An update on microbiological causes of canine otitis externa in Campania Region,Italy
Luisa De Martino, Francesca Paola Nocera, Karina Mallardo, Sandra Nizza, Eleonora Masturzo,Filomena Fiorito, Giuseppe Iovane, Piergiorgio CatalanottiDepartment of Veterinary Medicine and Animal Production,University of Naples“Federico II”,Via F. Delpino ,8037 Naples,ItalyDepartment of Experimental Medicine,Second University of Naples,Via Luigi de Crecchio 7,808 Naples,Italy
An update on microbiological causes of canine otitis externa in Campania Region,Italy
Luisa De Martino1*, Francesca Paola Nocera1, Karina Mallardo1, Sandra Nizza1, Eleonora Masturzo1,
Filomena Fiorito1, Giuseppe Iovane1, Piergiorgio Catalanotti21Department of Veterinary Medicine and Animal Production,University of Naples“Federico II”,Via F. Delpino 1,
80137 Naples,Italy
2Department of Experimental Medicine,Second University of Naples,Via Luigi de Crecchio 7,80128 Naples,Italy
ABSTRACT
Objective:To update the recent knowledge of the microbiological causes of canine otitis externa in Campania Region(Italy)and the antibiotic susceptibility patterns of the isolated strains.
Methods:A total of 122 dogs were examined by otoscopy, and auricular swab samples were collected from both ears in 74 dogs presenting clinical bilateral otitis and from single ears in 48 dogs displaying clinical unilateral otitis. Cytological examination, bacteriological analysis and antimicrobial susceptibility tests were performed.
Results:Thirty-one out of 122 dogs were positive for yeast species(25.4%, 95%confidence interval(CI): 18.2%–34.2%)with a higher prevalence of Malassezia pachydermatis(21/31 isolates, 67.7%, CI: 48.5%–82.7%), and a total of 91 out of 122 dogs were positive for bacterial species(74.6%;CI: 65.8%–81.8%)with a higher prevalence of Staphylococcus pseudintermedius(45/143 isolates, 31.5%, CI: 24.1%–39.8%). These results are the first description of Streptococcus agalactiae-associated otitis. The yeasts isolated showed high levels of susceptibility to all antifungal agents tested;on the contrary all the isolated bacterial strains were highly resistant to at least four out of ten antimicrobial classes. Both Gram-positive and Gram-negative bacteria showed high resistance to amoxicillin/clavulanate and kanamycin hence they are not recommended as initial empirical therapy for the otitis treatment.
Conclusions:This update illustrates an increase in antibiotic resistances providing an insight into the current knowledge of the therapeutic procedures followed on canine otitis externa in Italy. It also emphasizes the importance of considering the results of the microbiological and sensitivity tests to decide on an appropriate antibiotic therapy.
ARTICLE INFO
Article history:
Received 13 Oct 2015
Receivedinrevisedform27 Oct2015
Accepted 2 Nov 2015
Available online 24 Mar 2016
Keywords:
Canine otitis externa
Malassezia pachydermatis
Staphylococcus pseudintermedius
Antimicrobial resistance
Original article http://dx.doi.org/10.1016/j.apjtb.2015.11.012
Tel: +39 081 2536180
Fax: +39 081 2536179
E-mail: luisa.demartino@unina.it
All experimental procedures involving animals were conducted in accordance to guide for use and care of animals that visit our clinic and approved by The Local Ethical Committee of The Faculty of Veterinary Medicine, University of Naples “Federico II”(authorisation reference number Prot. 2011/0123330).
Foundation Project: Supported in part by the Second University of Naples and University of Naples“Federico II”, Fondi di Ateneo, by a fellowship from the University of Naples‘Federico II’(2012-4/STV-Project FORGIARE), and co-funded by “Compagnia San Paolo”of Turin, Italy.
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
1. Introduction
Otitis externa is the most common ear disease of dogs, being up to 20%of the dog population affected by this disease. It has a multifactorial etiology but it is predominantly a microbial infection. Clinical signs, such as exudates and frequently erythema, oedema, offensive odour and pruritus are seen. Microorganisms belonging to the normal microbial flora of the auricular area can become pathogenic when environmental and/or primary conditions determine faster expression of their virulence factors.
