A New Strategy to Probe and Compare the Binding Modes of Two Perfluorocarboxylic Acids with Human Serum Albumin Based on Spectroscopic and Molecular Docking Methods
HU Tao-ying, FANG Qing, JIN Ye, ZHOU Shan-shan,2, LIU Ying,2*
1. College of Life and Environmental Sciences, Minzu University of China, Beijing 100081, China
2. Beijing Engineering Research Center of Food Environment and Public Health, Minzu University of China, Beijing 100081, China
A New Strategy to Probe and Compare the Binding Modes of Two Perfluorocarboxylic Acids with Human Serum Albumin Based on Spectroscopic and Molecular Docking Methods
HU Tao-ying1, FANG Qing1, JIN Ye1, ZHOU Shan-shan1,2, LIU Ying1,2*
1. College of Life and Environmental Sciences, Minzu University of China, Beijing 100081, China
2. Beijing Engineering Research Center of Food Environment and Public Health, Minzu University of China, Beijing 100081, China
Perfluorocarboxylic acids (PFCAs) have been widespread used for over half century as surfactants in commercial and industrial products because of their hydrophilic and hydrophobic peculiarity. Perfluoroundecanoic acid (PFUnA) and perfluorotridecanoic acid (PFTriA) are two representatives of long-chain PFCAs, and they were detected more frequently in human body recent years, however, the two PFCAs were found to express endocrine disruption effects, developmental toxicity and teratogenicity. In this study, we established a new strategy to probe the binding modes of PFUnA (PFTriA) with the most abundant protein human serum albumin (HSA) based on spectroscopic and molecular docking methods. Results showed that both PFUnA and PFTriA can quench the intrinsic fluorescence of HSA with one binding site by means of dynamic and static quenching procedure with a strong affinity and the order is PFUnA>PFTriA. On the basis of thermodynamic results, we knew that the main driving force of the interaction between PFUnA and HSA was electrostatic force (ΔH=-26.32 kJ·mol-1, ΔS=21.76 J·mol-1·K-1), while van der Waals interaction and halogen-bond played major roles in complexation process of PFTriA-HSA (ΔH=-39.69 kJ·mol-1, ΔS=-25.66 J·mol-1·K-1). The binding distance (r<8 nm) indicated that the non-radioactive energy transfer came into being from HSA to PFUnA (PFTriA). The binding process of PFUnA (PFTriA) with HSA caused conformational and some micro-environmental changes of HSA, but also led to a loss of helical stability through three-dimensional fluorescence and circular dichroism spectra (CD). Furthermore, site markers competitive experiments and molecular docking revealed that PFUnA and PFTriA had a high affinity into hydrophobic pocket of subdomain IIA in HSA through polar force, hydrophobic interaction and halogen-bond and so on, and the fluorophore Trp residues was located in the binding position which proved further the quenching of PFUnA and PFTriA on HSA fluorescence. The accurate and full basic data in the work are beneficial to clarify the binding mechanism of long-chain perfluorocarboxylic acids with serum protein in vivo, and provide essential theoretical clues for their toxicity assessment and toxicologic research.
Perfluoroundecanoic acid; Perfluorotridecanoic acid; Human serum albumin; Spectroscopic method; Molecular docking
Introduction
Human serum albumin (HSA), the most abundant protein in the circulatory system of a wide variety of organisms, increases the apparent solubility of hydrophobic ligands in plasma and modulates their delivery to cell in vivo and in vitro[1]. Perfluorocarboxylic acids (PFCAs), which are composed of a hydrophobic perfluorinated alkyl chain and a hydrophilic carboxylic functional group, have been widespread used for over half century as surfactants in commercial and industrial products[2]. Perfluoroundecanoic acid (PFUnA) and perfluorotridecanoic acid (PFTriA) are common long-chain members of PFCAs. The high energy carbon-fluorine (C-F) bond renders PFUnA (PFTriA) resistant to hydrolysis, photolysis, microbial degradation and metabolism, increasing their biomagnifications and bioaccumulations through the food-chain. Interestingly, PFUnA (PFTriA) preferentially accumulates in serum, liver and kidneys instead of lipids and fatty tissue because of their hydrophilic nature[2]. More importantly, they are also detected in emerging personal care products (termed PCPs in 1999), which are one large class of the active ingredients receiving comparatively little attention but used in large amounts throughout the world[3]. In recent years, PFUnA and PFTriA were common detected in human maternal plasma, breast milk and liver, and unfortunately they were found to exhibit endocrine disruption effects, developmental toxicity and teratogenicity[4]. Thus, the binding of PFUnA and PFTriA with HSA may impede the transport of endogenous substances and affect conformation, activity and physiological function of HSA. So far, however, there is no report on the interaction of PFTriA-HSA and only one report on the binding of PFUnA with serum albumin, and the binding site, driving force and structural changes of albumin caused by PFUnA have not previously been identified[5]. The three aspects hinder our proper and comprehensive understanding of the interaction between long-chain PFCAs and protein.
