四元体系Na+,K+//Br-,SO42--H2O 373 K相平衡
崔瑞芝,桑世华(成都理工大学材料与化学化工学院,四川 成都60059;矿产资源化学四川省高校重点实验室,四川 成都60059)
四元体系Na+,K+//Br-,SO42--H2O 373 K相平衡
崔瑞芝1,2,桑世华1,2
(1成都理工大学材料与化学化工学院,四川 成都610059;2矿产资源化学四川省高校重点实验室,四川 成都610059)
摘要:采用等温溶解平衡法研究了四元体系Na+,K+//Br-,SO42--H2O在373 K条件下的相平衡关系,测定了平衡溶液的溶解度和密度,并根据实验数据绘制相应的相图、水图和密度图。研究发现:交互四元体系Na+,K+//Br−,SO42--H2O在373 K温度下,有复盐钾芒硝Na2SO4·3K2SO4生成,相图由3个共饱和点、7条单变量曲线和5个结晶区组成。其中,5个结晶区分别对应单盐:K2SO4,KBr,NaBr,Na2SO4和复盐Na2SO4·3K2SO4(Gla)。关键词:地下卤水;相平衡;溶解性;溶液;钾盐;溴盐
2015-07-22收到初稿,2015-10-19收到修改稿。
联系人:桑世华。第一作者:崔瑞芝(1988—),女,博士研究生。
Received date: 2015-07-22.
Foundation item: supported by the National Natural Science Foundation of China (41373062),the Specialized Research Fund for the Doctoral Program of Higher Education of China (20125122110015) and the Scientific Research and Innovation Team in Universities of Sichuan Provincial Department of Education(15TD0009).
引 言
四川盆地地下卤水分布广泛,资源丰富,除NaCl,卤水还含有K+、Br-、I-、B3+、Li+、Sr2+等多种有用组分[1],是世界上罕见和国家短缺的液态矿产资源,加强该卤水开发和回收利用研究的力度,必将产生重大的社会效益和经济效益。