Malassezia genus are commonly isolated in auricular canal of dogs and cats, having otitis externa or in cases of dermatitis disorders. The yeasts of the Malassezia genus areopportunistic microorganisms and can cause both human [1,2]and animal infections[3]. Particularly Malassezia pachydermatis(M. pachydermatis)has been reported as the predominant causative agent of canine otitis externa[4].
Furthermore, among bacterial agents, it is known that Staphylococcus, Pseudomonas, Escherichia, and Proteus species are considered important pathogens causing otitis externa in dogs[5]. Among Gram-positive bacteria, Staphylococcus pseudintermedius,(S. pseudintermedius)a coagulase-positive staphylococcal species, is frequently associated with pyoderma, otitis externa, urinary tract infections, and opportunistically infected sites in dogs[6]. Among Gram-negative bacteria, Pseudomonas aeruginosa(P. aeruginosa)plays a major role in otitis externa also because of the increasing number of multiresistant strains[7].
Regular treatment at home with disinfecting ear washes should become part of the pet's grooming-routine with a correct antimicrobial therapy. It has been described a high sensitivity to beta-lactams and aminoglycoside-aminocyclitols for Gram-positive bacteria, while for Gram-negative bacteria has been suggested the use of aminoglycoside-aminocyclitols, polymyxin B and enrofloxacin[8].
In recent decades it was reported that the high frequency of multidrug-resistant of the isolated bacteria could become a high risk factor for owners and veterinary professionals. Therefore, a rational policy of antibiotic prescription in order to prevent the selection of resistant strains is needed.
The purpose of the present study is to evaluate the presence of microorganisms involved in otitis externa in dogs from Campania Region, in southern Italy, in order to update data and to investigate the presence of multi-resistant bacterial strains[9].
2. Materials and methods
2.1. Samples
One hundred and twenty-two dogs with clinical signs of otitis externa, such as local pain, pruritus, erythema, ear discharge and desquamation of the epithelium, in at least one ear, were selected tocollectauricularswabs,duringaperiodoftwoyears,andatotal of 196 culture swabs were obtained. Precisely, samples were collectedfrombothearsin74dogswithclinicalbilateralotitisand unilaterally from 48 dogs with clinical unilateral otitis. Sterile cotton-tipped applicator was used to collect samples of ear exudatesbyinsertingswabsintoearcanal,rotatingoncethrough360°and then rolling it out, and immediately transferred to Amies transport medium(Oxoid Ltd, UK)and maintained at 4°C(not longerthan24h)untilprocessing.Thesampleswerepresentedfor screening at the Microbiology Laboratory of the Department of Veterinary Medicine and Animal Production, University of Naples Federico II(Italy). The canine population studied aged from 2 months to 16 years and included 75 males and 47 females.
Dogs were excluded from the trial if they had previously received(i)topical treatment(within 10 days),(ii)systemic treatment with an antibiotic, antifungal or nonsteroidal antiinflammatory(within 10 days),(iii)a steroidal anti-inflammatory treatment(within 14 days), or(iv)a long acting steroidal antiinflammatory drug systemically(within 60 days). Informed consent was obtained from the owners of all dogs prior to their participation in the study. All experimental procedures were approved by The Local Ethical Committee of The Faculty of Veterinary Medicine, University of Naples Federico II(authorisation reference number Prot. 2011/0123330).
2.2. Cytological analysis
For cytological analysis cotton-tipped swabs of ear canal exudates were streaked onto glass slides, which were then heat fixed and stained with a modified Wright's stain(Diff Quik, Dade Behring, Deerfield, IL). All stained slides were examined under a Nikon Eclipse E600 Microscopy(Nikon Instruments Inc., Melville, NY)at 40×magnification. At least 10 fields were examined, and a number of yeast cells per field(>5 yeast/field)represented excessive colonization by the organism and were considered positive(infection). A number of bacteria 25 per microscopy field(40×)were considered positive(infection)as previously described by Ginel et al.[10].