Therefore, major goal in this paper is dedicated to further elucidating the underlying binding mechanism, thermodynamic characterization and structural changes of HSA upon binding PFUnA (PFTriA) by spectroscopic and molecular docking methods. The study can clarify the binding mechanism of PFUnA (PFTriA) with HSA at a molecular level, and contribute to understand its effect on protein structure and function in vivo.
1 Materials and methods
HSA (Sigma, USA) working solution was prepared with the concentration of 2.0×10-5mol·L-1. PFUnA and PFTriA (Shanghai Aijie Biological Technology Co., Ltd., China) was dissolved and diluted to 5.0×10-4mol·L-1with ultrapure water as stock solutions. The stock solutions of phenylbutazone and ibuprofen were prepared to 5.0×10-4mol·L-1. All above solutions were kept in the dark at 0~4 ℃. Phosphate buffer solution (mixture of 75 mmol·L-1NaH2PO4and 75 mmol·L-1Na2HPO4(19∶81,V/V), pH 7.40).
Fluorescence spectroscopy, elimination of the inner filter effects, CD measurements and molecular modeling are seen literature [6].
2 Results and discussions
2.1 The mechanism of fluorescence quenching
The fluorescence quenching measurement of HSA in the absence and presence of PFUnA (PFTriA) at 298 K were performed. Fig.1 shows the effect of PFUnA and PFTriA on the fluorescence emission spectra of HSA at 280 nm excitation wavelength. The fluorescence intensity of HSA remarkably decreased regularly with increasing PFUnA (PFTriA) concentrations. The maximum emission wavelength upon the binding of PFUnA and PFTriA shifted from 351 to 345 nm

Fig.1 Effects of PFUnA (PFTriA) on HSA fluorescence
cHSA=2.0×10-6mol·L-1;cPFUnA(×10-6mol·L-1)(1~7): 0, 1.6, 3.2, 4.8, 6.4, 8.0, 9.6;cPFTriA(×10-6mol·L-1)(1~7): 0, 1.2, 2.4, 3.6, 4.8, 6.0, 7.2;T=298 K
and 348 nm, respectively, which indicated the microenvironment changes for residues of HSA and the formation of PFUnA (PFTriA)-HSA complex.
Fluorescence quenching is usually classified as static quenching and dynamic quenching. To elucidate the type of fluorescence quenching mode, the fluorescence quenching data were analyzed with the Stern Volmer equation[7]
(1)
whereF,F0,KSV,kq,τ0and [Q] are seen literature [7]. As shown in Fig.2, the slope of Stern Volmer curves (KSV) increased accordingly with the increasing temperature, which suggested that the predominant quenching process was dynamic quenching mechanism. However, the static quenching effect of complex formation could not be completely precluded in the present study. On the one hand, spectroscopic studies above have confirmed the formation of PFUnA (PFTriA)-HSA complex. On the other hand, the maximum scatter collision quenching constant (kq) of various quenchers with the biopolymer is 2.0×1010L·mol-1·s-1[8]while that of HSA quenching procedure initiated by PFUnA and PFTriA, 8.17×1012L·mol-1·s-1and 7.44×1012L·mol-1·s-1at 298 K, respectively, was far greater than this value. Therefore, in conclusion, the fluorescence quenching mechanism of HSA by PFUnA and PFTriA was a combination of dynamic quenching with ground complex formation.

