown that the deposited ZnO structure is of various crystalline shapes. These controlling morphology and structure can also be sticks, needles, pentagonal flakes, etc. Further orientation of columns could be random, a well-oriented direction, well-aligned and well-perpendicular to the surface of substrates. They can be of discrete column and high packed columns[7-17]. All these morphology and structures of metal oxides are of great importance for above applications and their implementation on the technological devices. Since nature is the most perfect and powerful by millions years of evolution, here a simulation growth of natural “Platanus Occidentalis”is considered and has been carried out to obtain a kind of special“fruiting ball”——“Platanus Occidentalis”made of Al2O3,Au and ZnO. This kind of complex ZnO material may have greater potential and signification in science and technology in the future.
1 Experimental
To obtain ZnO needles, the electrodeposition method needs a three electrode electrochemical cell containing an aqueous solution of ZnCl2, and KClO4or KCl as supporting electrolyte with dissolved oxygen[7]. Alumina ceramic substrates covered with Au layer by sputtering were located near the referential cathode at approximately 1 cm in the cell. A potentio/galvanostat was used to keep a constant potential during the deposition. The electrochemical cell was placed in a thermoregulated bath and the deposition temperature was changed between 20 and 90 ℃. After deposition, the ZnO films were subsequently rinsed with distilled water and acetone.
Scanned electron microscopy (SEM) images and quantitative elemental analysis were obtained by using a JSM 6300. The photoluminescence was excited by a He—Cd laser operating at 325 nm and captured by a charge coupled device (CCD) camera through a monochromator. A small chamber which could be cooled down to 7 K was used to perform low temperature PL.
2 Results and discussion
The prepared sample looks like the assembled “Platanus Occidentalis”in photographs of SEM. Fig.1, Fig.2 and Fig.3 show SEM images about “Platanus Occidentalis”made of Al2O3,Au and ZnO respectively for Sample A, B and C. Clearly, the surface of “fruiting ball”consists of controlled density of numerous tiny seeds on which needles and hexagonal columns of ZnO are grown. Following our experiments’ process, the spherical core is Al2O3and the thin shell is Au. Numerous tiny seed-like Au compose the shell and they control a density of needles of ZnO. The mono disperse needles (or hexagonal columns) of ZnO are on the Au seed layer. The single needle-resolved Al2O3-Au-ZnO nanostructure was obtained.
As is known to us, electrochemical procedures permit the synthesis of ZnO films on different morphologies depending on the solvent, concentrations of the solutions, temperature, electrical potential, and substrate. Until now,these have been systematically investigated. In Fig.1 ZnO (sample A) was deposited in aqueous solvent with 0.1 mmol·L-1ZnCl2, 100 mmol·L-1KCl, -0.9 V potential and 90 (℃) temperature. Fig.1(a) shows the surface area of the sample A by SEM photographs. Further zoom of photograph (a) was done and it is shown in Fig.1(b). In SEM of the ball with columns,randomized, independent and lower density ZnO columns nearly perpendicular to the substrate of the ball are observed. From Fig.1(b) it is noted that the columns have two kinds of crystals, hexagonal rod style (&) and needle like (#) like our previous work [16].
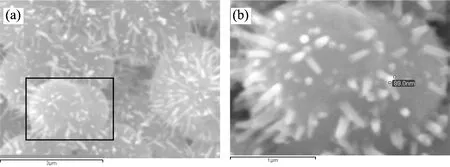
Fig.1 Some SEM images of “Platanus Occidentalis”made of Al2O3,Au and ZnO (Sample A). ZnO was deposited in aqueous solvent with 0.1 mmol·L-1ZnCl2, 100 mmol·L-1KCl, -0.9 V potential and 90 (℃) temperature
(a):SEM image (×20 000, scale bar is 3 μm) of position Ⅱ; (b): Further zoom of (a), SEM: ×50 000, scale bar is 1 μm
Similarly, SEM photographs of “Platanus Occidentalis”in Fig.2 coming from Sample B in which ZnO was deposited in aqueous solvent with 5 mmol·L-1ZnCl2, 100 mmol·L-1KCl, -0.9 V potential and 90 (℃) temperature. Photograph of Fig.2 (b) is the further zoom of image (a). Independent and higher density ZnO columns nearly perpendicular to the substrate of the ball were observed.