2.3. Microbiological analysis
Collected samples were plated on blood agar base supplemented with 5%sheep blood, selective medium used for the isolation of Gram-positive microorganisms, on mannitol-salt agar, selective medium to identify staphylococci, and on Mac-Conkey agar, selective and differential medium to grow Gramnegative bacteria, which were incubated aerobically at 37°C for 24–48 h. Sabouraud's dextrose agar with chloramphenicol was used to grow fungal flora and was kept at 30°C for 7 days, then stained with Gram stain and examined microscopically. The plates were all microbiological media from Oxoid Ltd, UK. In the case of yeasts of the genus Candida, we performed subculture on Oxoid chromogenic candida agar media to differentiate species of Candida. Further confirmation of genus and species of yeasts were obtained by biochemical identification using Remel RapID™yeast plus identification panel after pure culture growth on Emmons agar(Oxoid).
Bacteria were identified by macroscopic observation of the colonies, Gram staining, standard laboratory methodologies (catalase, staphylocoagulase tube test, aesculin), and miniaturized biochemical tests API system(bioM´erieux SA, Marcy L'Etoile, France). The species identification by miniaturized biochemical tests was accepted when probability was>88%.βhaemolytic cocci strains were identified as group B[Streptococcus agalactiae(S. agalactiae)]or group C Streptococcus dysgalactiae(S. dysgalactiae), using a streptococcus grouping kit(Streptex, Mitsubishi Chemical Medience Corporation, Tokyo, Japan). The presence of the mecA gene was detected by growth on oxacillin-containing media(2 mg/L), agar diffusion with oxacillin disks(5 μg)and positive latex agglutination test (PBP2′Test. Oxoid Ltd, UK).
2.4. Antimicrobial susceptibility testing
The antimicrobial susceptibility patterns of isolated bacterial strains were determined by disk diffusion test using Mueller–Hinton agar(Oxoid Ltd, UK). The inhibitory zone diameters obtained around the antibiotic disks were measured after incubation for 24 h at 37°C and evaluated according to the Clinical and Laboratory Standards Institute[11]. An oxacillin (methicillin)susceptibility test of all isolates was performed by oxacillin disk diffusion and if assayed, the minimal inhibitory concentration was determined by standard methods.
Ten drug classes were included in this study, using commercial disks containing the drugs(Oxoid Ltd, UK). Penicillins were represented by benzylpenicillin(penicillin G)(10 IU),ampicillin(10 μg), amoxicillin-clavulanic acid combination (20 + 10 μg), and oxacillin(1 μg). Furthermore, cephalosporins (first generation)were represented by cefadroxil(30 μg), cephalosporins(third generation)were represented by ceftriaxone(30 μg),fluoroquinolones by enrofloxacin(5 μg)and norfloxacin(10 μg), aminoglycosides by gentamicin(10 μg), kanamycin(30 μg), neomycin(30 μg)and streptomycin(10 μg), glycopeptides by vancomycin(30 μg), macrolides by erythromycin(15 μg), and polypeptides by bacitracin(0.05 μg)and colistin sulfate(10 μg). In addition, tetracyclines were assayed as doxycycline(30 μg)and tetracycline(10 μg)and lincosamides as lincomycin(2 μg). Finally, we assayed also a sulphonamide(sulfamethoxazole/trimethoprim, 50 μg). The choice of drugs depended by strains and clinical use. The strains were categorized as sensitive or resistant to the drug, intermediate susceptibility was regarded as resistant, and only the resistances are reported in the results.
Antifungal susceptibility was performed by disc diffusion method using antimycotic sensitivity test agar. Discs used were amphotericin B(100 IU),fluconazole(10 μg), clotrimazole (10 μg), itraconazole(10 μg), miconazole(10 μg)was measured as for the instruction manual(HiMedia). ATCC strain of Candida albicans was used as control.
