Synthesis of hierarchical dendritic micro-nano structure ZnFe2O4 and photocatalytic activities for water splitting☆
Zhongping Yao,Yajun Zhang,Yaqiong He,Qixing Xia,Zhaohua Jiang
School of Chemical Engineering and Technology,State Key Laboratory of Urban Water Resource and Environment,Harbin Institute of Technology,Harbin 150001,China
1.Introduction
Hydrogen is of high energy capacity,environmentally-friendly and renewable[1].Since Fujishima found electrochemical photolysis of water onn-type TiO2semiconductor electrode,the photocatalysis of water splitting for hydrogen generation has provided an alternative way to solve the current energy and environmental crisis[2].Consequently,the preparation,characterization and modification of the suitable photocatalysts forwatersplitting have long been the central contents during the research processes of semiconductor photoelectric chemistry and photocatalysis[3].
There is much interest about spinel zinc ferrite(ZnFe2O4)semiconductordue to the low resistivity,and fascinating electricaland magnetic properties among the ferrite[4-8].Zinc ferrite has been widely investigated in the fields of ferro fluid,medical imaging,drug targeting,magnetic data storage,lithium ion batteries,gas sensor and catalysis[9-15].Up to now various ZnFe2O4particles and films have been researched by many researchers[16-19].As a semiconductor material,spinel zinc ferrite is of visible-light response characteristics(the bandgap:1.9 eV),and has the outstanding photochemical stability,strong magnetism and low cost[1,20,21].Therefore,much research on ZnFe2O4based composites have been conducted in the photocatalytic process[22].However,pure ZnFe2O4as photocatalystforwatersplitting is rarely reported to our knowledge.
Besides,the morphology and size of the materials have great influences on the properties of the photocatalysts[23].Dendrite is a hierarchical structure,which consists of main stems in micrometer size and branches in nanometer size,like a pine-tree structure[24].Dendritic materials have attracted much attention for potential applications in catalysis[25,26].In this work,a hierarchical dendritic micro-nano structure ZnFe2O4was prepared by electrodeposition and thermal oxidation for the first time.The composition and structure of the catalysts were characterized and the photocatalytic activity for water splitting was investigated in the presence of Na2S/Na2SO3as sacri ficial electron donors under Xe lamp light irradiation.
2.Experimental Section
2.1.Preparation of the samples
The hierarchical dendritic micro-nano structure ZnFe2O4was prepared by electrodeposition and thermal oxidation.Firstly,hierarchical dendritic micro-nano structure ZnxFe1-xalloys(x=0.1,0.35,0.7)was prepared by reduction of ZnSO4and FeSO4in aqueous solution at room temperature.xis the concentration of Zn2+in the solution,while the total concentration of Zn2+and Fe2+is 1 mol·L-1.
The electrodeposition was conducted in a cylinder electrolyzer which was divided into two parts by a concentric cylinder anion perm-selective membrane.The detailed description of the electrodeposition device can be seen from the previous work in our lab[24].40 ml 1 mol·L-1ZnSO4and FeSO4mix aqueous solution and 2 mlethanolwas in the inner cylinder electrolyser,the outside cylinder electrolyser was filled with 250 ml 0.1 mol·L-1H2SO4solution.The current density was 30.0 A·cm-2and the reaction time was 20 s to prepare alloys.The alloys was then cleaned with ethanol and vacuum dried at 60°C.In the end,the dried alloys were oxidized at 350°C in air tube furnace for1 h.The sample prepared withx=0.1 is named after S-1,the sample prepared withx=0.35 is named after S-2,and the sample prepared withx=0.7 is named after S-3.
2.2.Characterization of morphology and structure
The composition and microstructure of the samples were characterized by X-Ray Diffraction(D/max-rB,RICOH,Japan),Raman microspectroscopy(Renishaw inVia,England),and Field-emission Scanning Electron Microscopy(FESEM Quanta 200F,America).BET specific surface areas and pore volumes were calculated from nitrogen adsorption-desorption isotherms determined at 77 K using a 3 H-2000PS1 surface analyzer(the sample was outgassed under vacuum at 200°C).
2.3.Optical properties of the samples
UV-Vis diffuse reflectance spectra were acquired by a spectrophotometer(UV-2450,SHIMAPZU)and were converted from reflection to absorbance by the standard Kubelka-Munk method.BaSO4was used as the reflectance standard.
2.4.Evaluation of the photocatalytic property
The photocatalytic reaction was performed in a closed gas-circulation system with a side window.The photocatalyst powder was dispersed in an aqueous solution(200 ml)containing Na2SO3(0.02 mol·L-1)and Na2S(0.1 mol·L-1)as electron donors.The reaction was carried out by irradiating the mixture with light from a Xe lamp(300 W).The amount of produced H2was measured by gas chromatography(SP-2100)with a thermal conductivity detector(TCD)and Ar as the carrier gas.
3.Results and Discussion
3.1.Morphology and structure of the samples
Fig.1 shows the field-emission scanning electron microscopy(FESEM)of the samples.It can be seen that the samples are all of hierarchical dendritic micro-nano structure.The dendrite consists of a main stemin micrometersize and branches in nanometersize.S-1 sample mainly consists of stems and short branches,which look like dead standing trees.S-2 and S-3 samples have different shapes of branches from S-1 sample.The former are of lush branches with leaves,which is like the vibrant trees.However,as the proportion of Zn in the alloys is increased,the size of the branches becomes larger,as seen in S-3 and partial branches further transfer into flake-like structure.
The different morphology differences of the samples are surely related to the composition,therefore the Raman spectrum was measured with the result shown in Fig.2.Clearly,S-1 sample is mainly composed of α-Fe2O3,but ZnFe2O4rarely exists.As for the samples S-2 and S-3,the peak shapes are similar,which illustrates that the samples both have ZnFe2O4.However,zinc oxide was not detected by Raman spectra although the high content of zinc ions was used in electrochemical reduction process in S-3.