Fig.2 Stern-Volmer plots for the quenching of HSA by PFUnA and PFTriA at different temperatures
2.2 Binding constants and binding sites
For the equilibrium between free and bound molecules, when small molecules bind independently to a set of equivalent sites on a macromolecules, the relationship between the binding constant (Ka) and the number of binding sites (n) could be described by the equation[7]
(2)
Kaandnwill be derived from a plot of log[(F0-F)/F] versus log[Q]. Table 1 shows the calculatedKaandn. The number of binding sites,n, was approximately 1, indicating the existence of just a single binding site in HSA for PFUnA (PFTriA) during the interaction. For PFUnA, the binding constant increased with the rising temperature, which indicated that the capacity of PFUnA binding to HSA was enhanced at high temperature. However, opposite to PFUnA, the binding constant of PFTriA decreased with rising temperature, which implied the existence of PFTriA-HSA complex and its stability became less stable with rising temperature. Furthermore, the affinity order is as follows: PFUnA>PFTriA.
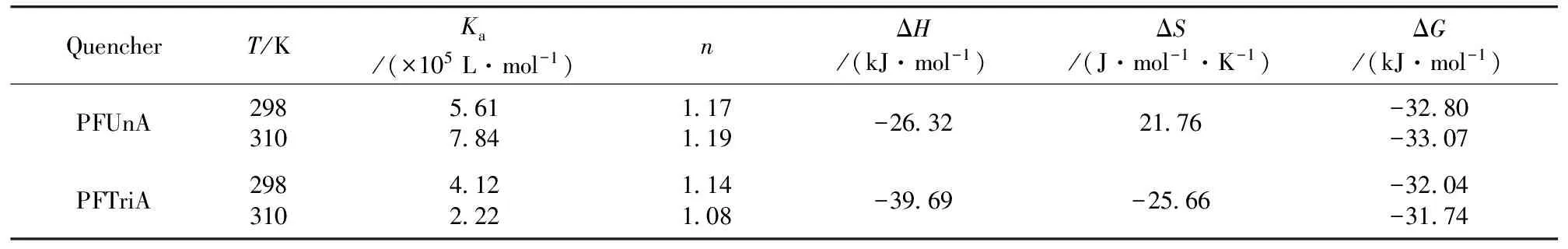
Table 1 Binding constants and relative thermodynamic parameters of PFUnA (PFTriA) HSA
2.3 Thermodynamic parameters and binding distance
According to van’t Hoff equation and thermodynamic equation[6], we obtained enthalpy change (ΔH), entropy change (ΔS) and free energy change (ΔG) at 298 and 310 K (Table 1). The negative sign for ΔGof PFUnA (PFTriA)-HSA meant that the interaction process was spontaneous. The negative ΔHand positive ΔSvalues for the association interaction between PFUnA and HSA implied that electrostatic force was the major driving force of the interaction, while halogen-bond and van der Waals force played major roles in the acting forces of PFTriA with HSA in terms of the both negative ΔHand ΔS.
The distancerbetween a protein residue (donor) and a bound ligand molecule (acceptor) can be calculated according to Föster non-radioactive energy transfer theory[9]. There was enough overlapping between the absorption spectrum of PFUnA (PFTriA) with the fluorescence emission spectrum of HSA, and their overlap integralJwere 1.04×10-14and 4.91×10-15cm3·L·mol-1, respectively. The binding distancerbetween PFUnA (PFTriA) and Trp residues were 3.66 and 3.32 nm (<8 nm), respectively, indicating that energy transfer from HSA to PFUnA or PFTriA occurred with high possibility.
2.4 Conformation investigation
Three-dimensional fluorescence spectrum can comprehensively exhibit the fluorescence information and conformational changes of the protein. The three-dimensional fluorescence spectra of HSA, PFUnA-HSA and PFTriA-HSA systems are shown in Fig.3. Peak a (λex=λem) is the Rayleigh scattering peak, peak b (λem=2λex) is the second-ordered scattering peak. Peak 1 mainly reveals the spectral feature of the polarity of Trp and Tyr residues microenvironment[7]. After the addition of PFUnA (PFTriA), the fluorescence intensity of peak 1 decreased markedly and both had a same obvious blue shift (from 350 to 347 nm). It indicated that the polarity of Trp and Tyr residues microenvironment decreased and almost all the hydrophobic amino acid residues of HSA were buried in hydrophobic pocket. Peak 2 chiefly exhibits the fluorescence spectra behavior of polypeptide chain backbone structures[7]. The fluorescence intensity of peak 2 reduced dramatically with a 3 nm blue shift after the addition of PFUnA (PFTriA), meaning that the conformation of the peptide backbone was altered. Thus we can conclude that the binding of PFUnA (PFTriA) to HSA induced some micro-environmental and conformational changes on HSA.