2.5. Statistical analysis
Statistical analysis of bacteriological examination results was performed by One-way ANOVA with Bonferroni post-test using GraphPad InStat Version 3.00 for Windows 95(GraphPad Software Inc., La Jolla, CA). P value of less than 0.05 was regarded as statistically significant.
3. Results
The screening in 196 samples performed by cytological examination showed a prevalence significantly higher(P<0.001)of bacterial compared to fungal otitis externa, both in monolateral(37/11)and bilateral infection(53/21). Furthermore, the cases of bilateral otitis(74/122)was significantly higher (P<0.001)respect to monolateral otitis(48/122)regardless of the microorganism that causes disease.
Yeasts were detected in a total of 53 swabs samples with M. pachydermatis presenting the highest prevalence(67.74%)followed by Candida parapsilosis(12.90%), as shown in Table 1. These diseased dogs after local therapy with miconazole and prednisolone acetate, for at least 2 weeks, showed a remission of symptoms.

Table 1 Number and percentage of isolated yeasts from dogs with otitis externa.
Table 2 shows the number and percentage of isolated bacteria. The most frequently isolated strain was S. pseudintermedius(31.5%;95%CI: 24.1%–39.8%)followed by P. aeruginosa (16.1%;95%CI: 10.7%–23.4%), Proteus mirabilis(P. mirabilis)(6.3%;95%CI: 3.1%–12.0%), and Staphylococcus aureus (S. aureus)(4.9%;95%CI: 2.2%–10.2%). Moreover, a total of four out of forty-five S. pseudintermedius(8.9%;95%CI: 2.9%–22.1%)were positive for themecA geneas confirmed byoxacillin agarscreening test,oxacillin diffusion testand latexagglutination test. A total of 10 bacteria belonging to Streptococcaceae group were identified and an interesting case of S. agalactiae-associated otitis was observed.
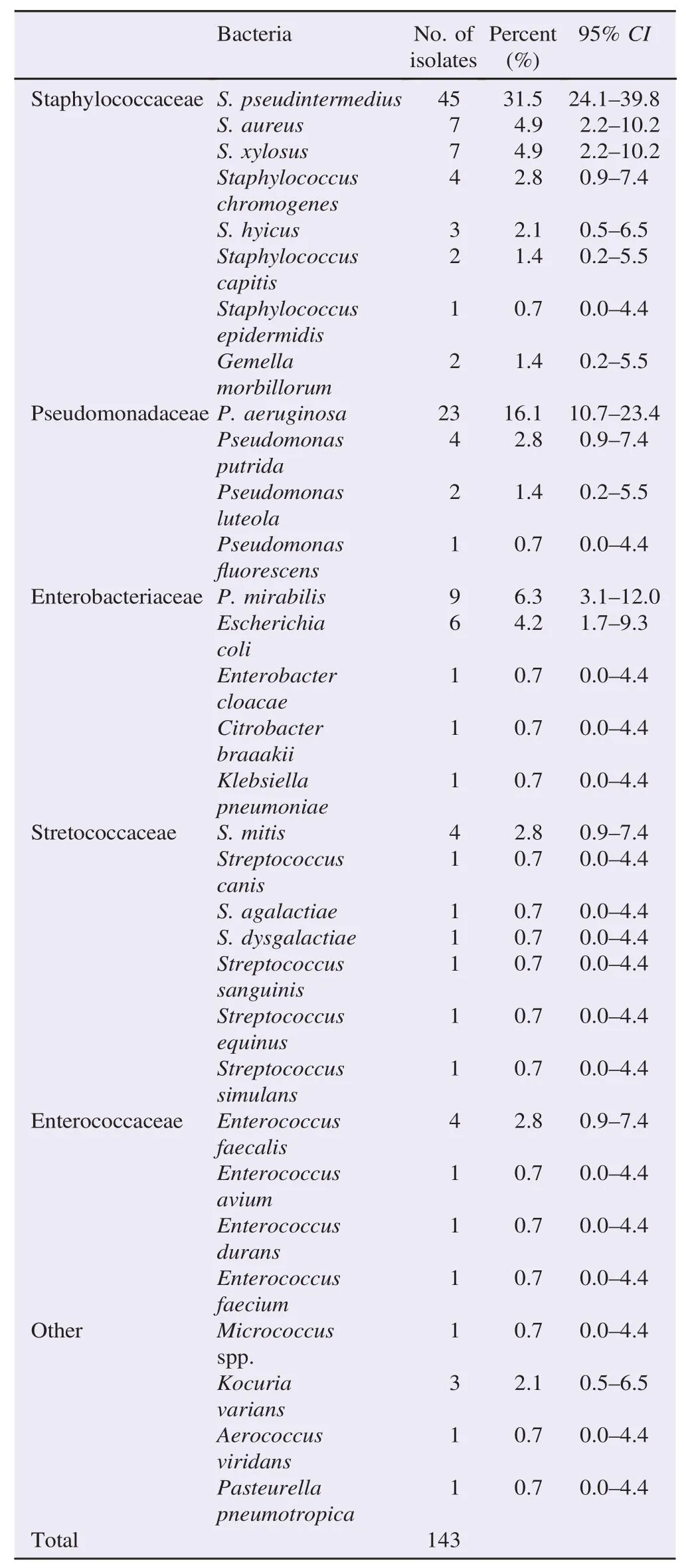
Table 2 Number and percentage of isolated bacteria from dogs with otitis externa.
Table 3 shows the results of the antimicrobial susceptibility tests of the most frequently isolated strains from dogs with otitis externa. However, S. pseudintermedius,S. aureus and S. xylosus, which represented 85.5%of staphylococcal strains, were variously resistant to all antimicrobial classes. Furthermore, 4/45(8.9%;95%CI: 2.9%–22.1%)S. pseudintermedius, 6/7(85.7%;95%CI: 42.0%–99.2%)S. xylosus, 3/4(75.0%;95%CI: 21.9%–98.7%)Staphylococcus chromogenes, and 3/3 (100%;95%CI: 31.0%–96.8%)S. hyicus strains isolates were methicillin/oxacillin