Susceptibility of Aedes flavopictus miyarai and Aedes galloisi mosquito species in Japan to dengue type 2 virus
Raweewan Srisawat, Thipruethai Phanitchat, Narumon Komalamisra, Naoki Tamori, Lucky Runtuwene,Kaori Noguchi, Kyoko Hayashida, Shinya Hidano, Naganori Kamiyama, Ikuo Takashima,Tomohiko Takasaki, Ichiro Kurae, Narihiro Narita, Takashi Kobayashi, Yuki EshitaFaculty of Tropical Medicine,Mahidol University,0/6 Rajvithi Road,Bangkok 000,ThailandFaculty of Medicine,Oita University,- Idaigaoka,Hasama,Oita 879-9,JapanLaboratory of Public Health,Department of Environmental Veterinary Sciences,Graduate School of Veterinary Medicine,Hokkaido University,Kita 8 Nishi 9,Kita-ku,Sapporo 060-088,JapanDepartment of Virology ,National Institute of Infectious Diseases,-- Toyama,Shinjuku-ku,Tokyo 6-860,JapanCultural Anthropology Laboratory,Department of Arts and Sciences,Ohkagakuen University,8 Takeji,Sakae,Toyoake-shi,Nagoya 70-9,Japan
Susceptibility of Aedes flavopictus miyarai and Aedes galloisi mosquito species in Japan to dengue type 2 virus
Raweewan Srisawat1*, Thipruethai Phanitchat1, Narumon Komalamisra1, Naoki Tamori2, Lucky Runtuwene2,
Kaori Noguchi2, Kyoko Hayashida2, Shinya Hidano2, Naganori Kamiyama2, Ikuo Takashima3,
Tomohiko Takasaki4, Ichiro Kurae4, Narihiro Narita5, Takashi Kobayashi2, Yuki Eshita21Faculty of Tropical Medicine,Mahidol University,420/6 Rajvithi Road,Bangkok 10400,Thailand
2Faculty of Medicine,Oita University,1-1 Idaigaoka,Hasama,Oita 879-5593,Japan
3Laboratory of Public Health,Department of Environmental Veterinary Sciences,Graduate School of Veterinary Medicine,Hokkaido University,Kita 18 Nishi 9,Kita-ku,Sapporo 060-0818,Japan
4Department of Virology 1,National Institute of Infectious Diseases,1-23-1 Toyama,Shinjuku-ku,Tokyo 162-8640,Japan
5Cultural Anthropology Laboratory,Department of Arts and Sciences,Ohkagakuen University,48 Takeji,Sakae,Toyoake-shi,Nagoya 470-1193,Japan
ARTICLE INFO
Article history:
Received 18 Oct 2015
Received in revised form 8 Nov, 2nd
revised form 9 Dec 2015
Accepted 27 Dec 2015
Available online 18 Mar 2016
Keywords:
Aedes flavopictus miyarai
Aedes galloisi
Aedes albopictus
Aedes aegypti
Dengue type 2 virus
Japan
Oral infection
Intrathoracic inoculation
ABSTRACT
Objective:To evaluate the potential of local mosquitoes to act as vectors for dengue transmission in Japan.
Methods:Serotype2ThNH28/93wasusedtotestthedenguesusceptibilityprofilesofAedes flavopictus miyarai(Ae. f. miyarai), Aedes galloisi(Ae. galloisi)and Aedes albopictus(Ae. albopictus),whichwerecollectedinJapan.WeusedAedesaegyptifromThailandasapositive control. The mosquitoes were infected with the virus intrathoracically or orally. At 10 or 14 days post infection, the mosquitoes were dissected and total RNA was extracted from their abdomens, thoraxes, heads and legs. Mosquito susceptibility to dengue virus was evaluated using RT-PCR with dengue virus-specific primers. Differences in the infection and mortality rates of the different mosquito species were tested using Fisher's exact probability test.