Fig.2.Raman shift of the samples(λ=632.8 nm).

Fig.1.FESEM image of the samples(a)S-1;(b)S-2 and(c)S-3.

Fig.3.XRD patterns of three samples.
In order to investigate the existing state of zinc ions in the sample,XRD analysis was conducted.Fig.3 shows the X-ray diffraction patterns of the three samples.The phase of S-1 sample is α-Fe2O3and ZnFe2O4,which is consistent with Raman results.The phase of S-2 sample is nearly pure ZnFe2O4.And S-3 consists of ZnFe2O4and ZnO.Therefore,three kinds of dendrites with different compositions and morphologies were successfully prepared by the electrochemical reduction and thermal oxidation treatment.According to the XRD analysis,the formation reactions of these substances are proposed by Eqs.(1)-(3).Due to the different molar ratios of Zn/Fe in the alloys obtained during the electrochemical reduction,in the thermal oxidation process ZnFe2O4and ZnO were generated whenx=0.7(Zn at excess);ZnFe2O4and Fe2O3were formed whenx=0.1(Fe at excess)and only whenx=0.35,the pure ZnFe2O4was formed.The lattice parameters of ZnFe2O4in three samples were calculated by using Jade software with the results shown in Table 1.Clearly,the cell volume of ZnFe2O4is increased a little under the condition of Fe at excess whereas the cell volume of ZnFe2O4is decreased a little under the condition of Zn at excess.This change of the lattice parameter may be associated with the radiuses of iron ions with different oxidation states(r(Fe2+)=0.078 nm;r(Fe3+)=0.055 nm).With the decrease of the ratio of Zn2+/Fe2+in the electrolyte,the oxidation degree of Fe in the alloys many be comparatively weaken and part of Fe2+is formed during the thermal oxidation process,which leads to the increase of the lattice parameters.


Table 1The lattice parameters of ZnFe2O4 in three samples
Different compositions of the samples influence the morphologies.Table 2 is the volume and the density of the related oxides and alloys.Using Jade software,the relative proportion of α-Fe2O3,ZnFe2O4and ZnO in three samples are also calculated,with the results shown as follows:for S-1,α-Fe2O3is 74.2 wt%and ZnFe2O4is 25.8 wt%sample;for S-3,ZnFe2O4is 51.1 wt%and ZnO is 48.9 wt%.S-2 is 100%ZnFe2O4.Therefore,based on the above data,it can be noted that all the volumes of the samples increase after the oxidation of the alloys,the increasing degree of the volume is different for different substances.During the oxidation process,S-2 expands uniformly due to the pure composition and therefore keeps the dendrite structure better than the other two samples.The flake structure in S-3 is corresponding to ZnO due to the high proportion in the sample[27].
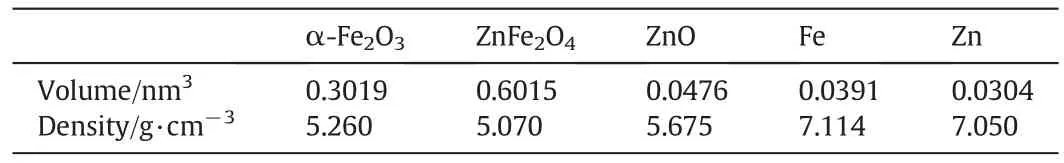
Table 2The volume and the density of the related oxides and alloys
N2adsorption-desorption isotherms for S-2 and the corresponding Barrett-Joyner-Halenda(BJH)pore-size distribution plots are shown in Fig.4.The hysteresis loop of adsorption-desorption isotherms belongs to Type H4,which means that the pores are formed due to the layer structure accumulation instead of the particle accumulation.Therefore,this also proves the dendritic hierarchical structure of the samples.The type of hysteresis loop shows that the isotherm curve is type IV and the absorbed volume increases sharply at the high pressure period,which means that the pores are large,with the size between 20 nmand 40 nm(shown in the insetin Fig.4).Since the only condition of the ratio of Zn2+/Fe2+was changed and all the other technique conditions were the same in the experimental process,the specific surface areas for three samples are similar,with the value of around 22 m2·g-1.