and penicillin resistant using disk diffusion tests and could be cultivated on oxacillin resistant screening agar base(data not shown). Among thirty Pseudomonas spp strains, the most frequently isolated P. aeruginosa(23 strains)presented evident multidrug resistant profiles, with over 73%resistance to penicillins(ampicillin and amoxicillin-clavulanate), over 65%to aminoglycosides(kanamycin, neomycin, streptomycin)and tetracyclines(doxycycline and tetracycline), and over 43%resistance to enrofloxacin, 100%to bacitracin and over 69%to lincomycin. Two isolates of Pseudomonas luteola and one of Pseudomonas fluorescens showed a substantial multi-resistance (data not shown). It is noteworthy that multidrug resistant P. mirabilis strains were observed.
The most of streptococcal strains(S. mitis, S. agalactiae and Streptococcus canis)were also multi-resistant. In addition, surprisingly, the only S. agalactiae strain, generally considered uniformly susceptible to penicillins, was not susceptible to penicillin G with a minimal inhibitory concentration of 128 μg/ L, and showed simultaneous resistance to ceftriaxone, erythromycin, tetracycline, lincomycin, sulfamethoxazole/trimethoprim, other than others β-lactams tested. S. dysgalactiae strain isolated was resistant to erythromycin and tetracycline(data not shown).
4. Discussion
Ear disease is one of the most common conditions present in pets, and the infections can be caused by both bacterial and fungal origin. Malassezia spp are normal commensals and occasional pathogens of the skin for many veterinary species, present in up to 40%of canine otitis cases[12]. Herein, we reported a prevalence of yeast species of 25.4%with a higher prevalence of M. pachydermatis(67.74%).
S. pseudintermedius represents the majority of all coccal microorganism isolated, whereas Gram-negative bacteria as P. aeruginosa, P. mirabilis and others, are also included among the most common isolates that may cause canine otitis externa [5,12]. In the present study, in agreement with the above mentioned findings, the most commonly identified bacteria were S. pseudintermedius, P. aeruginosa and P. mirabilis. Furthermore, most S. pseudintermedius, S. xylosus, and S. hyicus strains isolates were methicillin/oxacillin and penicillin resistant. We reported no isolation of Staphylococcus schleiferi which is considered as a potential pathogen in dogs and is often oxacillin resistant[13].