Results:The infection ratesfor dengue virus administered intrathoracicallyto Ae.f. miyarai, Ae.galloisiandAedesaegyptimosquitoeswereidenticalbyRT-PCRonDay10postinfection. All of the body parts we testedwere RT-PCR-positive for dengue virus. For the orally administeredvirus,theinfectionratesinthedifferentbodypartsoftheAe.f.miyaraimosquitoeswere slightly higher than those of Ae. albopictus mosquitoes, but were similar to the control mosquitoes(P>0.05).ThemortalityratesforAe.f.miyaraiandAe.albopictusmosquitoeswere similar(P=0.19).Ourdataindicatedthatdengueviruswasabletoreplicateanddisseminateto secondaryinfectionsitesinallofthefourmosquitospecies(Japaneseand Thai).
Conclusions:Ae. albopictus is a well-known candidate for dengue transmission in Japan. However, our data suggest that Ae. f. miyarai from Ishigaki Island(near Okinawa Island)and Ae. galloisi from Hokkaido(Northern Japan)should also be regarded as potential vectors for dengue transmission in these regions. Further studies on these mosquitoes should be conducted.
Original article http://dx.doi.org/10.1016/j.apjtb.2016.03.003
Tel: +66(0)2306 9176
E-mail: raweewan.sri@mahidol.ac.th
Foundation Project: Supported by the Matsumae International Foundation in Japan for Raweewan Srisawat, Faculty of Tropical Medicine grant, Grants-in-Aid(Kiban-B, #25300053)from Japan Society for the Promotion of Science(JSPS), Research Program on Emerging and Re-emerging Infectious Diseases(H26-shinkou-jitsuyouka-007)from the Japan Agency for Medical Research and Development(AMED).
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
1. Introduction
Dengue fever(DF), dengue hemorrhagic fever(DHF), and dengue shock syndrome are medically important arthropodborne viral diseases causing high morbidity in humans. It is estimated that 50–200 million infections, resulting in 500000 cases of DHF occur annually, and the disease has spread extensively throughout the tropical regions of the world[1]. About half of the world's population is now at risk. Thedisease is transmitted to humans by the bite of a dengue-infected mosquito vector[2].
Between 1942 and 1945, Japan experienced dengue outbreaks in the cities of Nagasaki, Hiroshima, Kobe, and Osaka[3]. Since then, dengue has not been epidemic in Japan. According to the Infectious Disease Control Law, 868 imported cases of DF were reported between 1999 and 2010, and there is an increasing trend each year[4]. Travel-associated cases have also occurred in Taiwan[5]and Madagascar[6], but local transmission has also occurred in these countries. The most recent dengue cases in Japan were all travelers who had visited Southeast Asia or South Asia[4,7]. Additionally, some foreign visitors who were infected in their own countries became ill with dengue virus while in Japan. Nearly 5 million Japanese people visit countries in tropical and subtropical areas of the world annually, and 2 million people visit Japan from these areas[8].
The first cases of locally contracted DF were reported in Tokyo in 2014(26th August–30th October)and the large number of reported cases(160)has caused panic in Japan. Its seriousness is underlined by the fact that local transmission of DF in Japan has not occurred in over 70 years. In almost all cases, the dengue patients had visited a large area of parkland in Tokyo called Yoyogi Park. Interestingly, a patient who stayed at a house about a 2–3 minutes' walk from the park was bitten by a mosquito, although he had not actually visited the park[9]. Therefore, it was suspected that a local Japanese Aedes vector had acquired the virus from a traveler who had visited a dengue endemic country and the virus had subsequently been distributed in mosquitoes around the park and caused the outbreak. From a public health perspective, DF and DHF are currently very important infectious diseases in Japan. Hence, identifying the vector that transmits dengue in Japan is crucial for control and management of the outbreak.
