Phylogeny and drug resistance of HIV PR gene among HIV patients receiving RT inhibitors in Iran
Kazem Baesi, Majedeh Moradbeigi, Mehrdad Ravanshad, Ashrafolnesa BaghbanHepatitis & AIDS Department,Pasteur Institute of Iran,Tehran,IranIranian Research Center for HIV/AIDS,Iranian Institute for Reduction of High Risk Behaviors,Tehran University of Medical Sciences,Tehran,IranDepartment of Virology,Faculty of Medical Sciences,Tarbiat Modares University,Tehran,Iran
Phylogeny and drug resistance of HIV PR gene among HIV patients receiving RT inhibitors in Iran
Kazem Baesi1, Majedeh Moradbeigi2, Mehrdad Ravanshad3*, Ashrafolnesa Baghban31Hepatitis & AIDS Department,Pasteur Institute of Iran,Tehran,Iran
2Iranian Research Center for HIV/AIDS,Iranian Institute for Reduction of High Risk Behaviors,Tehran University of Medical Sciences,Tehran,Iran
3Department of Virology,Faculty of Medical Sciences,Tarbiat Modares University,Tehran,Iran
ARTICLE INFO
Article history:
Received 20 Oct 2015
Receivedinrevisedform26 Nov,2nd
revised form 2 Dec 2015
Accepted 20 Dec 2015
Available online 19 Mar 2016
Keywords:
HIV
Highly active antiretroviral therapy
Transmitted drug resistance
Protease inhibitor
ABSTRACT
Objective:To survey the level and patterns of reverse transcriptase-based drug resistance and subtype distribution among antiretroviral-treated HIV-infected patients receiving only reverse transcriptase inhibitors in Iran.
Methods:A total of 25 samples of antiretroviral therapy experienced patients with no history of using protease inhibitors were collected. After RNA extraction, reverse transcriptase-nested PCR was performed. The final products were sequenced and then analysed for drug-resistant mutations and subtypes.
Results:No drug resistant mutations were observed among the 25 subjects. The results showed the following subtypes among patients: CRF 35_AD(88%), CRF 28_BF(8%), and CRF 29_BF(4%).
Conclusions:A significant increase in drug resistance has been noted in recentlyinfected patients worldwide. Subtype distributions are needed to perform properlydesigned surveillance studies to continuously monitor rates and patterns of transmitted drug resistance and subtypes to help guide therapeutic approaches and limit transmission of these variants.
Original article http://dx.doi.org/10.1016/j.apjtb.2015.12.020
Tel: +98 21 82883836
Fax: +98 21 82883581
E-mail: ravanshad@modares.ac.ir
The study protocol was performed according to the Helsinki Declaration and approved by Tarbiat Modares University ethical committee(Reg No. 1389-9). Informed written consent was obtained from all patients.
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
1. Introduction
The use of combinations of three or more antiretroviral drugs from two drug classes has proven remarkably effective in controlling the progression of HIV and has reduced mortality in HIV-infected individuals[1]. These benefits can be compromised by the development of transmitted HIV-1 drug resistance. This type of resistance is the consequence of mutations that emerge in the viral proteins targeted by antiretroviral agents[2–9].
The implementation of antiretroviral therapy(ART)more than a decade ago has improved the quality and life expectancy of HIV-infected patients. Factors such as low-adherence rates by patients, inappropriate ART regimens, incorrectly-prescribed drugs, extensive genetic variation, toxicity of medication, high pill burden, and HIV genomic mutations as the main cause of virological and treatment failure have led to the emergence and subsequent spread of antiviral drug resistance in HIV-infected patients[5,10–15]. Surveillance studies have shown that approximately 10%of new HIV-1 infections involve drugresistant strains, indicating that treated individuals are involved in the spread of new infections. It has also been shown that individuals infected de novo with drug-resistant viruses can serve as a source of subsequent infection and contribute to the spread of drug-resistant HIV[16].
The increase in the prevalence of transmitted drug resistance in low- and middle-income countries varies by area according to the nature of the ART programs implemented. This justifies the need for HIV-1 genotyping resistance testing before the initiation of ART to monitor antiretroviral treatment of HIV-1 patients as a major factor for therapeutic regimens selection [3,10,17–19]. Sequence analysis of the HIV-1 pol gene provides important information about antiretroviral drug resistanceassociated mutations affecting the susceptibility of HIV-1strains to protease(PR)and reverse transcriptase(RT)inhibitors and on subtype diversity[20]. The present study surveyed the level and patterns of PR-based drug resistance and subtype distribution among antiretroviral-treated HIV-infected patients who receive only RT inhibitors in Iran.