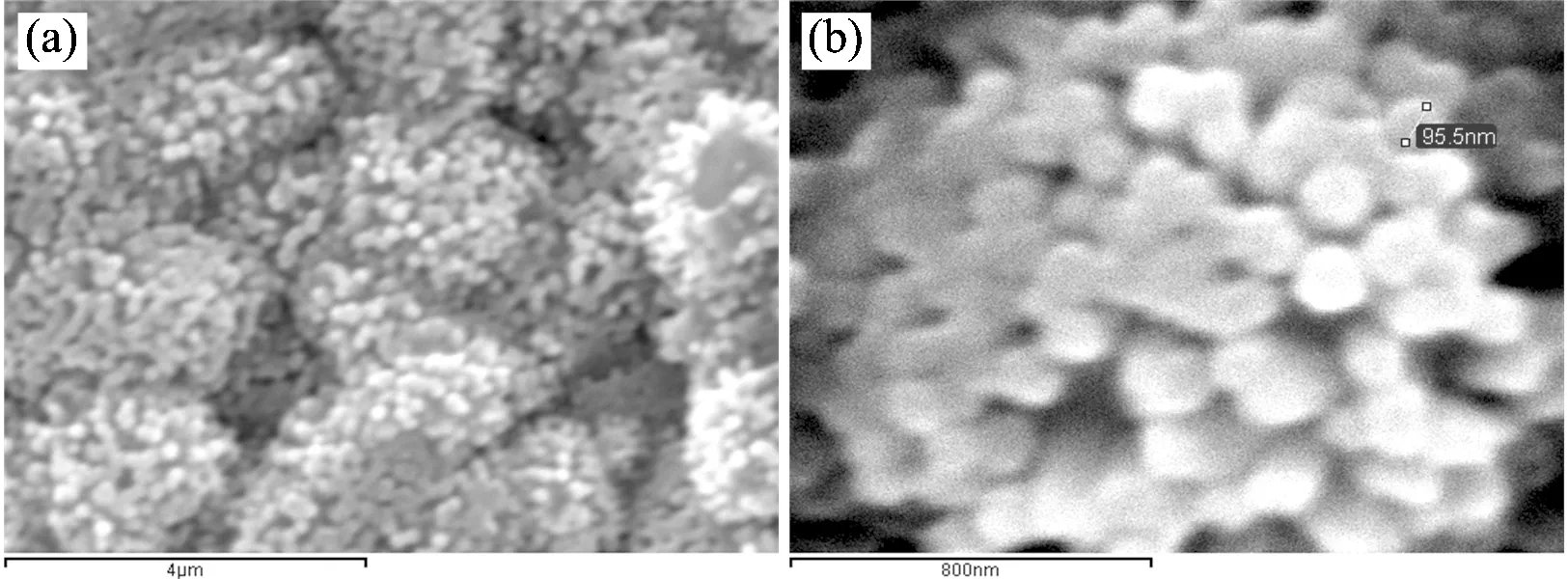
Fig.2 SEM images of “Platanus Occidentalis”made of Al2O3,Au and ZnO (Sample B). ZnO was deposited in aqueous solvent with 5 mmol·L-1ZnCl2, 100 mmol·L-1KCl, -0.9 V potential and 90 (℃) temperature
(a):Photograph of SEM (×15 000, scale bar is 4 μm); (b):Photograph of further zoom of (a), SEM: ×75 000, scale bar is 800 nm
In order to understand the forming process of the “Platanus Occidentalis”micro/nano structrures, we carried out the experiments by varying the ZnO deposition time too. In sample C, ZnO was deposited in aqueous solvent with 5 mmol·L-1ZnCl2, 100 mmol·L-1KCl, -0.8 V potential and 90 (℃) temperature. Fig.3(a) is the SEM image of surfaces Au/Al2O3(base) substrate. Fig.3(b), (c) and (d) are photograph of SEM for 130 seconds, 510 seconds and 1 280 seconds ZnO deposition time respectively. Fig.3(e) and (f) are photographs of further zoom of (c) and (d) respectively. Compared with Fig.3 (a), photograph of surfaces Al2O3/Au substrate investigated by AFM and their images about Al2O3substrate and surface of “Platanus Occidentalis”ball are shown in Fig.4. Fig.4(a) is the surfaces image of Al2O3substrate. Zoom of the surface of one Al2O3ball in Fig.4(a) is shown in Fig.4(b) and further zoom of the Au covered surface of one Al2O3ball is Fig.4(c). Further more, by aqueous ZnO electrodeposition and Au physical vapor deposition, the“fruiting ball”is assembled for a core/shell-shaped structure. It is possible to control the shape, well-oriented ZnO films with hexagonal columns and well-perpendicular to the substrate had been obtained on Au/ITO (base) substrate in aqueous solvent of electrolyte[17].