对于含溴相平衡体系的研究,唐宗薰等[2]针对盐卤资源锂钾卤化物研究了常温298 K条件下三元体系NH4Br-LiBr-H2O和LiI-LiBr-H2O相平衡关系,王静康等[3-4]为了开发老挝钾盐矿中的溴资源而进行了298 K、313 K和333 K条件下三元体系NaCl-NaBr-H2O和KCl-KBr-H2O相平衡研究。针对川西盆地的地下卤水,本课题组在前期工作中已经完成了298 K、323 K、348 K和373 K条件下三元体系KBr-K2B4O7-H2O的相平衡研究[5-8],323 K、348 K和373 K条件下四元体系KCl-KBr-K2SO4-H2O的相平衡研究[9-11],323 K条件下四元体系NaCl-NaBr-Na2SO4-H2O、KCl-KBr-K2B4O7-H2O和Na2B4O7-Na2SO4-NaBr-H2O的相平衡研究[12–14],以及323 K和348 K条件下五元体系KCl-KBr-K2SO4-K2B4O7-H2O的相平衡研究[15]。
四川盆地西部的地下气田卤水组成可简化为复杂多组分六元体系K+,Na+//Cl-,Br-,SO42-,B4O72--H2O,要完成该六元体系的相平衡研究,应首先进行其子体系的研究,本文报道的四元体系Na+,K+//Br−,SO42--H2O即为上述六元复杂体系的一个子体系。由于该卤水储层水温113.81℃[16],卤水层温度分布随着地层深度变化而变化,因此,开展该卤水体系从常温到高温的多温相平衡与相图研究对于揭示该卤水的溶解规律和变化更有实际意义,本课题组已开展了该体系323 K 相平衡研究和理论计算[17-18],本文选择了100℃即373 K作为研究的温度条件。373 K条件下该四元体系的三元子体系Na2SO4-K2SO4-H2O[19]、NaBr-Na2SO4-H2O、NaBr-KBr-H2O[20]、KBr-K2SO4-H2O[21]均已见文献报道,在前期研究工作的基础上,本文详细研究了四元体系Na+,K+//Br−,SO42--H2O在373 K条件下的相平衡关系,并绘制出该体系的相图,对于开发卤水资源、揭示卤水地球化学平衡过程具有一定的指导意义。
1 实验材料和方法
1.1实验试剂和仪器
实验所用试剂为分析试剂,分别为:KCl、KBr、K2SO4、NaCl、NaBr和Na2SO4,实验所用的水为去离子水,其电导率小于1.5×10-4S·m-1。
AL104型电子天平(Mettler-Toledo公司,精度值0.0001 g); SHA-GW数显恒温油浴振荡器(金坛市国旺实验仪器厂,使用精密温度计二次标定,±0.1 K)。
1.2实验方法