2. Materials and methods
2.1. Sample collection
This cross-sectional study examined 25 HIV-infected patients undergoing ART at the Iranian Research Centre for HIV/AIDS of Imam Khomeini Hospital in Tehran, Iran. All patients received a combination of ART apart from PR inhibitors. A total of 25 individuals were enrolled in the study(100%participation). The study protocol was approved by the Medical Research Ethics Committee of Tarbiat Modares University in Tehran. All participants provided written informed consent prior to sample collection.
Initially, 5 mL blood samples were collected in ethylenediaminetetraacetic acid blood collection tubes. The plasma was separated by centrifugation at 3000 r/min and frozen at -70°C until use for RNA extraction. The HIV pol regions, which include viral protease genes, were amplified and sequenced to determine the subtype and antiretroviral-resistant mutations.
2.2. HIV RNA extraction and cDNA synthesis
HIV RNA was extracted from 140 μL of plasma using the column purification method(QIAamp Viral RNA Mini Kit, Qiagen, Germany)according to manufacturer instructions. Following RNA viral denaturation at 70°C for 10 min, cDNA synthesis was performed at 42°C for 1 h using 200 IU of MMuLV reverse transcriptase(Fermentas)and 2 μL of antisense outer primers(Table 1)plus 2 μL dNTP and 0.5 μL RNAse inhibitor(Fermentas).
2.3. Nested PCR amplification
First-round PCR was performed using 5 μL cDNA, 1×PCR buffer, 0.5 μL of 10 mmol/L dNTP, Pfu polymerase, 0.75 μL 50 mmol/L MgCl2, 0.5 mmol/L of 10 pmol/μL primer solution containing outer primers(Table 1), and 15 μL of double-distilled water. Briefly, the amplification profile consisted of denaturation at 95°C for 5 min, followed by 30 cycles of denaturation at 94°C for 50 s, annealing at 58°C for 40 s, extension at 72°C for 50 s, followed by a final extension phase at 72°C for 5 min. An aliquot(about 2.5 μL)of the primary PCR product was used for 35 cycles of nested PCR as follows: initial denaturation at 95°C for 5 min, 20 cycles of denaturation at 94°C for 50 s, annealing at 58°C for 40 s, and polymerization at 72°C for 50 s, with a final elongation at 72°C for 5 min. An Eppendorf gradient PCR system thermal cycler was used for all PCR reactions. The results were checked by electrophoresis of the nested PCR products on 1.5%agarose gel and visualized with ethidium bromide under UV light.
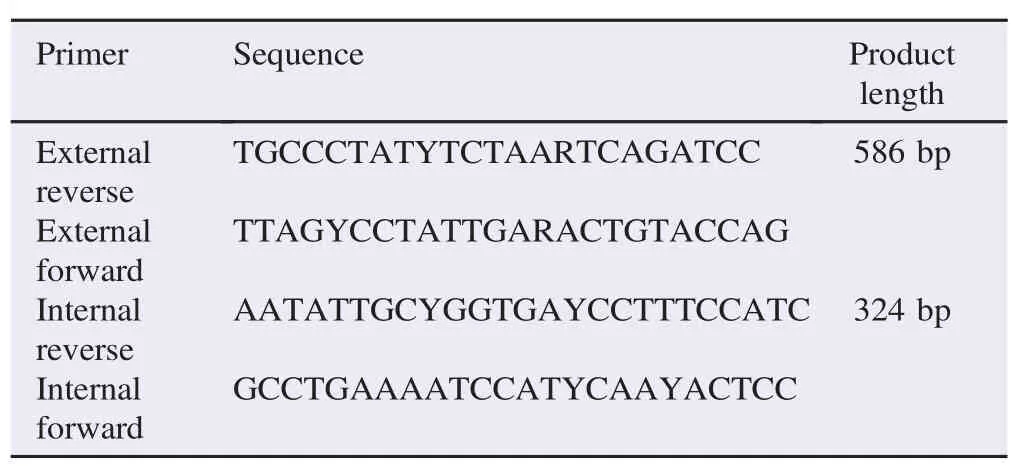
Table 1 The sequence of primers.
2.4. Purification and DNA sequencing
The PCR products were purified using a gel purification kit (Bioneer, Global Genomics Partner)according to manufacturer instructions and sequenced on both strands(bi-directionally)using the dideoxy chain termination method(ABI PRISM 3700 DNA Analyser Automated Sequencer, Applied Biosystems, USA).