Dengue viruses are transmitted by infected Aedes(Stegomyia)mosquitoes, the vectors of this virus. Aedes aegypti(Ae. aegypti)is the most prevalent vector in the human-mosquito cycle in tropical and sub-tropical regions, while Aedes albopictus(Ae. albopictus)is regarded as a secondary vector. In Japan, the numerous Aedes(Stegomyia)subgenus mosquito species include the following: Ae. albopictus,Aedes flavopictus, Aedes riversi, Aedes galloisi(Ae. galloisi), Aedes flavopictus daitensis, Aedes flavopictusdownsi,Aedes flavopictusmiyarai(Ae.f.miyarai)and Aedes wadai. Only Ae. albopictus, Aedes flavopictus, Aedes riversi and a different genus, Ocherotatus dorsalis, have been shown to be susceptible to dengue in Japan[10]. No other vector specieshavebeenconfirmedasbeingsusceptibleto denguevirus.
Ae. f. miyarai and Ae. galloisi belong to the Stegomyia subgenus of the scutellaris group and have not been reported as vectors of dengue virus in Japan. Here, we aimed to determine the susceptibility of Ae. f. miyarai and Ae. galloisi mosquitoes to dengue type 2 virus.
2. Materials and methods
2.1. Mosquito strains
The laboratory colonies tested for susceptibility to a type 2 dengue virus(ThNH28/93)were as follows: Ae. f. miyarai (collected by Dr. Motoyoshi Mogi, Saga University, Ishigaki Island, Japan), Ae. albopictus colonized by Yuki Eshita in Kurume, Fukuoka, and field-collected Ae. galloisi mosquitoes from Sapporo, Hokkaido, Japan. Ae. aegypti(collected by Yuki Eshita in Nakhon Phanom, Thailand)was used as a comparative control. All of Aedes species were collected in larval stage. Eggs were submerged in oxygen-free water. Eighty larvae were reared in each pan(30 cm×20 cm)containing 500 mL of tap water. These larvae were fed on a mixed diet of yeast extract(EBIOS, Asahi Food & Healthcare Co., Ltd., Tokyo, Japan)and mouse feed powder(CLEA Japan Inc., Tokyo, Japan)in an equal ratio. Pupae were transferred to a wet Kimwipe®(Kimberly–Clark Ltd., Irving, TX, USA)in 200 mL plastic cups and kept in adult cages. Adult mosquitoes were maintained with 4%sucrose immersed cotton. All their developmental stages were kept at (25±1)°C under 60%–80%relative humidity and a 16:8 h (light: dark)daily photoperiod in an insectary.
2.2. Virus strain
ThNH28/93, a strain of dengue serotype 2 was used for all the mosquito susceptibility experiments. It was isolated from patients diagnosed as DHF grade II-positive at Nakhon Phanom Provincial Hospital(Thailand)during the dengue outbreak in 1993, and was kindly provided by Dr. Akira Igarashi of the Institute of Tropical Medicine, Nagasaki University. The stock virus was prepared using Ae. albopictus C6/36 cells(5th passages). The viral titer of the stock measured by focus formation using Vero cells on 96-well plates was 3.2×106fluorescence forming units(FFU)(FFU/mL). The viral stock solution was divided into aliquots and stored at -80°C until use.
2.3. Mosquito infections
2.3.1. Intrathoracic inoculations
Seven-day-old female mosquitoes were inoculated intrathoracically with~0.17 μL of 3.2×106FFU/mL dengue virus solution, according to the procedure described by Rosen and Gubler[11]. The mosquitoes were maintained at(28±1)°C for 10 days. Live female mosquitoes were sacrificed by freezing and examined for the presence of the dengue viral genome in various body parts by RT-PCR.