本体系的研究采用等温溶解平衡法,即从373 K次级三元体系共饱点开始逐渐加入第三种盐配制料液,并将所配料液放入密封性良好的磨口玻璃瓶中,再将玻璃瓶置于恒温油浴(373 K ± 0.1 K)中振荡,静置。以液相化学组成不变作为达到平衡的标志,实验表明达到平衡的时间为5 d,静置3 d,平衡后取液相及湿渣样进行分析。用X射线粉晶衍射确定平衡固相,平衡液相的密度采用密度瓶法测定;平衡液相的组成采用化学分析法确定。
1.3分析方法
Br-含量:硝酸银容量法 (±0.5%);SO42-含量:茜素红-S法 (±0.5%);K+含量:四苯硼钠-季胺盐返滴定法 (±0.5%);Na+含量:离子平衡差减法[22]。
2 实验结果与讨论
由表1及图1可知,该四元体系有复盐Na2SO4·3K2SO4(Gla)生成,无固溶体生成,其等温溶解度图存在3个共饱和点,分别为点F、点G和点H,其中,共饱和点F对应的平衡固相为Na2SO4·3K2SO4(Gla) + K2SO4+ KBr,平衡液相组成为w(K+) = 0.1435,w(Na+) = 0.0145,w(SO42-) = 0.0028,w(Br-) = 0.3387;共饱和点G对应的平衡固相为Na2SO4·3K2SO4(Gla) + NaBr + KBr,平衡液相组成为w(K+)=0.0634,w(Na+)= 0.0837,w(SO42-) = 0.0039,w(Br-) = 0.4138;共饱和点H对应的平衡固相为Na2SO4·3K2SO4(Gla) + NaBr + Na2SO4,平衡液相组成为w(K+) = 0.1025,w(Na+) = 0.0336,w(SO42-) = 0.0025,w(Br-) = 0.4209;7条单变量曲线,分别为AF、BH、CF、FG、GH、EH和DG,其中,曲线AF对应的平衡固相为K2SO4+ Na2SO4·3K2SO4(Gla),曲线BH对应的平衡固相为Na2SO4+ Na2SO4·3K2SO4(Gla),曲线CF对应的平衡固相为K2SO4+ KBr,曲线FG对应的平衡固相为KBr + Na2SO4·3K2SO4(Gla),曲线GH对应的平衡固相为NaBr + Na2SO4·3K2SO4(Gla),曲线EH对应的平衡固相为NaBr + Na2SO4,曲线DG对应的平衡固相为NaBr + KBr;5个相区,分别为K2SO4、Na2SO4、NaBr、KBr和Na2SO4·3K2SO4(Gla),其中,Na2SO4·3K2SO4(Gla)的结晶区域最大,对应于Na2SO4·3K2SO4(Gla)的溶解度最小,NaBr的结晶区域最小,对应于NaBr的溶解度最大。说明溴化物较硫酸盐易溶,且溴化物对硫酸盐有强烈的盐析作用。