3. Results
At the time of the study, the plasma samples were selected retrospectively from 25 ART patients attending the Iranian Research Centre for HIV/AIDS at Imam Khomeini Hospital in Tehran in Iran. Of these, 19(76%)were males and 6(24%)were females. The primary routes of HIV/AIDS transmission were intravenous drug use(80%)and sexual contact(20%).
All 25 patients received highly active antiretroviral therapy for at least 1 year. The therapy regimen contained nucleoside reverse transcriptase inhibitor and non-nucleoside reverse transcriptase inhibitor. None of the patients had experienced the use of protease inhibitors(PIs). The CD4 cell count was below 250–300 cells/m3. In all isolates, the PR segment of the pol gene amplified and produced useful sequences in this region. Phylogenetic analysis of the PR sequences derived from all isolates revealed no drug resistant mutations associated with the PIs.
In this study, recombinant CRF 35_AD(88%)was found to be the predominant HIV subtype, followed by subtypes CRF 28_BF in 8%and CRF 29_BF in 4%of patients failing treatment in Tehran. It was found that subtype CRF 35_AD of HIV was the most prevalent among HIV-infected patients in Iran. This study also showed that the major route of HIV/AIDS transmission was through injection drug users in Iran.
4. Discussion
The number of adults living with HIV in Iran was estimated to be 26125 at the end of 2012 and was comprised of 89.8% males and 10.2% females[21]. Most participants were intravenous drug addicts, which is in accordance with results of recent studies from different areas of Iran[22].
This study focused on mutations of the PR gene that confer resistance to ART drugs in patients not previously treated with PIs. The prevalence of transmitted PR resistance-associated mutations in the sample of 25 patients receiving ART in Tehran was investigated and it was found that none of the ART patients had major resistance mutations. This is in agreement with global studies showing minimal prevalence of antiretroviral drug resistance in individuals experiencing ART[23–25].
No major mutations associated with drug resistance were observed in this study, although other studies performed in Iranshowed a prevalence ranging from 10.3%to 45%[26–28]. Previous studies on the prevalence of transmission of drugresistant HIV found the prevalence to be as follows: North America(12.9%), Europe(10.9%), Latin America(6.3%), Africa(4.7%), and Asia(4.2%)[29].
Previous studies have indicated that subtypes A1 and B are most prevalent in Iranian HIV-positive patients[10,30,31]. The present study found recombinant CRF 35_AD(88%)to be the predominant HIV subtype, followed by subtypes CRF 28_BF in 8%and CRF 29_BF in 4%of patients failing treatment in Tehran, which is consistent with other reports of HIV in Iran [3,32–35]. The results support recent findings on the emergence of new predominant HIV subtypes/CRFs, demonstrating the dynamic and rapid evolution of HIV. Recombinants are currently estimated to be responsible for at least 20%of HIV-1 infections worldwide[36,37].
There is no compelling evidence that the HIV-1 subtype should be considered during the choice of ART regimens for first-line or second-line therapy. Consideration of cost, effectiveness, toxicity, and tolerability are more important in lowincome and middle-income countries[34].
The significant increase in drug resistance in recentlyinfected HIV patients and subtype distribution requires performance of properly-designed surveillance studies to continuously monitor rates and patterns of transmitted drug resistance and subtypes to help guide therapeutic approaches and limit transmission of these variants.
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1]Shafer RW. Genotypic testing for human immunodeficiency virus type 1 drug resistance. Clin Microbiol Rev 2002;15: 247-77.
[2]Clavel F, Hance A. HIV drug resistance. N Engl J Med 2004;350: 1023-35.
[3]MousaviSM,HamkarR,GouyaMM,SafaieA,ZahraeiSM,YazdaniZ, etal. Surveillance of HIV drug resistancetransmissionin Iran: experiencegainedfromapilotstudy.Arch Virol2009;155:329-34.
[4]Valle-Bahena OM, Ramos-Jim´enez J, Ortiz-L´opez R, Revol A, Lugo-Trampe A, Barrera-Saldaña HA, et al. Frequency of protease and reverse transcriptase drug resistance mutations in na¨ıve HIV-infected patients. Arch Med Res 2006;37: 1022-7.
[5]Ali A, Bandaranayake RM, Cai Y, King NM, Kolli M, Mittal S, et al. Molecular basis for drug resistance in HIV-1 protease. Viruses 2010;2: 2509-35.