The members of the Staphylococcus genus have a high frequency of conjugation and frequently acquire plasmids that encode antimicrobial resistance, and the production of the enzyme β-lactamase is the major mechanism by which staphylococci acquire resistance[14]. The reservoir of mecA gene is likely larger in coagulase-negative-staphylococci than in S. aureus[15], as more times reported, both in human clinical isolates[16]and in various animal species[17,18].

Table 3 Antimicrobial susceptibility of the most frequently isolated strains from dogs with otitis externa.
The literature reports a higher interest for coagulase-positive staphylococci, but also the coagulase-negative staphylococci, whichhavelongbeenconsideredasnonpathogenic,recentlyhave assumed an important role as pathogens because of their increasing incidence as cause of bacteremia. Interestingly, amongour isolates we identified only one strain of methicillin/oxacillinresistant Staphylococcus epidermidis resistant to 6/17 antimicrobial drugs, which represents serious evidence of emergence of multidrug-resistant strains in dog infections. A substantial multiresistance was also found in others Gram-positive bacteria isolates, as streptococci and enterococci. The only S. agalactiae strain showed a reduced susceptibility to penicillin G. There is little information on the nature of mechanism of resistance. The most likely hypothesis isthe modificationof amino acid sequence ofpenicillin-bindingprotein[19].However,S.agalactiae(group B streptococci)is an emerging pathogen in immunocompromised adults and a major cause of neonatal pneumonia, sepsis, and meningitis;it has been isolated in bovine mammary gland infections, and in companion animals. Its surface-localized penicillin-binding protein 1a, encoded by ponA, is known to be an important virulence factor in infection[20]. Traditionally,βlactams are the first-line agents against S. agalactiae and resistance is rarely reported[21,22]. Our case demonstrated that the resistances, overall to β-lactams and cephalosporins, of this strain request further studies.
The rods involved in canine otitis externa are frequently P. aeruginosa, although P. mirabilis, or other Gram-negative bacteria such as Escherichia coli,Corynebacterium species or, infrequently, Klebsiella and Enterobacter spp., may occur. Our resultsindicatedthatinourregion,thepredominant Gram-negative bacteria were P.aeruginosa and P. mirabilisdisplaying a high rate ofresistancetoeachoftheantimicrobialstested.Themostfrequent antimicrobial classes used in small animals in veterinary medicine include penicillins, cephalosporins, macrolides, lincosamides, fusidic acid, tetracyclines, chloramphenicol, potentiated sulphonamides, aminoglycosides and fluoroquinolones. Aminoglycosides, such as gentamicin and neomycin, are routinely used for topical therapy in canine otitis mainly caused by P. aeruginosa [5,7,12,23], but our Pseudomonas spp. isolates presented multidrug resistant profiles with very high resistance to aminoglycosides, other than penicillins and tetracyclines. Our results combined with data from the literature support the premise that antimicrobials should be selected basically on bacterial culture and antimicrobial susceptibility test. Until recently, multiresistance was overall seen in Gram-positive bacteria[24,25]. However, the recent rapid international spread of multi-resistant Gram-negative bacteria[26], is much more ominous, and the optimal management of serious infections with these bacteria remains to be determined. A recent study has reported that administration of a topical bacteriophage mixture leads to lysis of P. aeruginosa in the ear without apparent toxicity and that might potentially became a convenient and effective treatment for P. aeruginosa-associated otitis in dogs[27].