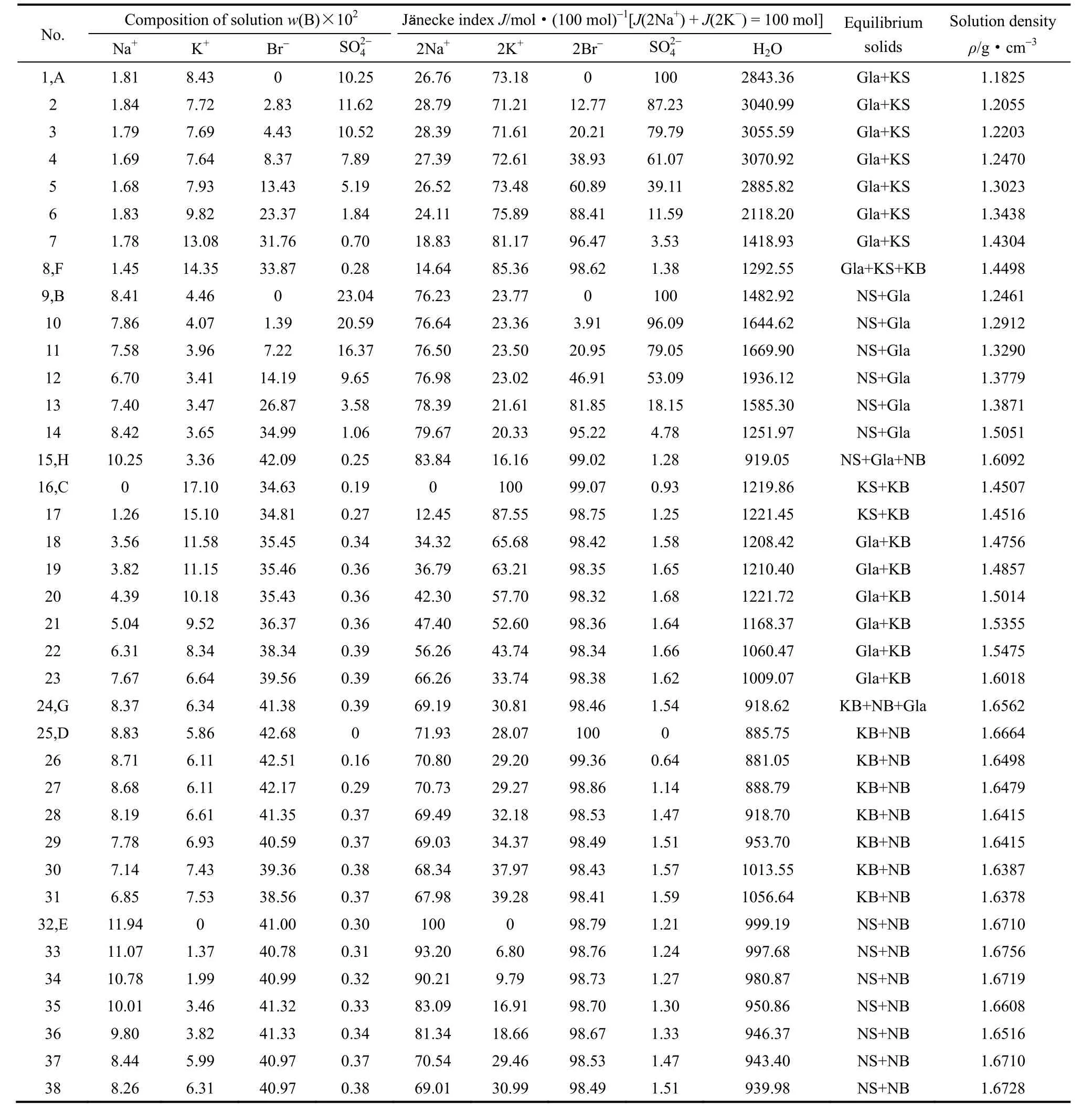
表1 四元体系Na+,K+//Br-,SO42-–H2O 373 K下平衡液相中各组分的溶解度和液相密度Table 1 Solubilities and densities of solution in quaternary system Na+,K+//Br-,SO42--H2O at 373 K
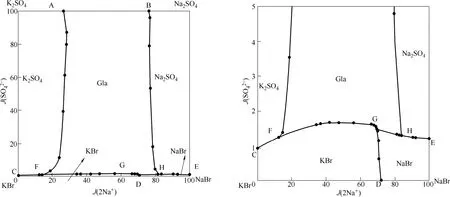
图1 四元体系Na+,K+// Br-,SO24--H2O在373 K下的干盐图及局部放大图Fig.1 Dry-salt solubility diagram and its enlarged bottom of quaternary system Na+,K+// Br-,SO24--H2O at 373 K
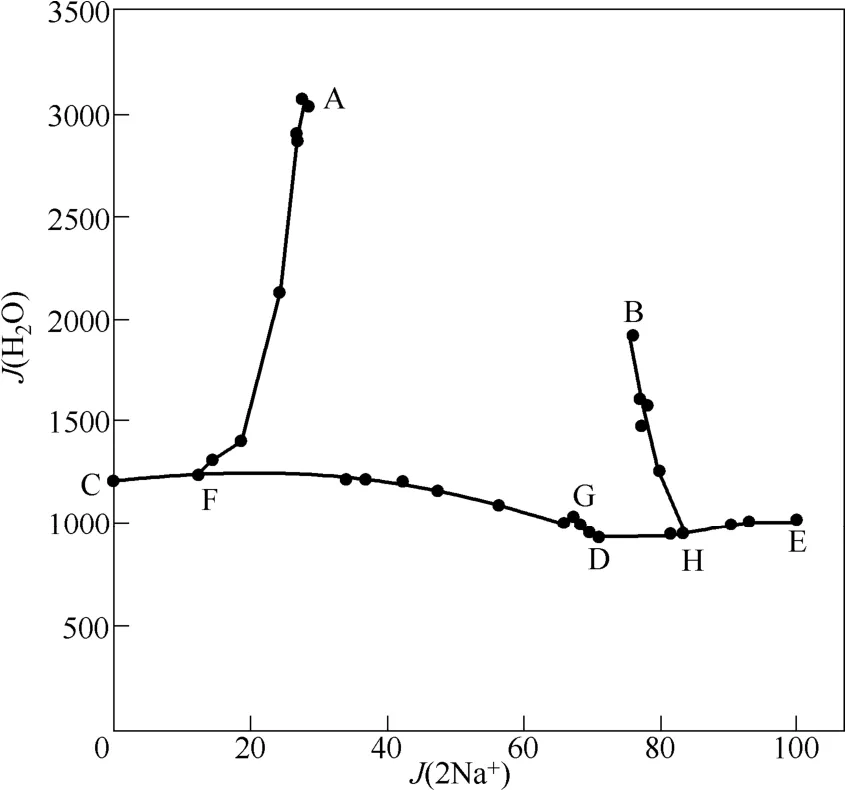
图2 四元体系Na+,K+//Br-,SO24--H2O在373 K下的水含量Fig.2 Water contents of saturated solutions in quaternary system Na+,K+//Br-,SO24--H2O at 373 K
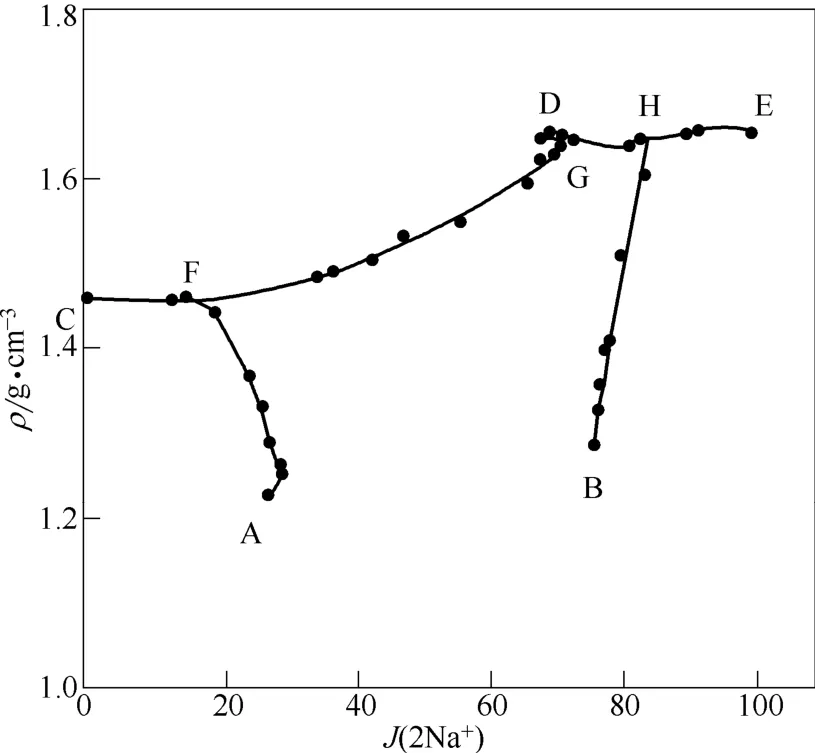
图3 四元体系Na+,K+//Br-,SO24--H2O在373 K下的密度变化趋势Fig.3 Density-composition relations of solutions in quaternary system Na+,K+//Br-,SO24--H2O at 373 K
由图2和图3可知,在点D处,体系中溴离子饱和,无硫酸盐存在,体系的溶解性强,总离子浓度最大,其水值最小,密度最大,达到1.6664 g·cm-3;在点A处,体系中无溴化物存在,体系的溶解性弱,总离子浓度最小,其水值最大,密度值最小,为1.1825 g·cm-3。
四元体系Na+,K+//Br-,SO42--H2O 323 K条件下的相平衡实验研究和计算已见文献报道[19-20],对照323 K和348 K这两个不同温度下的相图,二者相图的形状相似,均含有3个共饱和点,7条单变量曲线和5个结晶区。