[6]Bahmani MK, Khosravi A, Mahboodi F, Sarrami-Forooshani R. Genotypic correlation of a virologic response to lamivudine, stavudine and nevirapine in patients for whom combination therapy had failed. Iran J Clin Infect Dis 2008;3: 215-9.
[7]Xing H, Ruan Y, Li J, Shang H, Zhong P, Wang X, et al. HIV drug resistance and its impact on antiretroviral therapy in Chinese HIV-infected patients. PLoS One 2013;8: e54917.
[8]Johnson JA, Li JF, Wei X, Lipscomb J, Irlbeck D, Craig C, et al. Minority HIV-1 drug resistance mutations are present in antiretroviral treatment-na¨ıve populations and associate with reduced treatment efficacy. PLoS Med 2008;5: e158.
[9]Hirsch MS, Gunthard HF, Schapiro JM, Brun-V´ezinet F, Clotet B, Hammer SM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an international AIDS society-USA panel. Clin Infect Dis 2008;47: 266-85.
[10]Baesi K, Ravanshad M, Hosseini Y, Haji Abdolbaghi M. Drug resistance profile and subtyping of HIV-1 RT gene in Iranian patients under treatment. Iran J Biotechnol 2012;10(1): 1-7.
[11]Maldarelli F, Kearney M, Palmer S, Stephens R, Mican J, Polis MA, et al. HIV populations are large and accumulate high genetic diversity in nonlinear fashion. J Virol 2013;87(18): 10313-23.
[12]World Health Organization. HIV drug resistance report 2012. Geneva: World Health Organization;2012.[Online]Available from: http://www.who.int/hiv/pub/drugresistance/report2012/en/ [Accessed on 19th October, 2013]
[13]Wittkop L, Gunthard HF, de Wolf F, Dunn D, Cozzi-Lepri A, de Luca A, et al. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV(EuroCoord-CHAIN joint project): a European multicohort study. Lancet Infect Dis 2011;11: 363-71.
[14]P´erez L, Kourí V, Alem´an Y, Abrahantes Y, Correa C, Aragon´es C, et al. Antiretroviral drug resistance in HIV-1 therapynaive patients in Cuba. Infect Genet Evol 2013;16: 144-50.
[15]Johnson VA, Calvez V, Gunthard HF, Paredes R, Pillay D, Shafer RW, et al. Update of the drug resistance mutations in HIV-1: March 2013. Top Antivir Med 2013;21: 6-14.
[16]Pingen M, Nijhuis M, de Bruijn JA, Boucher CA, Wensing AM. Evolutionary pathways of transmitted drug-resistant HIV-1. J Antimicrob Chemother 2011;66: 1467-80.
[17]Paydary K, Khaghani P, Emamzadeh-Fard S, Seyed-Alinaghi SA, Baesi K. The emergence of drug resistant HIV variants and novel anti-retroviral therapy. Asian Pac J Trop Biomed 2013;3: 515-22.
[18]Thompson MA, Aberg JA, Cahn P, Montaner JS, Rizzardini G, Telenti A, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS society-USA panel. JAMA 2010;304: 321-33.
[19]Bontell I, Cuong D, Agneskog E, Diwan V, Larsson M, S¨onnerborg A. Transmitted drug resistance and phylogenetic analysis of HIV CRF01_AE in Northern Vietnam. Infect Genet Evol 2012;12: 448-52.
[20]Soares RO, Batista PR, Costa MGS, Dardenne LE, Pascutti PG, Soares MA. Understanding the HIV-1 protease nelfinavir resistance mutation D30N in subtypes B and C through molecular dynamics simulations. J Mol Graph Model 2010;29: 137-47.
[21]National AIDS Committee Secretariat, Ministry of Health and Medical Education. Islamic Republic of Iran AIDS progress report: on monitoring of the United Nations general assembly special session on HIV and AIDS. Tehran: National AIDS Committee Secretariat, Ministry of Health and Medical Education;2015. [Online]Available from: http://www.unaids.org/sites/default/files/ country/documents/IRN_narrative_report_2015.pdf [Accessed 11th November, 2015]
[22]Naderi HR, Tagliamonte M, Tornesello ML, Ciccozzi M, Rezza G, Farid R, et al. Molecular and phylogenetic analysis of HIV-1 variants circulating among injecting drug users in Mashhad-Iran. Infect Agents Cancer 2006;1: 4.