2.3.2. Oral infection of mosquitoes
Seven-day-old female mosquitoes were starved for several hours at 25°C then allowed to feed on virus blood sugar(VBS)solution through a cotton pad for 1–2 h at 28°C. The VBS solution contained stock dengue virus solution(1 part), phosphate buffer solution-rinsed and packed human red blood cells (1 part), and 4%sucrose(Wako Co., Tokyo, Japan). The VBS cotton pad was left for addition 12 h at 28°C to get more engorged female. Only the VBS fed mosquitoes were transferred to small cardboard containers and kept at 28°C for 10 or 14 days. Live females were killed by freezing and examined for the presence of the dengue viral genome by RT-PCR.
2.4. Detection of the dengue viral genome
2.4.1. Extraction of total RNA
Total RNA was extracted from the fully separated body parts (abdomen, thorax, head and legs)of individual mosquitoes using TRIzol®LS reagent(Invitrogen, Carlsbad, CA, USA)following the manufacturer's instructions. Next, the total RNA was purified using an RNeasy Mini kit(Qiagen, Hilden, Germany)following the manufacturer's instructions, and eluted into a 50 μL volumeof RNase-free water. Individual eluates(5 μL)were used as template RNA for RT-PCR assays.
2.4.2. RT-PCR assays
The universal primers DC1(5′-TCA ATA TGC TGA AAC GCG CGA GAA ACC G-3′)and DC2(5′-TTG CAC CAA CAG TCA ATG TCT TCA GGT TC-3′), which were designed to target the gene encoding the structural protein from dengue virus were used in this work[12]. The PCR mixture contained a total volume of 12.5 μL in a 200 μL microfuge tube with 10 pmol of each primer, 1X SuperScript™One step RT-PCR with PLATINUM Taq reaction mixture(Gibco BRL), and 5 μL of template RNA derived from homogenates of the partial body parts from the mosquitoes. Reactions, conducted in a thermal cycler(MJ Research Inc., Watertown, MA, USA)consisted of 50°C for 30 min(first strand cDNA synthesis), 94°C for 2 min(inactivation of reverse transcriptase and denaturation), followed by 35 repetitive PCR cycles at 94°C for 30 s, 53°C for 30 s and 68°C for 1 min, followed by 68°C for 2 min. PCR products were subjected to 30 min electrophoresis at 100 V on a 1.5%agarose gel and then visualized by ethidium bromide staining of the gel. The dengue viral PCR product was expected to be 511 base pairs. RNA of dengue serotype 2 from cell culture was extracted and used as template for positive control.
2.5. Data analysis
Dengue infection and dissemination rates were calculated from the number of RT-PCRs that were positive for the presence of the dengue viral genome in the mosquito samples. Infection rates were determined by the number of mosquito abdomens infected with virus as a percentage of the total number of mosquitoes tested. We defined the dissemination rate as the number of viruses that had escaped through the midgut and disseminated throughout the hemocoel. Thereafter, the dissemination rate was calculated from the number of mosquitoes infected(positive leg or thorax)expressed as a percentage of the total number of mosquitoes tested. Infection and mortality rate differences between the mosquito species were analyzed using Fisher's exact probability test in the Statistical Package for the Bioscience software version 8.8(Winesteen Institute of Community Medicine, Saitama, Japan). Differences were considered statistically significant when P value was less than 0.05.
3. Results
3.1. Intrathoracically-inoculated mosquito infections
The infection rates of Ae. galloisi, Ae. f. miyarai and Ae. aegypti inoculated intrathoracically with dengue type 2 virus were identical(100%, P = 1.00)for the individual body parts(abdomen, thorax, head and legs)that were analyzed separately.
3.2. Orally infected mosquitoes
As there was no statistically significant difference between the infection rates at 10 and 14 days post-infection, these data were combined as shown in Table 1. After the 10 and 14 days incubation periods, there were no significant differences among three species. Even though the infection rates(30%)for Ae. f. miyarai were slightly higher than those(17%)of Ae. albopictus, they were similar to the control(Ae. aegypti).