此外,两个温度条件下单变量曲线GH所对应的平衡固相不同,点G和点H所对应的平衡固相也有所不同,323 K条件下曲线GH对应的平衡固相为KBr + Na2SO4,373 K条件下曲线GH对应的平衡固相为NaBr + Na2SO4·3K2SO4(Gla);323 K条件下共饱和点G对应的平衡固相为KBr + Na2SO4+ Na2SO4·3K2SO4(Gla),共饱和点H对应的平衡固相为KBr + NaBr·2H2O + Na2SO4,373 K条件下共饱和点G对应的平衡固相为Na2SO4·3K2SO4(Gla)+ NaBr + KBr,共饱和点H对应的平衡固相为Na2SO4·3K2SO4(Gla) + NaBr + Na2SO4。此外,由于324 K时,溶液中析出无水溴化钠结晶,低于324 K则生成二水物,因此,323 K条件下溴化钠带2个结晶水,为NaBr·2H2O,373 K条件下溴化钠的结晶水消失。
373 K条件下四元体系Na+,K+//Cl-,SO42--H2O相平衡研究也已见文献报道[19]。通过对比373 K条件下两个相图可知,二者形状相似,单变量曲线、固相结晶区以及共饱点数量一致,其中钾芒硝有最大结晶区,在平衡液相中有最小溶解度,卤化物均对应有较小结晶区,在平衡液相中有较大的溶解度,说明在两个体系中,卤化物均对硫酸盐有较强的盐析作用。但是卤化物的结晶区面积大小有变化,KCl的结晶区面积大于KBr的结晶区面积,这是由于373 K条件下在该四元体系中,KBr的溶解度大于KCl的溶解度。
3 结 论
本文采用等温溶解平衡法对交互四元体系K+,Na+//Br−,SO42--H2O 373 K时的相平衡进行了研究。研究发现,373 K条件下交互四元体系Na+,K+//Br−,SO42--H2O有复盐Na2SO4·3K2SO4(Gla)生成,无固溶体生成,其等温溶解度图存在3个共饱和点,7条单变量曲线,5个相区,分别为:K2SO4、Na2SO4、NaBr、KBr和Na2SO4·3K2SO4(Gla),NaBr在该四元体系中结晶区最小,溶解度最大,Na2SO4·3K2SO4(Gla)在该四元体系中结晶区最大,溶解度最小。
References
[1] 林耀庭,陈绍兰. 四川地下卤水勘探开发前景展望 [J]. 盐湖研究,2008,16(1): 1-7. LIN Y T,CHEN S L. Exploration and development prospect of underground brine in Sichuan Basin [J]. Journal of Salt Lake Research,2008,16(1): 1-7.