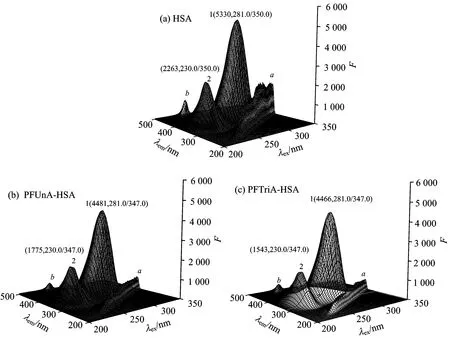
Fig.3 Three-dimensional fluorescence spectra of HSA, PFUnA HSA and PFTriA HSA systems
2.5 Changes of HSA’s secondary structure
To monitor the global structural changes of HSA upon the binding of PFUnA (PFTriA), CD measurements were carried out in the presence of different concentrations of PFUnA (PFTriA) (Fig.4). As shown in Fig.4, the CD spectra of HSA in the absence and presence of PFUnA (PFTriA) were similar in shape, indicating that the structure of HSA was also predominantlyα-helix. Theα-helix contents in the secondary structure of HSA were calculated from mean residue ellipticity (MRE) values at 209 nm using the following two equations[6]
(3)
(4)
where the parameters ofCp,n(n=585, for HSA),l, MRE209, 4 000, and 33 000 are seen literature [6]. From the above equations, the quantitative analysis results of the secondary structure in HSA were obtained (Table 2). It was noted that the free HSA had anα-helix content of 48.4%. When the molar ratio of HSA to PFUnA was 1∶15 and 1∶20, theα-helix content was reduced to 42.2% and 38.7%, respectively. When the molar ratio of HSA to PFTriA was 1∶20 and 1∶30, theα-helix content was reduced to 40.7% and 38.2%, respectively. It reflected the impaired secondary structure of HSA by PFUnA (PFTriA). These results suggested that the higher concentration of PFUnA (PFTriA) induced larger extent of structural damage of HSA. The more pronounced decrease inα-helix content with the greater binding ability of PFUnA than that of PFTriA indicated that the binding affinities of PFUnA and PFTriA were closely associated with the structural alteration of HSA.
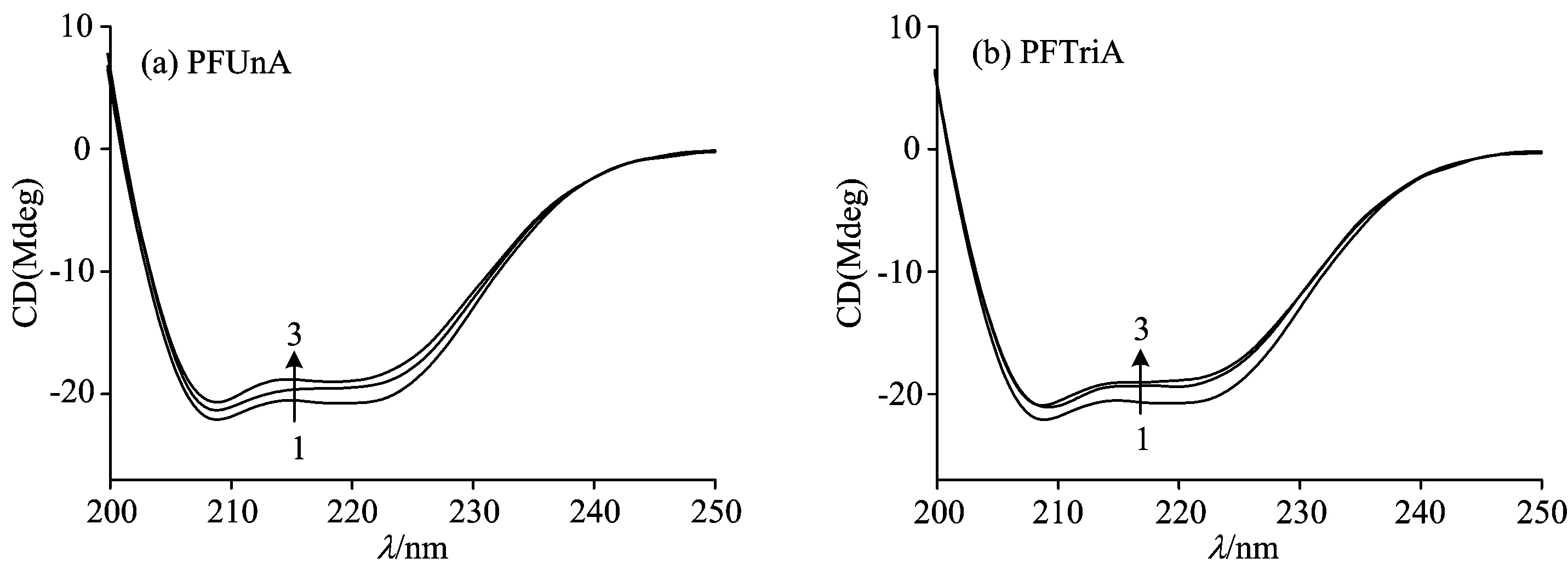
Fig.4 CD spectra of HSA in the absence and presence of PFUnA (PFTriA) at room temperature
Table 2 Secondary structure of HSA in the absence and presence of PFUnA (PFTriA) determined by CONTIN

Molarratiosα⁃helix/%β⁃sheet/%β⁃turn/%Random/%FreeHSA48 48 216 826 7HSA∶PFUnA=1∶1542 212 713 234 0HSA∶PFUnA=1∶2038 711 514 835 0HSA∶PFTriA=1∶2040 711 514 233 6HSA∶PFTriA=1∶3038 213 114 334 4