For each mosquito body part(abdomen, thorax, head and legs), the infection rates did not differ between Ae. f. miyarai and Ae. albopictus(P = 0.46, P = 0.40, P = 0.66, P = 0.66, respectively)and the comparative species Ae. aegypti(P = 1.00, P = 0.74, P = 1.00, P = 1.00, respectively). However, the infection rates in the abdomens of Ae. f. miyarai and Ae. albopictus were higher than those of the thorax head and leg.
The Ae. f. miyarai mortality rate was similar to that of Ae. albopictus(P = 0.19);however, it was significantly lower than that of the Ae. aegypti mosquito control(P = 0.01). The mortality rate for Ae. albopictus was similar to that of the Ae. aegypti control(P = 0.55)(Table 1). Also, for Ae. f. miyarai and Ae. albopictus, the infection rates were higher than the dissemination rates.
We analyzed the RT-PCR results to determine whether orally administered dengue virus type 2 had been successful at infecting each tissue or had been retarded by any barrier(s)in the mosquitoes(Table 2). The viral dissemination rate was determined from the infected mosquitoes. Among the nine Ae. f. miyarai mosquitoes that were positive for dengue virus, two (sample No. 27, 30)had infected abdomens only. Two mosquitoes(sample No. 28 and 53)had virus-positive abdomens and thoraxes. Five mosquitoes(sample No. 26, 29, 35, 40, 52)were virus-positive for all of the body tissues screened herein. Of the two individual infected Ae. albopictus, one(sample No. 55)was positive for dengue virus in its abdomen, while the other was virus-positive for all the body tissues we screened. Out of the eight infected Ae. aegypti controls, three were dengue viruspositive in the abdomen only, one was positive for both the abdomen and thorax, one was positive for the abdomen, head and legs, while three were positive in all the body tissues we screened.
The PCR products derived from the dengue type-2 virus genome were faint in mosquito No. 6, 20 of Ae. aegypti and 28 of Ae. f. miyarai(Table 2). We assumed that the quantity of the virus that had multiplied in each mosquito tissue was reflected by the intensity of the PCR products that were obtained.
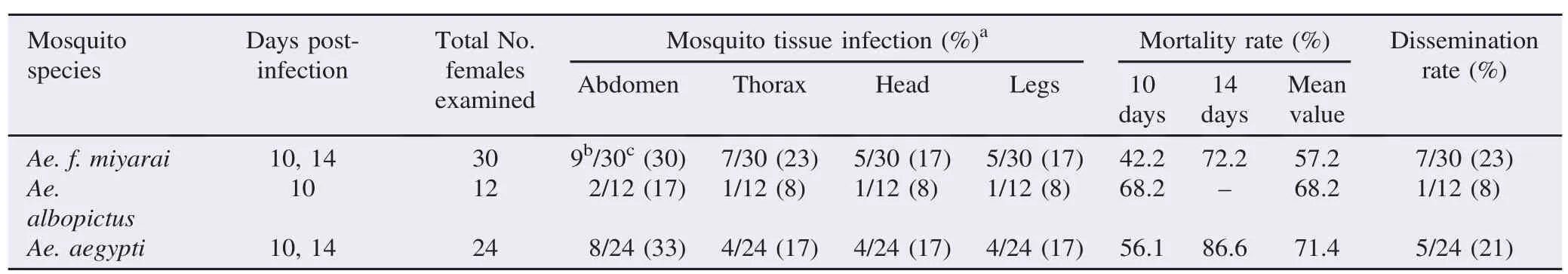
Table 1 Oral infection of Aedes mosquitoes with type 2 dengue virus and tissue dissemination rates.

Table 2 Infected mosquitoes where dengue type 2 virus escaped the midgut barrier and disseminated.