[2] 唐宗薰,张逢星,郭志箴,等. 盐湖资源锂钾卤化物水体系研究(Ⅳ):三元体系NH4I-LiI-H2O,LiI-LiBr-H2O和NH4Br-LiBr-H2O 298.2 K 溶解度 [J]. 盐湖研究,1993,1(3): 9-13. TANG Z X,ZHANG F X,GUO Z Z,et al. A study on the systems of lithium and potassium halides for salt lake and bittern resources(Ⅳ): The ternary systems NH4I-LiI-H2O,LiI-LiBr-H2O and NH4Br-LiBr-H2O at 298.2 K [J]. Journal of Salt Lake Science,1993,1(3): 9-13.
[3] 翁延博,王静康,尹秋响,等. NaCl-NaBr-H2O三组分物系的固液平衡 [J]. 石油化工,2007,36(4): 358-361. WENG Y B,WANG J K,YIN Q X,et al. Solid-liquid equilibrium of NaCl-NaBr-H2O ternary system [J]. Petrochemical Technology (China),2007,36(4): 358-361.
[4] 翁延博,王彦飞,王静康,等. K+//Cr–,Br–-H2O三元体系298 K,313 K,333 K时的相平衡 [J]. 高校化学工程学报,2007,21(4): 695-699. WENG Y B,WANG Y F,WANG J K,et al. Phase diagram for the ternary system of K+//Cl–,Br–-H2O at 298 K,313 K and 333 K [J]. Journal of Chemical Engineering of Chinese Universities,2007,21(4): 695-699.
[5] 桑世华,殷辉安,倪师军,等. 三元体系KBr-K2B4O7-H2O在298 K的相平衡研究 [J].成都理工大学(自然科学版),2006,4: 414-416. SANG S H,YIN H A,NI S J,et al. A study on equilibrium solubilities and properties of solutions in the ternary system K2B4O7-KBr-H2O at 298 K [J]. Journal of Chengdu University of Technology (Science & Technology Edition),2006,4: 414-416.
[6] 赵向阳,桑世华,孙明亮. 三元体系KBr-K2B4O7-H2O在323 K的相平衡研究 [J]. 盐湖研究,2011,19(1): 35-39. ZHAO X Y,SANG S H,SUN M L. Phase equilibrium of the ternary system K2B4O7-KBr-H2O at 323 K [J]. Journal of Salt Lake Research,2011,19(1): 35-39.
[7] 桑世华,李婷,崔瑞芝.三元水盐体系KBr-K2B4O7-H2O 348 K相平衡研究 [J]. 盐湖研究,2013,21(2): 29-32. SANG S H,LI T,CUI R Z. A study on phase equilibria in the ternary salt-water system KBr-K2B4O7-H2O at 348 K [J]. Journal of Salt Lake Research,2013,21(2): 29-32.
[8] CUI R Z,SANG S H,HU Y X,et al. Phase equilibria in the ternary systems KBr-K2B4O7-H2O and KCl-K2B4O7-H2O at 373 K [J]. Acta Geologica Sinica 2012,87: 1668-1673.