2.6 Site marker competitive experiments
To identify the binding sites of PFUnA (PFTriA) on HSA, site marker competitive experiments were performed using phenylbutazone (that explicitly binds to site Ⅰ in the subdomain ⅡA) and ibuprofen (that explicitly binds to site Ⅱ located in the subdomain ⅢA) as our site probes[10]. The changes induced by the site probes are presented in Fig.5. The fluorescence of the complex was remarkably affected in presence of phenylbutazone, but remained invariant with ibuprofen. The observations demonstrated that phenylbutazone displaced PFUnA (PFTriA) from the binding site, while ibuprofen had a little effect on the binding of PFUnA (PFTriA) to HSA. Hence, it can be concluded that PFUnA (PFTriA) was likely to be bound to site Ⅰ in the subdomain ⅡA of HSA.

Fig.5 Effects of site marker probes on the fluorescence of PFUnA-HSA and PFTriA-HSA systems
2.7 Molecular docking study
To acquire the more information about the PFUnA (PFTriA) interaction with HSA, we had run a docking program to simulate the binding mode between PFUnA (PFTriA) and HSA. The crystal structure of HSA was taken from the Protein Data Bank (entry codes 2BXN). The best energy ranked results are shown in Fig.6. It was noted that both PFUnA and PFTriA were bound in subdomain ⅡA, and the inside wall of the pocket of subdomain ⅡA was formed by hydrophobic side chains, whereas the entrance to the pocket was surrounded by positively charged residues (PFUnA with Arg 218, Arg 257, Lys199, His 242; PFTriA with Arg 218, Arg 257, Lys 195, Lys 199, His 242) [Fig.6(c)]. Thus we concluded that PFUnA (PFTriA) were able to fit well within the hydrophobic cavity of subdomain IIA. The docking results showed the existence of polar force, hydrophobic interaction and halogen-bond between PFUnA (PFTriA) and HSA (Table 3). For example, C 2 of PFUnA and C 4 of PFTriA interacted with Trp 214 through hydrophobic interaction, which also elucidated that PFUnA and PFTriA can quench the intrinsic fluorescence of HSA.