4. Discussion
Ae. aegypti and Ae. albopictus are well-known primary and secondary vectors of DF in Southeast Asia. Currently, there are no data on Ae. aegypti infestations in urban residential areas in Japan[14], even if Ae. aegypti was detected at Tokyo Narita International Airport in 2012[15]. Therefore, only Ae. albopictus is suspected to be a vector of dengue virus in this country. Our results show that Ae. f. miyarai and Ae. albopictus had similar infection rates. Furthermore, susceptibility to dengue virus tended to be high in Ae. f. miyarai compared with Ae. albopictus, which also had a low mortality rate. This suggests that Ae. f. miyarai may have relatively high longevity, thereby making contact with humans and spread of the virus more likely. Hence, in common with Ae. albopictus, Ae. f. miyarai may be an efficient vector of dengue virus, and a potential vector of this virus in Japan. However, this conclusion is based mainly on our laboratory data.
We used intrathoracic inoculation as a preliminary experiment to determine whether dengue virus was susceptible to the live mosquito cells of Ae. f. miyarai and Ae. galloisi post 10 days inoculation. There is no report on the susceptibility to dengue virus of these species. If the virus is unable to replicate in them, it is not likely that they will serve as biological vectors of dengue [16]. The intrathoracic inoculation was more useful for susceptible experiment than oral infection when the limited number of mosquito was collected in case of Ae. galloisi. If they refused to blood feeding, it will have no susceptible information. This phenomenon always occurs in field-collected mosquito. However, the results showed that both mosquito species became infected with the virus. However, this does not necessarily indicate that they are susceptible to oral infection. Therefore, Ae. f. miyarai, Ae. albopictus and the Ae. aegypti control from Thailand were investigated further using the oral infection route. Because colony of Ae. galloisi was unable to maintain, further oral infections were not done with this species.
Based on the results of the oral infections, the infection rates in the mosquitoes were quite low at less than 50%when compared with intrathoracic inoculation. When a mosquito ingests dengue virus-infected blood, the virus replicates in the midgut, then disperses in the circulating hemolymph and disseminates to infect secondary target organs. Once the salivary glands become infected, the virus can be transmitted to vertebrates through blood feeding. However, there are multiple barriers to productive vector infection in the vector-arbovirus system. Several barriers to productive infection in the vector are known or hypothesized to be present at the midgut and salivary glands[17]. Our data have revealed a midgut barrier whereby the virus could not infect the midgut cells, as shown by the negative RT-PCR results obtained for the presence of the dengue viral genome. The exact mechanism for such a barrier is not known, although many hypotheses have been proposed to explain it. The barrier can be by-passed by intrathoracic inoculation exception of salivary gland barrier[18], a method that achieves a higher infection rate than oral infection.
We found 22.2%, 50.0%and 37.5%of Ae. f. miyarai, Ae. albopictus and Ae. aegypti, had midgut escape barriers, respectively, allowing viral replication in the midgut;however, the virus could not exit the midgut to disseminate the infection elsewhere[midgut escape barriers were calculated as follows: (No. of mosquitoes with infected abdomens only×100)/Total No. of mosquitoes with infected abdomens]. Dissemination rates were 77.8%, 50.0%and 62.5%in Ae. f. miyarai, Ae. albopictus and Ae. aegypti, respectively, based on mosquitoes with virusinfected abdomens.
For Ae. aegypti mosquito No. 2(Table 2), no dengue viral genome PCR product was amplified from the thorax, although the other organs were dengue-positive. One explanation is that of viral RNA degradation caused by the lack of an additional RNase inhibitor. Alternatively, there may have been an inhibitor of PCR in the thorax of this mosquito. It was clear that three mosquitoes(No. 6, 28, 53)were not infected with dengue virus in the head and legs, although their abdomens and thoraxes were infected. Clearly, additional unknown barriers in the mosquito head and/or inhibitors of RT-PCR should be investigated further.
The PCR product intensities were used as a proxy for the viral load in the mosquitoes, but they were not used for inferring the dissemination rates. We found no relationship among them. For example,five mosquitoes(No. 1, 22, 27, 30 and 55, Table 2)had strong abdomen-derived PCR product bands, but the virus was unable to exit their midguts. In contrast, two mosquitoes (No. 6, 28), which produced faint PCR bands, may have been infected with low viral titers, although the viruses escaped from the midguts to the secondary organs. Our findings are consistent with those of Bennett et al.[19]in that high viral titers in the midgut are not correlated with viral dissemination rates.