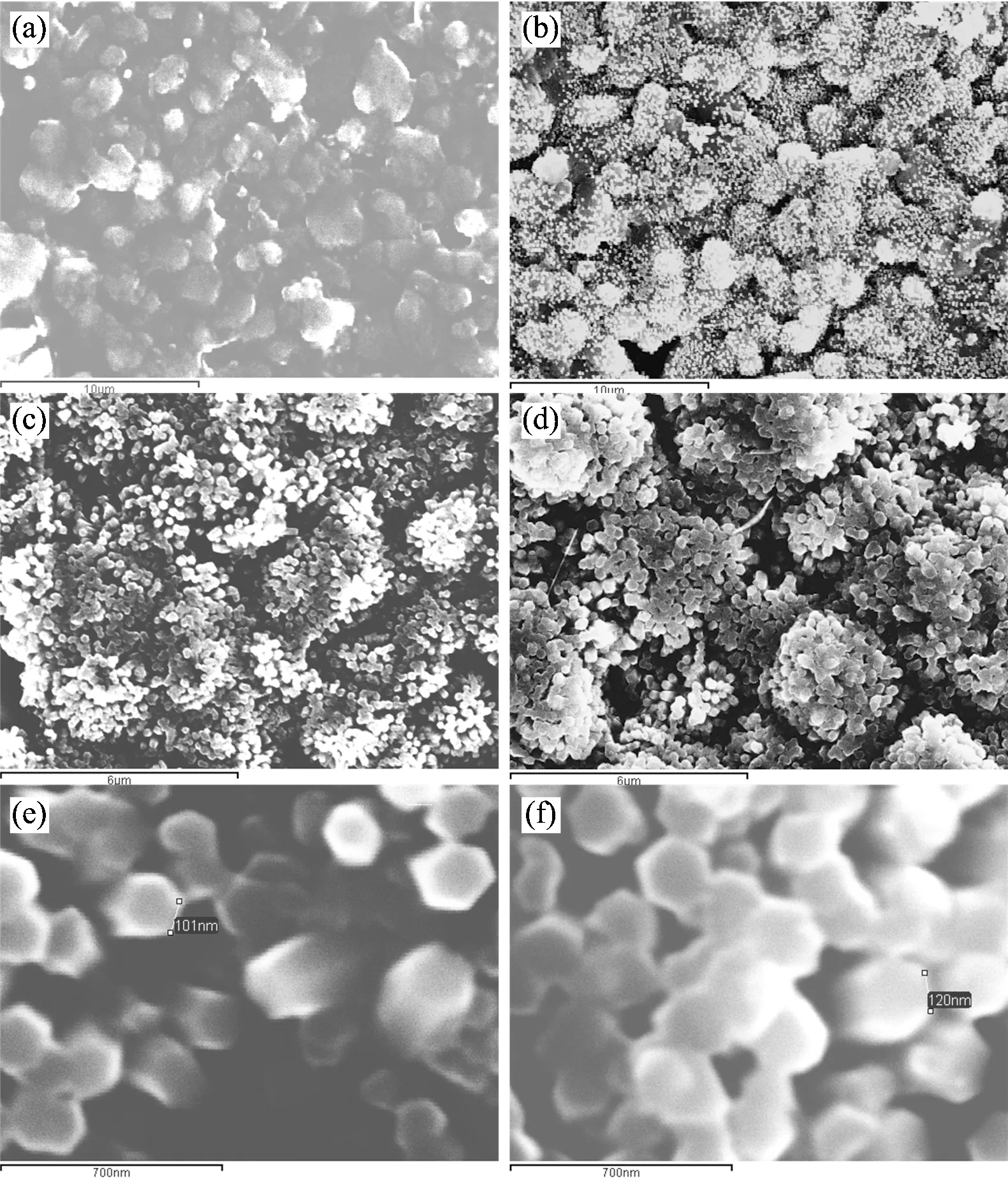
Fig.3 SEM images about “Platanus Occidentalis”made of Al2O3,Au and ZnO (Sample C) in different deposition time. ZnO was deposited in aqueous solvent with 5 mmol·L-1ZnCl2, 100 mmol·L-1KCl, -0.8 V potential and 90 (℃) temperature
(a):Photograph of surfaces Al2O3/Au substrate (×5 000, scale bar is 10 (m); (b):Photograph of SEM for 130 seconds (×5 000, scale bar is 10 (m) ZnO deposition time; (c):Photograph of SEM for 510 seconds (×10 000, scale bar is 6 (m) ; (d):Photograph of SEM for 1 280 seconds (×10 000, scale bar is 6 (m) ; (e):Photograph of further zoom of (c) (×80 000, scale bar is 700 nm); (f):Photograph of further zoom of (d) (×80 000, scale bar is 700 nm)
Well-oriented ZnO films with hexagonal columns and well-perpendicular to the substrate had been obtained on Au/ITO(base) substrate in aqueous solvent of electrolyte[17]. The crystalline characteristic of ZnO columnar structures on Au/ITO(base) and ITO/glass (base) substrate was analyzed and discussed by our previous XRD measurement[16-17]. The results showed that there are five peaks located at 34.4°, 36.3°, 47.6°, 56.6°and 63.5°, which correspond to the (0 0 2), (1 0 1),(1 0 2), (1 1 0) and (1 0 3) directions of the ZnO hexagonal wurtzite structure.
Since optical properties of the oxide films are connected to their morphology and structure, the photoluminescence (PL) spectra of complex ZnO “fruiting ball”were measured at 7 K temperature. PL spectra of samples before and after annealing at 300 ℃ for 60 min are shown in Fig.5. It reveals the existence of three components, a strong band-edge emission (NBE) 378.6 nm and a broader deep level emission (DLE) band with two centers 510 and 660 nm in Fig.5(a). The band 378.6 nm corresponds to one kind of varieties of free exciton (FE: 357 nm) plus one longitudinal optical (LO) phonon replica by the calculation of 72 meV phonon energy[18]. The NBE emission had