Consistent with previous reports, this study is a further confirmation that coagulase-positive species of staphylococcal strains are the most causative microorganisms of otitis externa in dogs. In particular, both S. pseudintermedius and methicillin/ oxacillin-resistant S. pseudintermedius strains represent a group of emerging pathogens to be monitored as opportunistic animal pathogens with zoonotic capabilities. In addition, to our knowledge, this is the first report of canine otitis correlated to S. agalactiae, a bacteria isolated mainly from humans and bovine sources. Surprisingly, this isolate presented a simultaneous resistance to more different classes of antibiotics other than β-lactams. Thus, our data emphasize the importance:(i)to study profiles and genetic characteristics of GBS colonizing the pet animals,(ii)to investigate the possible transmission from human to dog, and(iii)to focus on the prevention and treatment of GBS infections.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This study was supported in part by the Second University of Naples and University of Naples“Federico II”, Fondi di Ateneo. Filomena Fiorito was supported by a fellowship from the University of Naples‘Federico II’(2012-4/STV-Project FORGIARE)and co-funded by“Compagnia San Paolo”of Turin, Italy. The authors would like to thank Salvatore Monnolo for his technical assistance.
References
[1]Zhang E, Tanaka T, Tsuboi R, Makimura K, Nishikawa A, Sugita T. Characterization of Malassezia microbiota in the human external auditory canal and on the sole of the foot. Microbiol Immunol 2012;56(4): 238-44.
[2]Buommino E, De Filippis A, Parisi A, Nizza S, Martano M, Iovane G, et al. Innate immune response in human keratinocytes infected by a feline isolate of Malassezia pachydermatis. Vet Microbiol 2013;163(1–2): 90-6.
[3]Nardoni S, Corazza M, Mancianti F. Diagnostic and clinical features of animal malasseziosis. Parassitologia 2008;50(1–2): 81-3.
[4]Bond R. Superficial veterinary mycoses. Clin Dermatol 2010;28(2): 226-36.
[5]Zamankhan Malayeri H, Jamshidi S, Zahraei Salehi T. Identification and antimicrobial susceptibility patterns of bacteria causing otitis externa in dogs. Vet Res Commun 2010;34: 435-44.
[6]Rubin JE, Chirino-Trejo M. Prevalence, sites of colonization, and antimicrobial resistance among Staphylococcus pseudintermedius isolated from healthy dogs in Saskatoon, Canada. J Vet Diagn Invest 2011;23: 351-4.
[7]Mekic S, Matanovic K,ˇSeol B. Antimicrobial susceptibility of Pseudomonas aeruginosa isolates from dogs with otitis externa. Vet Rec 2011;169(5): 125.
[8]Petrov V, Mihaylov G, Tsachev I, Zhelev G, Marutsov P, Koev K. Otitis externa in dogs: microbiology and antimicrobial susceptibility. Rev Med Vet 2013;164(1): 18-22.
[9]Tenover FC. Mechanism of antimicrobial resistance in bacteria. Am J Infect Control 2006;34: S3-10.
[10]Ginel PJ, Lucena R, Rodriguez JC, Ortega J. A semiquantitative cytological evaluation of normal and pathological samples from the external ear canal of dogs and cats. Vet Dermatol 2002;13(3): 151-6.
[11]Clinical and Laboratory Standards Institute(CLSI). Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals;approved standard. 4th ed. Wayne: CLSI;2013.
[12]Bugden DL. Identification and antibiotic susceptibility of bacterial isolates from dogs with otitis externa in Australia. Aust Vet J 2013;91(1–2): 43-6.
[13]Cain CL, Morris DO, Rankin SC. Clinical characterization of Staphylococcus schleiferi infections and identification of risk factors for acquisition of oxacillin-resistant strains in dogs: 225 cases (2003–2009). J Am Vet Med Assoc 2011;239(12): 1566-73.
[14]van Hoek AHAM, Mevius D, Guerra B, Mullany P, Roberts AP, Aarts HJM. Acquired antibiotic resistance genes: an overview. Front Microbiol 2011;2: 203.
[15]Fluit AC, Carpaij N, Majoor EA, Bonten MJ, Willems RJ. Shared reservoir of ccrB gene sequences between coagulase-negative staphylococci and methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 2013;68: 1707-13.
[16]Velasco V, Buyukcangaz E, Sherwood JS, Stepan RM, Koslofsky RJ, Logue CM. Characterization of Staphylococcus aureus from humans and a comparison with isolates of animal origin, in North Dakota, United States. PLoS One 2015;10(10): e0140497.