The assessment of infected mosquito's saliva was omitted in this study. And it would be strong evidence for dengue viral transmission of these mosquitoes. We operate dengue infectedmosquitoes in Biological Safety Level 3 laboratory, however it would be more risky for us in Japan where local dengue transmission had suddenly occurred in 2014.
Our results should provide useful information for the vector control program in Japan, especially if dengue virus from a viremic human patient is introduced into areas where dengue and Ae. albopictus are not present, such as Ishigaki Island and Sapporo, where Ae. f. miyarai and Ae. galloisi are distributed. So, both species could not be found in Tokyo. The transmission cycle of the mosquito vector should be broken as quickly as possible. Therefore, the data provided here about putative vectors for dengue are important. With the lack of specific treatment or a vaccine for dengue available, the prevention and control of a dengue outbreak relies solely on controlling the vector population[5].
Our findings need to be supplemented with additional physiological and ecological information on the vectors of dengue, such as their density, biting rates, host preferences, extrinsic incubation periods and longevities. Importantly, whether Ae. f. miyarai can transmit dengue virus as a vector in nature needs answering[20], because the infectivity of colonized mosquitoes analyzed in a laboratory setting may not have any relationship with the infectivity of natural populations[21].
This is the first report that Ae. f. miyarai can replicate dengue type 2 virus and that this virus can spread to the hemocoel and infect secondary organs. This virus could also replicate in Ae. galloisi, although it is worth noting that the mosquitoes involved were inoculated intrathoracically with the virus. Colonization of Ae. galloisi should be investigated by additional oral infection laboratory experiments and oral infections in field-collected Ae. f. miyarai with DEN-1 that only one serotype found in Japan should also be further studied.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We thank Hiroyuki Takaoka and staff of the Department of Infectious Diseases, Oita University, Japan for their kind assistance, and Somjai Leemingsawat, Narumon Komalamisra and Yupha Rongsriyam of the Department of Medical Entomology, Faculty of Tropical Medicine, Mahidol University for their valuable suggestions and comments. This work was supported by the Matsumae International Foundation in Japan for Raweewan Srisawat, Faculty of Tropical Medicine grant, Grants-in-Aid(Kiban-B, #25300053)from Japan Society for the Promotion of Science(JSPS), Research Program on Emerging and Reemerging Infectious Diseases(H26-shinkou-jitsuyouka-007)from the Japan Agency for Medical Research and Development (AMED).
References
[1]Gubler DJ. The economic burden of dengue. Am J Trop Med Hyg 2012;86(5): 743-4.
[2]World Health Organization. Dengue and severe dengue. Geneva: World Health Organization;2015.[Online]Available from: http:// www.who.int/mediacentre/factsheets/fs117/en/[Accessed on 7th November, 2015]
[3]Hotta S.[Dengue vector mosquitoes in Japan: the role of Aedes albopictus and Aedes aegypti in the 1942–1944 dengue epidemics of Japanese Main Islands]. Med Entomol Zool 1998;49: 267-74. Japanese.
[4]Takasaki T. Imported dengue fever/dengue hemorrhagic fever cases in Japan. Trop Med Health 2011;39: 13-5.
[5]Kuan MM, Lin T, Chuang JH, Wu HS. Epidemiological trends and the effect of airport fever screening on prevention of domestic dengue fever outbreaks in Taiwan, 1998–2007. Int J Infect Dis 2010;14: e693-7.
[6]Savini H, Gautret P, Gaudart J, Field V, Castelli F, L´opez-V´elez R, et al. Travel-associated diseases, Indian ocean islands, 1997–2010. Emerg Infect Dis 2013;19: 1297-301.