mostly been attributed to the radiative transition between the electron (in conduction band) and the hole (in valence band) recombination process, and it corresponds to the bound exciton (BE)[17]. Compared with Fig.5(a), prominent NBE band at 380.4 nm corresponds to one kind of varieties of free exciton (FE: 357 nm) plus two LO phonon replica while DLE bands decrease little in Fig.5(b). After annealing. It shows us that there is stable optical quality of ZnO in “fruiting ball”, and defect states of ZnO columns were removed a lot after annealing. The appearance of sharp BE emission line and the LO -phonon replicas imply that the ZnO nanocolumns are of high quality. No further broadening of NBE band in Fig.5(a) and (b) are mainly due to the good quality of crystallographic structure and the stable of order/disorder in the semiconductor.
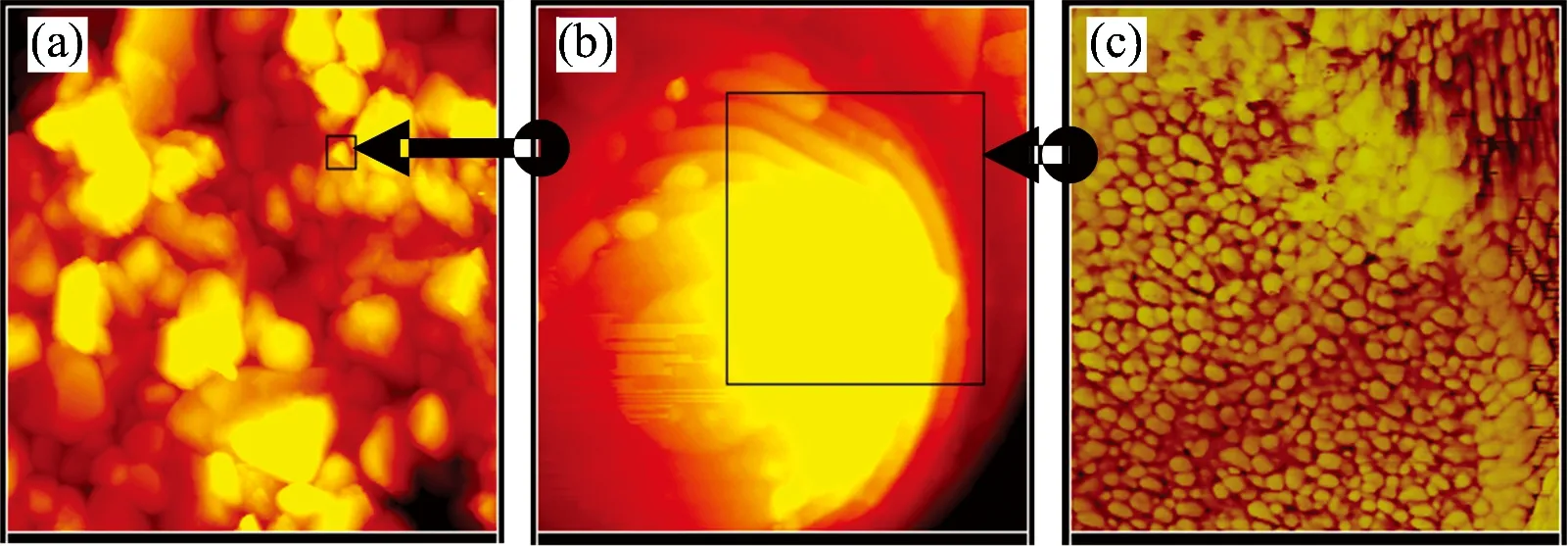
Fig.4 AFM images about Al2O3 substrate and surface of “Platanus Occidentalis”ball
(a): Surfaces photograph of Al2O3substrate; (b): Zoom of the surface of one Al2O3ball in )a); (c): Further zoom of the Au covered surface of one Al2O3ball in (b)

Fig.5 Photoluminescence (PL) spectra of complex ZnO “fruiting ball ”that were measured at 7 K temperature
(a): The sample before annealing at 300 ℃; (b): The sample after annealing at 300 ℃; (c): As comparing, the PL spectra of ZnO films on Au/ITO substrate.
After further study of two components of DLE band in Fig.5(a), one band centering around 510 nm related to oxygen vacancies in inner ZnO crystallites[19-20]. Another emission band at around 660 nm is observed in the PL spectra of ZnO, indicating the existence of macrocrystalline ZnO and confirming the high dispersion (discrete column) of zinc oxide (or no density)[21]. The DLE involves deeply trapped charged carriers in ZnO. In addition some of the possible explanations for the red DLE include the presence of oxygen vacancies[20]and zinc