Fig.6 (a) The binding site of PFUnA (PFTriA) on HSA. HSA is shown in cartoon and PFUnA (PFTriA) is represented using spheres; (b) Enlarged binding mode between PFUnA (PFTriA) and HSA. HSA is shown in cartoon, the interacting side chains of HSA are displayed in surface mode and PFUnA (PFTriA) is represented using balls and sticks; (c) Molecular modeling of the interaction between PFUnA (PFTriA) and HSA. The atoms of PFUnA (PFTriA) are blue
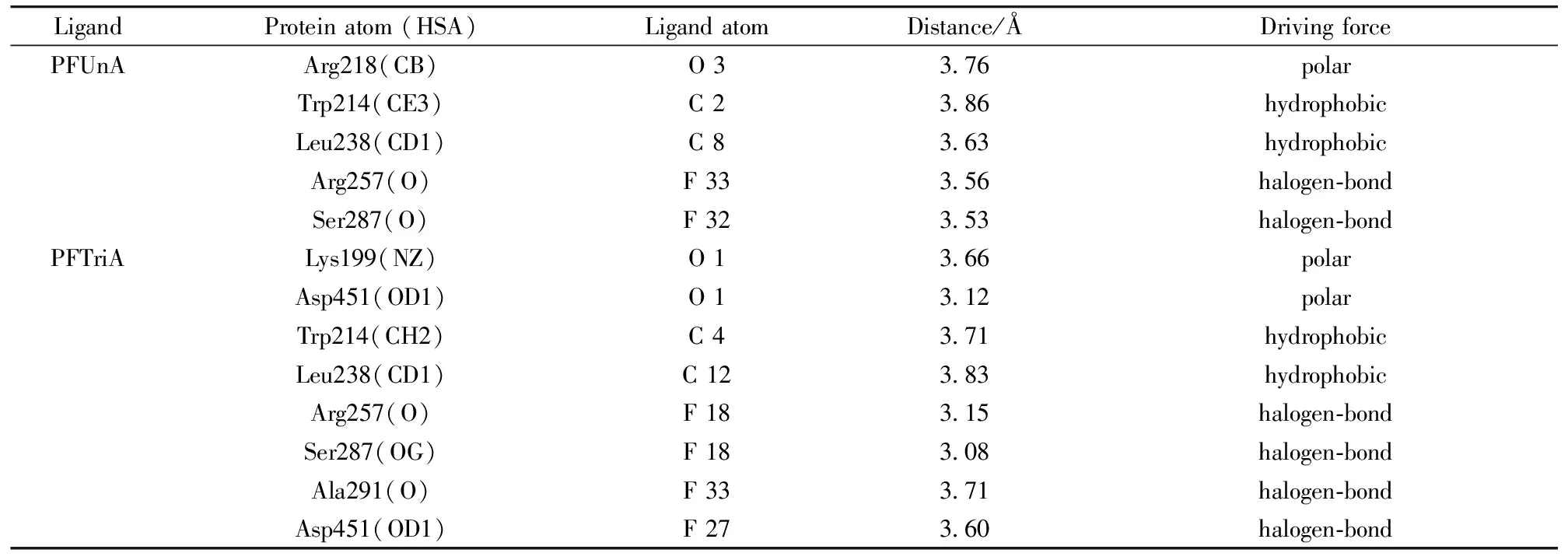
Table 3 The distances and driving forces between the PFUnA (PFTriA) atoms
3 Conclusions
In this paper, we probed and compared the interaction of PFUnA (PFTriA) with HSA at a molecular level by using spectroscopic and molecular docking methods. The experimental results indicated that PFUnA (PFTriA) can quench the HSA fluorescence by a combined process of dynamic quenching and ground-complex formation, and the complex formation had a high affinity of 105L·mol-1. The main driving forces for the interactions of PFUnA-HSA and PFTriA-HSA were electrostatic force (ΔH<0, ΔS>0), van der Waals interaction and halogen-bond (ΔH<0, ΔS<0), respectively. The distances (r=3.66 and 3.32 nm) revealed the occurrence of the energy transfer from HSA to PFUnA and PFTriA. Three-dimensional fluorescence and CD spectra showed that the binding of PFUnA and PFTriA can led to decrease of the polarity of Trp and Tyr residues microenvironment and conformational changes of HSA. By performing site marker competitive experiments, the specific binding of PFUnA and PFTriA in the vicinity of site Ⅰ of HSA was clarified. The molecular modeling further confirmed the specific binding site of PFUnA (PFTriA) on HSA. This paper can provide insights with the binding mode of PFUnA (PFTriA)-HSA and salient biophysical and biochemical clues on elucidating the transport, distribution of PFUnA (PFTriA) in vivo.