[17]De Martino L, Lucido M, Mallardo K, Facello B, Mallardo M, Iovane G, et al. Methicillin-resistant staphylococci isolated from healthy horses and horse personnel in Italy. J Vet Diagn Invest 2010;22: 77-82.
[18]Mallardo K, Nizza S, Fiorito F, Pagnini U, De Martino L. A comparative evaluation of methicillin-resistant staphylococci isolated from harness racing-horses, breeding mares and ridinghorses in Italy. Asian Pac J Trop Biomed 2013;3: 169-73.
[19]Haenni M, Galofaro L, Ythier M, Giddey M, Majcherczyk P, Moreillon P, et al. Penicillin-binding protein gene alterations in Streptococcus uberis isolates presenting decreased susceptibility to penicillin. Antimicrob Agents Chemother 2010;54(3): 1140-5.
[20]Klinzing DC, Ishmael N, Dunning Hotopp JC, Tettelin H, Shields KR, Madoff LC, et al. The two-component response regulator LiaR regulates cell wall stress responses, pili expression and virulence in group B Streptococcus. Microbiology 2013;159: 1521-34.
[21]Longtin J, Vermeiren C, Shahinas D, Tamber GS, McGeer A, Low DE, et al. Novel mutations in a patient isolate of Streptococcus agalactiae with reduced penicillin susceptibility emerging after long-term oral suppression therapy. Antimicrob Agents Chemother 2011;55: 2983-5.
[22]Pinto TC, Costa NS, Corrˆea AB, de Oliveira IC, de Mattos MC, Rosado AS, et al. Conjugative transfer of resistance determinants among human and bovine Streptococcus agalactiae. Braz J Microbiol 2014;45(3): 785-9.
[23]Penna B, Thom´e S, Martins R, Martins G, Lilenbaum W. In vitro antimicrobial resistance of Pseudomonas aeruginosa isolated from canine otitis externa in Rio de Janeiro, Brazil. Braz J Microbiol 2011;42: 1434-6.
[24]De Martino L, Nizza S, de Martinis C, Foglia Manzillo V, Iovane V, Paciello O, et al. Streptococcus constellatus-associated pyoderma in a dog. J Med Microbiol 2012;61: 438-42.
[25]Giannakaki V, Miyakis S. Novel antimicrobial agents against multi-drug-resistant gram-positive bacteria: an overview. Recent Pat Antiinfect Drug Discov 2012;7(3): 182-8.
[26]Schmiedel J, Falgenhauer L, Domann E, Bauerfeind R, Prenger-Berninghoff E, Imirzalioglu C, et al. Multiresistant extendedspectrum β-lactamase-producing Enterobacteriaceae from humans, companion animals and horses in central Hesse, Germany. BMC Microbiol 2014;14: 187.
[27]Hawkins C, Harper D, Burch D, Anggård E, Soothill J. Topical treatment of Pseudomonas aeruginosa otitis of dogs with a bacteriophage mixture: a before/after clinical trial. Vet Microbiol 2010;146: 309-13.
*Corresponding author:Prof. Luisa De Martino, Department of Veterinary Medicine and Animal Production, University of Naples“Federico II”, Via F. Delpino 1, 80137 Naples, Italy.
 Asian Pacific Journal of Tropical Biomedicine2016年5期
Asian Pacific Journal of Tropical Biomedicine2016年5期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Phylogeny and drug resistance of HIV PR gene among HIV patients receiving RT inhibitors in Iran
- Susceptibility of Aedes flavopictus miyarai and Aedes galloisi mosquito species in Japan to dengue type 2 virus
- Antibacterial activity of Bixa orellana L.(achiote)against Streptococcus mutans and Streptococcus sanguinis
- Antimicrobial properties of sea anemone Anthopleura nigrescens from Pacific coast of Costa Rica
- Emergence of non-albicans Candida species and antifungal resistance in intensive care unit patients
- Primary oral and nasal transmissible venereal tumor in a mix-breed dog