vacancies[22-23]. In our samples, there is no clear variety for NBE band and the green DLE band during annealing. Good optical quality of ZnO column of “fruiting ball”before and after annealing was demonstrated with the PL measurement. As a comparison, the PL spectra of ZnO films on Au/ITO substrate were measured and it is shown in Fig.5 (c). One broader band centering at 650 nm can be observed in all the visible PL spectra. All these tell us that the three samples of Fig.5 have similar optical quality but different defect states.
3 Conclusion
A simulation growth of natural “Platanus Occidentalis”is considered and it has been carrying out. The assembled ball is a kind of nanostructure of Al2O3-Au core-shell with outside needle-shaped ZnO. As to the surface of “fruiting ball ”there are needles (or hexagonal columns) of ZnO with controlled density. The microscopy photos prove natural platanus occidentalis and simulation grown balls with different densities of needles. Studies of PL spectra tell us that there is stable optical quality of ZnO in “fruiting ball”, and defect states of ZnO columns were removed much after annealing. The appearance of sharp BE emission line and the LO -phonon replicas implies that the ZnO nanocolumns are of high quality.
Acknowledgements:Authors would like to thank Prof M. Mollar(Universitat Politècnica de València) for helpful discussions.
[1] Lee J H, Kwon Y H. J. Electrochem. Soc., 2012,159(2):H102.
[2] Cui J. Materials Characterization, 2012,64:43.
[3] Kushiya K, Ohshita M, Hara I. Solar Energy Materials and Solar Cells, 2003,75(6):179.
[4] Han X, Liu R, Xu Z. Thin Solid Films,2009,517:5653.
[5] Pauport′e T, Lincot D. Appl. Phys. Lett., 1999, 75: 3817.
[6] Liu R, Vertegel A A, Bohannan E W. Chem. Mater., 2001, 13: 508.
[7] Tortosa M, Mollar M, Marí B. Journal of Crystal Growth,2007,304(97):97.
[8] Sahal M, Hartiti B, Ridah A. Microelectronics Journal, 2008,39(12):1425.