[1] Yang B J, Hao F, Li J R, et al. Food Chem. Toxicol., 2014, 65: 227.
[2] Bischel H N, MacManus-Spencer L A, Luthy R G. Environ. Sci. Technol., 2010, 44: 5263.
[3] Christian G D, Thomas A T. Environ. Health Persp., 1999, 107: 907.
[4] Jo A, Ji K, Choi K. Chemosphere, 2014, 108: 360.
[5] MacManus-Spencer L A, Tse M L, Hebert P C, et al. Anal. Chem., 2010, 82: 974.
[6] Hu T Y, Liu Y. J. Pharm. Biomed. Anal., 2015, 107: 325.
[7] Sun H W, Wu Y J, Xia X H, et al. J. Lumin., 2013, 134: 580.
[8] Lakowicz J R, Weber G. Biochemistry, 1973, 12: 4161.
[9] Förster T. Modern Quantum Chemistry. Academic Press: New York, 1965.
[10] Sudlow G, Birkett D J, Wade D N. Mol. Pharmacol., 1975, 11: 824.
O657.3
A
光谱法和分子对接法研究和比较两种全氟羧酸与人血清白蛋白的结合模式
胡涛英1, 方 庆1, 金 叶1, 周珊珊1,2, 刘 颖1,2*
1. 中央民族大学生命与环境科学学院, 北京 100081
2. 中央民族大学北京市食品环境与健康工程技术研究中心, 北京 100081
全氟羧酸(PFCAs)由于具有既亲水又疏水的表面活性剂特性, 被广泛应用于工业和生活产品中。 全氟十一酸(PFUnA)和全氟十三酸(PFTriA)是长链PFCAs类的典型代表, 但近年来它们越来越频繁的在人体中检测到, 并且发现表现出内分泌干扰效应、 发育毒性和致畸性。 本文以光谱学和分子对接为基础, 探索PFUnA和PFTriA与人体最丰富的蛋白人血清白蛋白(HSA)的结合模式。 结果表明, PFUnA和PFTriA均通过动静态猝灭过程猝灭HSA的内源荧光, 与HSA只有一个强亲和位点, 且PFUnA与HSA的结合比PFTriA更紧密。 根据热力学计算结果, 可知PFUnA与HSA结合的焓变、 熵变分别为-26.32 kJ·mol-1和21.76 J·mol-1·K-1, 其结合作用主要依靠静电引力, 而PFTriA主要通过范德华力和卤键与HSA结合, 是放热熵减过程, 其焓变和熵变分别为-39.69 kJ·mol-1和-25.66 J·mol-1·K-1。 计算得到的结合距离(r<8 nm)显示从HSA到PFUnA和PFTriA发生了非辐射能量转移。 三维荧光光谱和圆二色谱表明, PFUnA和PFTriA与HSA的结合不仅可以改变HSA的构象和微环境, 还可以引起α-螺旋稳定性降低。 取代实验和分子对接进一步显示PFUnA 和PFTriA通过极性键、 疏水作用力和卤键等与HSA的亚域ⅡA疏水腔有高亲和性, 且荧光团Trp残基处于结合位置中, 进一步证明PFUnA和PFTriA可以猝灭HSA的荧光。 本文研究结果为阐明长链PFCAs在机体内与血清蛋白的结合机理提供了完整可靠的数据, 并为长链PFCAs的毒性评价和毒理学研究提供了理论依据。
全氟十一酸; 全氟十三酸; 人血清白蛋白; 光谱法; 分子对接
2015-06-03,
2015-11-20)
2015-06-03; accepted: 2015-11-20
The National Natural Science Foundation of China (21177163), 111 Project B08044, First-class University First Class Academic Program of Minzu University of China (YLDX01013), Coordinate Development of First-Class and First-Class University Discipline Construction Funds(10301-0150200604), The Academic Team Construction Project of Minzu University of China (2015MDTD25C&13C), First-class Universities and First-class Discipline Construction Transitional Funds Under Special Funding (10301-01404031, 2015), 2015MDTD08C
10.3964/j.issn.1000-0593(2016)08-2698-07
Biography: HU Tao-ying, (1989—), Master of College of Life and Environmental Sciences, Minzu University of China e-mail: hty0945020@163.com *Corresponding author e-mail: liuying4300@163.com
*通讯联系人