[9] 王丹,桑世华,曾晓晓,等. KCl-KBr-K2SO4-H2O 四组分物系323 K相平衡 [J]. 石油化工,2011,40(3): 285-288. WANG D,SANG S H,ZENG X X,et al. Phase equilibrium of KCl-KBr-K2SO4-H2O quaternary system at 323 K [J]. Petrochemical Technology,2011,40(3): 285-288.
[10] ZHANG K J,SANG S H,LI T,et al. Liquid-solid equilibria in the quaternary system KCl-KBr-K2SO4-H2O at 348 K [J]. Journal of Chemical & Engineering Data,2013,58: 115-117.
[11] CUI R Z,SANG S H,HU Y X. Solid-liquid equilibria in the quaternary systems KCl-KBr-K2B4O7-H2O and KCl-KBr-K2SO4-H2O at 373 K [J]. Journal of Chemical & Engineering Data,2013,58: 477-481.
[12] SANG S H,CUI R Z,HU J W,et al. Measurements of the solid-liquid equilibria in the quaternary system NaCl-NaBr-Na2SO4-H2O at 323 K [J]. Journal of Solution Chemistry,2013,42: 1633-1640.
[13] HU Y X,SANG S H,CUI R Z,et al. Solid-liquid equilibria in the quaternary system KCl-KBr-K2B4O7-H2O at 323 K [J]. Journal of Chemical & Engineering Data,2014,59: 1886-1891.
[14] SANG S H,ZHANG H,ZHONG S Y,et al. Experimental study of the solubilities of salts in the systems Na2B4O7-NaBr-H2O and Na2B4O7-Na2SO4-NaBr-H2O at 323 K [J]. Fluid Phase Equilibria,2014,361: 171-174.
[15] 崔瑞芝,桑世华,李婷,等. 五元体系KCl-KBr-K2SO4-K2B4O7-H2O 323 K和348 K的相平衡 [J]. 化工学报,2013,64(3): 804-810. CUI R Z,SANG S H,LI T,et al. Phase equilibria in the quinary system KCl-KBr-K2SO4-K2B4O7-H2O at 323 K and 348 K [J]. CIESC Journal,2013,64(3): 804-810.
[16] 张淑彬,徐廷谅,徐恩孝,等. 川西某构造富钾卤水成因及分析规律研究 [J]. 中国井矿盐,2003,34(2): 23-26. ZHANG S B,XU T L,XU E X,et al. Study on the occurrence of potassium-rich brine in a geological stracture in west sichuan and the analytical patterns [J]. China Well and Rock Salt,2003,34(2): 23-26.
[17] 桑世华,孙明亮,李恒,等. Na+,K+//Br-,SO24--H2O四元体系323 K相平衡研究 [J]. 无机化学学报,2011,27(5): 845-849. SANG S H,SUN M L,LI H,et al. A Study on equilibria of the quaternary system Na+,K+//Br-,SO24--H2O at 323 K [J]. Chinese Journal of Inorganic Chemistry,2011,27(5): 845-849.
[18] 曾晓晓,桑世华,王丹,等. 交互四元体系Na+,K+//Br-,SO24--H2O 323 K相平衡的理论计算 [J]. 化学工程,2012,40(5): 32-35. ZENG X X,SANG S H,WANG D,et al. The theoretical calculations of phase equilibrium in the interactive quaternary system Na+,K+//Br-,SO42--H2O at 323 K [J]. Chemical Engineering,2012,40(5): 32-35.
[19] 牛自得,程芳琴. 水盐体系相图及其应用[M]. 天津: 天津大学出版社,2002. NIU Z D,CHENG F Q. The Phase Diagram of Salt-Water System and Its Application[M]. Tianjin: Tianjin University Press,2002.