[7]Nakamura N, Arima Y, Shimada T, Matsui T, Tada Y, Okabe N. Incidence of dengue virus infection among Japanese travellers, 2006 to 2010. West Pac Surveill Response J 2012;3: 39-45.
[8]Takahashi M, Miwa T, Yamada K, Sato Y, Ikawa K, Matsumoto Y, et al. Detection of dengue virus-infected patients among passengers at the quarantine station of the New Tokyo International Airport. Jpn J Infect Dis 2002;55: 215-6.
[9]Kojima G. Autochthonous dengue fever imported to England from Japan, 2014. Emerg Infect Dis 2015;21: 182-4.
[10]Eshita Y.[Experimental studies on the transmission of dengue virus by Japanese mosquitoes]. Teikyo Med J 1982;5: 17-27. Japanese.
[11]Rosen L, Gubler D. The use of mosquitoes to detect and propagate dengue viruses. Am J Trop Med Hyg 1974;23: 1153-60.
[12]Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol 1992;30: 545-51.
[13]Pilitt DR, Jones JC. A qualitative method for estimating the degree of engorgement of Aedes aegypti adults. J Med Entomol 1972;9: 334-7.
[14]Kobayashi M, Komagata O, Yonejima M, Maekawa Y, Hirabayashi K, Hayashi T, et al. Retrospective search for dengue vector mosquito Aedes albopictus in areas visited by a German traveler who contracted dengue in Japan. Int J Infect Dis 2014;26: 135-7.
[15]Sukehiro N, Kida N, Umezawa M, Murakami T, Arai N, Jinnai T, et al. First report on invasion of yellow fever mosquito, Aedes aegypti, at Narita International Airport, Japan August 2012. Jpn J Infect Dis 2013;66: 189-94.
[16]Beaty BJ, Woodring WC. The biology of disease vectors. Boulder: University Press;1996, p. 51-72.
[17]Franz AW, Kantor AM, Passarelli AL, Clem RJ. Tissue barriers to arbovirus infection in mosquitoes. Viruses 2015;7: 3741-67.
[18]Manley R, Harrup LE, Veronesi E, Stubbins F, Stoner J, Gubbins S, et al. Testing of UK populations of Culex pipiens L. for Schmallenberg virus vector competence and their colonization. PLoS One 2015;10: e0134453.
[19]Bennett KE, Olson KE, Muñoz Mde L, Fernandez-Salas I, Farfan-Ale JA, Higgs S, et al. Variation in vector competence for dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States. Am J Trop Med Hyg 2002;67: 85-92.
[20]Armstrong PM, Rico-Hesse R. Differential susceptibility of Aedes aegypti to infection by the American and Southeast Asian genotypes of dengue type 2 virus. Vector Borne Zoonotic Dis 2001;1: 159-68.
[21]Tardieux I, Poupel O, Lapchin L, Rodhain F. Variation among strains of Aedes aegypti in susceptibility to oral infection with dengue virus type 2. Am J Trop Med Hyg 1990;43: 308-13.
*Corresponding author:Raweewan Srisawat, Faculty of Tropical Medicine, Mahidol University, 420/6 Rajvithi Road, Bangkok 10400, Thailand.
 Asian Pacific Journal of Tropical Biomedicine2016年5期
Asian Pacific Journal of Tropical Biomedicine2016年5期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Phylogeny and drug resistance of HIV PR gene among HIV patients receiving RT inhibitors in Iran
- An update on microbiological causes of canine otitis externa in Campania Region,Italy
- Antibacterial activity of Bixa orellana L.(achiote)against Streptococcus mutans and Streptococcus sanguinis
- Antimicrobial properties of sea anemone Anthopleura nigrescens from Pacific coast of Costa Rica
- Emergence of non-albicans Candida species and antifungal resistance in intensive care unit patients
- Primary oral and nasal transmissible venereal tumor in a mix-breed dog