[9] Marí B, Cembrero J, Mollar M. Phys. Stat. Sol. (c), 2008,5(2):555.
[10] Damonte L C, Hernández‐ Fenollosa M A, Meyer M. Structural Physica B,2007,doi:10.1016/j.physb.2007.04.085.
[11] Donderis V, Hernandez-Fenollosa M A, Damonte L C. Superlattices and Microstructures,2007,42:461.
[12] Tortosa M, Mollar M,Marí B. Journal of Crystal Growth,2007,304:97.
[13] Hernández M A, Damonte L C, Marí B. Superlattices and Microstructures, 2005,38(4-6):336.
[14] Peiró A, Domingo C, Peral J. Thin Solid Films,2005,483:79.
[15] Manjón F J, Marí B, Serrano J. Journal of Applied Physics,2005,97:053516.
[16] Cui H, Mollar M, María B. Optical Materials, 2011,33(3):327.
[17] Sun S, Marí B, Wu H. Applied Surface Science, 2010,257(3):985.
[18] Dai J, Su H, Wang L. J. Cryst. Growth, 2006, 290: 426.
[19] Chen H, Shi J, Chen H. Opt. Mater., 2004, 25: 79.
[20] Vanheusden K, Warren W L, Seager C H. J. Appl. Phys., 1996, 79: 7983.
[21] Chen J, Feng Z, Yingand P. J. Phys. Chem. B, 2004, 108:12669.
[22] Zhao Q, Klason P, Willander M. Appl. Phys. Lett., 2005, 87: 211912.
[23] Liu M, Kitai A H, Mascher P. J. Lumin., 1992, 54: 35.
*通讯联系人
O462.3
A
氧化铝、金和氧化锌构成的类“法国梧桐树”坚果球表面上氧化锌针的生长和特性
朱 琳1,崔海宁1*, B. Marí2, 王钇心1,王 荣1*,李晓霞1
1. 吉林大学物理学院,肇庆学院(526061),吉林 长春 130021
2. Departament de Física Aplicada-IDF. Universitat Politècnica de València, Camí de Vera s/n, 46022 València, Spain
复杂的氧化锌复合材料在光电设备方面有着很大的潜在应用。报道一种特殊 “果球”的模拟生长——由Al2O3,Au和ZnO制成的天然 “法国梧桐悬铃坚果”。这种“法国梧桐悬铃坚果”表面有许多小的、溅射在Al2O3表面上的金纳米粒子层。通过电沉积技术合成的ZnO针型在金纳米粒子层上可以很好的生长。单色散针(或者六角形纳米柱)的获得有着有不同的分布密度。所制备的样品在扫描电子显微镜(SEM)下观看,像组合起来的“法国梧桐悬铃坚果”。还研究了6 000 ℃下样品的光致发光(PL)和其他特征。尖锐的束缚激子(BE)的和纵光学声子LO-phonon 的光谱线意味着ZnO微纳结构有着高品质的光学特性。
ZnO的六角形纳米柱; Al2O3-Au-ZnO的纳米结构; 光致发光; 电沉积
2015-07-18,
2015-11-20)
Foundation item:Jointly Funded Project (61179055) of Chinese Civil Aviation Authority and National Natural Science Foundation of China, Major State Basic Research Development Program (2014CB921300) and Talent Grant (5030450104) of Educational Commission of Guangdong Province, China
10.3964/j.issn.1000-0593(2016)10-3394-05
Received:2015-07-18; accepted:2015-11-20
Biography:ZHU Lin, (1988—), a Master degree student studing in Department of Optical Information Science and Technology, College of Physics, Jilin University *Corresponding authors e-mail: cuihaining2009@126.com
- 光谱学与光谱分析的其它文章
- Gd靶激光等离子体光源离带辐射及其等离子体演化的研究
- Probing the Binding of Torasemide to Pepsin and Trypsin by Spectroscopic and Molecular Docking Methods
- Mn(Ⅱ)-5-Br-PADAP共沉淀-火焰原子吸收光谱法测定虾、贝样中的镉
- Near Infrared Spectroscopy Study on Nitrogen in Shortcut Nitrification and Denitrification Using Principal Component Analysis Combined with BP Neural Networks
- 内蒙古草原植被最大光能利用率取值优化研究
- 健康和糖尿病大鼠红细胞荧光光谱非线性程度差异