[20] 崔瑞芝,桑世华,胡咏霞. 三元体系NaBr-Na2SO4-H2O 和NaBr-KBr-H2O 373 K相平衡 [J]. 高校化学工程学报,2014,28(5): 939-943. CUI R Z,SANG S H,HU Y X. Phase equilibria of two ternary systems NaBr-Na2SO4-H2O and NaBr-KBr-H2O at 373 K [J]. Journal of Chemical Engineering of Chinese Universities,2014,28(5): 939-943.
[21] 卢啟富,桑世华,张海,等. 三元体系KBr-K2SO4-H2O在373 K的相平衡研究 [J]. 无机盐工业,2015,47(2): 13-15. LU Q F,SANG S H,ZHANG H,et al. Solid-liquid equilibria in ternary systemKBr-K2SO4-H2O at 373 K [J]. Inorganic Chemicals Industry,2015,47(2): 13-15.
[22] 中科院青海盐湖所. 卤水和盐的分析方法[M].北京: 科技出版社,1984: 75-78. Institute of Qinghai Salt-Lake,Chinese Academy of Sciences. Analytical Methods of Brines and Salts[M].Beijing: Science and Technology Press,1984: 75-78.
Phase equilibria in quaternary system Na+,K+//Br−,SO42--H2O at 373 K
CUI Ruizhi1,2,SANG Shihua1,2
(1College of Materials and Chemistry & Chemical Engineering,Chengdu University of Technology,Chengdu 610059,Sichuan,China;2Mineral Resources Chemistry Key Laboratory of Sichuan Higher Education Institutions,Chengdu 610059,Sichuan,China)
Abstract:According to the composition of the brine resources in the west of Sichuan Basin,phase equilibria in the quaternary system Na+,K+//Br-,SO42--H2O at 373 K is measured by the isothermal solution saturation method,and the solubilities and densities of the solution are determined experimentally. Using the experimental results,the dry salt phase diagram,water diagram and the densities versus composition diagram are obtained. In the phase diagram of quaternary system Na+,K+//Br-,SO42--H2O at 373 K,the double salt Na2SO4·3K2SO4is found. There are three invariant points,seven uninvariant curves and five crystallization fields in the quaternary system. The five crystallization fields correspond to NaBr,Na2SO4,K2SO4,KBr and Na2SO4·3K2SO4(Gla),respectively. The crystallization field of NaBr has the smallest crystallization area,whereas the double salt Na2SO4·3K2SO4(Gla) has the biggest crystallization field in the quaternary system. It means that the double salt Na2SO4·3K2SO4(Gla) has the smaller solubility,and it can be easiest separated from solution. Compared with the two phase diagrams of quaternary system Na+,K+//Br-,SO42--H2O at 323 K and 373 K,the result shows that the numbers of invariant points,crystallization fields and unvariant curves are the same. The double salt all forms in the phase diagrams of quaternary systems at two different temperatures. But univariant curve GH of the corresponding balance solid phase is different and the crystal water of sodium bromide has disappeared at 373 K. In comparison with the quaternary system Na+,K+//Cl-,SO42--H2O and the quaternary system Na+,K+//Br-,SO42--H2O at 373 K,the twophase diagrams have very similar shapes,each of them having three invariant points,seven univariant curves and five crystallization fields. The crystallization field of the salt NaBr is apparently smaller than that of NaCl. It is also found that halide has the salting-out effect on sulfates. The water content and the density transformation rules are discussed simply. The water content is lower with the higher solution of bromine and the density is higher with the higher solution of bromine.
Key words:underground brine; phase equilibria; solubility; solution; potassium salt; bromine salt
DOI:10.11949/j.issn.0438-1157.20151183
中图分类号:O 642
文献标志码:A
文章编号:0438—1157(2016)04—1123—06
基金项目:国家自然科学基金项目(41373062);高等学校博士学科点专项科研基金项目(20125122110015);四川省高校科研创新团队项目(15TD0009)。
Corresponding author:Prof. SANG Shihua,sangsh@cdut.edu.cn