[23]Callegaro A, Svicher V, Alteri C, Lo Presti A, Valenti D, Goglio A, et al. Epidemiological network analysis in HIV-1 B infected patients diagnosed in Italy between 2000 and 2008. Infect Genet Evol 2011;11: 624-32.
[24]Hamers RL, Wallis CL, Kityo C, Siwale M, Mandaliya K, Conradie F, et al. HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. Lancet Infect Dis 2011;11: 750-9.
[25]DiazGranados CA, Mantilla M, Lenis W. Antiretroviral drug resistance in HIV-infected patients in Colombia. Int J Infect Dis 2010;14: e298-303.
[26]Hamkar R, Mohraz M, Lorestani S, Aghakhani A, Truong HM, McFarland W, et al. Assessing subtype and drug-resistanceassociated mutations among antiretroviral-treated HIV-infected patients. AIDS 2010;24(Suppl 2): S85-91.
[27]Baesi K, Ravanshad M, Ghanbari Safari M, Saberfar E, Hajiabdolbaghi M. Survey of human immunodeficiency virus-1 protease gene drug resistance among HIV infected patients in an Iranian research center for HIV/AIDS. Modares J Med Sci Pathobiol 2012;14: 13-21.
[28]Baesi K, Ravanshad M, Ghanbarisafari M, Saberfar E, SeyedAlinaghi S, Volk JE. Antiretroviral drug resistance among antiretroviral-na¨ıve and treatment experienced patients infected with HIV in Iran. J Med Virol 2014;86(7): 1093-8.
[29]Frentz D, Boucher CA, van de Vijver DA. Temporal changes in the epidemiology of transmission of drug-resistant HIV-1 across the world. AIDS Rev 2012;14: 17-27.
[30]Baesi K, Ravanshad M, Hosseini SY, Haji Abdolbaghi M, Shahzamani K. Phylogenetic analysis of HIV-1 pol gene(RT sequences)among Iranian HIV infected patients. Sci J Iran Blood Transfus Organ 2010;7: 94-100.
[31]Sarrami-Forooshani R, Das SR, Sabahi F, Adeli A, Esmaeili R, Wahren B, et al. Molecular analysis and phylogenetic characterization of HIV in Iran. J Med Virol 2006;78: 853-63.
[32]Jahanbakhsh F, Ibe S, Hattori J, Monavari SHR, Matsuda M, Maejima M, et al. Molecular epidemiology of HIV type 1 infection in Iran: genomic evidence of CRF35_AD predominance and CRF01_AE infection among individuals associated with injection drug use. AIDS Res Hum Retroviruses 2013;29(1): 198-203.
[33]Jahanbakhsh Sefidi F, Hattori J, Ibe S, Monavari S, Memarnejadian A, Aghasadeghi M, et al. Trends in transmitted HIV drug resistance in Iran from 2010 to 2011. J Int AIDS Soc 2012;15: 18225.
[34]Baesi K, Moallemi S, Farrokhi M, Alinaghi SAS, Truong HHM. Subtype classification of Iranian HIV-1 sequences registered in the HIV databases, 2006–2013. PLoS One 2014;9(9): e105098.
[35]Baesi K, Moallemi S, Ravanshad M. Phylogenetic analysis of HIV-1 pol gene:first subgenomic evidence of CRF29-BF among Iranian HIV-1 patients. Asian Pac J Trop Dis 2014;4(Suppl 2): S617-20.
[36]Hemelaar J. Implications of HIV diversity for the HIV-1 pandemic. J Infect 2012;66(5): 391-400.
[37]Lessells RJ, Katzenstein DK, de Oliveira T. Are subtype differences important in HIV drug resistance?Curr Opin Virol 2012;2: 636-43.
*Corresponding author:Mehrdad Ravanshad, Department of Virology, Faculty of Medical Sciences, Tarbiat Modares University, P.O. Box 14115-331, Tehran, Iran.
 Asian Pacific Journal of Tropical Biomedicine2016年5期
Asian Pacific Journal of Tropical Biomedicine2016年5期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Susceptibility of Aedes flavopictus miyarai and Aedes galloisi mosquito species in Japan to dengue type 2 virus
- An update on microbiological causes of canine otitis externa in Campania Region,Italy
- Antibacterial activity of Bixa orellana L.(achiote)against Streptococcus mutans and Streptococcus sanguinis
- Antimicrobial properties of sea anemone Anthopleura nigrescens from Pacific coast of Costa Rica
- Emergence of non-albicans Candida species and antifungal resistance in intensive care unit patients
- Primary oral and nasal transmissible venereal tumor in a mix-breed dog