Fig.4.Nitrogen adsorption/desorption isotherms,and the inset figure is pore diameter distribution of S-2 sample.
3.2.Optical properties of the samples
Fig.5 shows UV-Vis absorption spectra of different samples.All the samples have a steep edge within the measure wavelength range,which indicates that their absorption relevant to the band gap is due to the intrinsic transition of these semiconductors and not from the transition from impurity levels[28].The absorption edge of S-1 sample is at650 nm in the visible lightregion,which is mainly corresponding to α-Fe2O3(its proportion is 74.2%in S-1).The large amount of α-Fe2O3is the main reason of the red color of the sample.The deep yellow S-2 sample has two absorption edges(575 nm and 518 nm),which may be due to the different oxidation degree or the un-uniformity of the electrodeposited Fe-Zn alloys.S-3 sample is light yellow and has three absorption edges(570 nm,510 nm and 410 nm).The first two edges are very close to that of S-2,and only a little blue shift.Besides,the absorption edge at 410 nm should belong to that of ZnO.For all three samples,the absorption edges gradually blue shift with the increase of the molar ratio of Zn2+.This further shows that there may be strong bonding effects between ZnFe2O4with ZnO orα-Fe2O3,which reversely influences the optical properties of the samples.

Fig.5.UV-Vis absorption spectra of the samples.
3.3.Photocatalytic property of the samples
Photocatalytic H2evolution test on the above-mentioned oxides is performed in 0.1 mol/L Na2S and 0.02 mol·L-1Na2SO3solution under Xe lamp irradiation.Fig.6 is the H2evolution versus time curve.The spectrum of Xe lamp is the inset figure of Fig.6.The light intensity is nearly focused on the whole range of visible light.The amount of H2increases nearly linearly under the present experimental conditions.The average H2evolution rate of S-1 sample,S-2 sample and S-3 sample is nearly 0.89 μmol·h-1,1.41 μmol·h-1and 1.29 μmol·h-1,respectively.Therefore,S-2,namely pure ZnFe2O4sample,presents the best photo-catalytic properties.
Photo-catalytic activities are related to the structure and optical properties of the samples.Firstly,the band structure of ZnFe2O4in S-2 sample(the CB is-0.39 eV)is suitable for the H2evolution from water splitting[29,30].The more the amount of ZnFe2O4,the better the photocatalytic property is.Secondly,S-2 sample with lush branches and leaves has larger specific surface area,which can provide more active sites for the photocatalytic reaction.Furthermore,the pure ZnFe2O4phase has no impurity energy levels,therefore reducing the recombination of the photo-excited holes and electrons.

Fig.6.H2 evolution of the samples.The inset figure is the spectrum of Xe lamp in experiment.
The photocatalytic activity of S-1 sample is better than that of S-3 sample,which may be related to the following reasons:(1)in general,the prepared various ZnO has impurity energy levels except for the synthesis by CVD technique[31,32],which are the recombination centers of the excited electrons and holes to worsen photocatalytic activity.Moreover,the wide bandgap of ZnO does little contribution on the hydrogen production based on the Xe lamp irradiation.(2)The color of S-1 sample is red,which is helpful for the absorption of more light for the catalytic process of ZnFe2O4.
4.Conclusions
(1)Hierarchical dendritic micro-nano structure ZnFe2O4was synthesized by electrodeposition and thermal oxidation.When the molar ratio of Zn2+/Fe2+is 0.35 during the electrochemical reduction process,the pure phase ZnFe2O4with lush branches was obtained.The molar ratios of Zn2+/Fe2+influences the crystal composition and microstructure and morphologies.
(2)The dendritic micro-nano structure samples exhibit photocatalytic activity for hydrogen production in the aqueous system with Na2SO3and Na2S as sacri ficial reagents under visible-light irradiation.The pure ZnFe2O4sample shows the best photocatalytic activity with H2evolution rate at 1.41 μmol·h-1.
[1]X.B.Chen,S.H.Shen,L.J.Guo,S.S.Mao,Semiconductor-based photocatalytic hydrogen generation,Chem.Rev.110(11)(2010)6503-6570.
[2]A.Fujishima,Electrochemical photolysis of water at a semiconductor electrode,Nature238(1972)37-38.
[3]T.Hisatomi,J.Kubota,K.Domen,Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting,Chem.Soc.Rev.43(22)(2014)7520-7535.
[4]Y.N.Zhang,Q.Shi,J.Schliesser,B.F.Wood field,Z.D.Nan,Magnetic and thermodynamic properties of nanosized Zn ferrite with normal spinal structure synthesized using a facile method,Inorg.Chem.53(19)(2014)10463-10470.
[5]Y.Hou,X.Y.Li,Q.D.Zhao,X.Quan,G.H.Chen,Electrochemicalmethod for synthesis of a ZnFe2O4/TiO2composite nanotube array Modified electrode with enhanced photoelectrochemical activity,Adv.Funct.Mater.20(13)(2010)2165-2174.
[6]F.Grasset,N.Labhsetwar,D.Li,D.C.Park,N.Saito,H.Haneda,O.Cador,T.Roisnel,S.Mornet,E.Duguet,J.Portier,J.Etourneau,Synthesis and magnetic characterization of zinc ferrite nanoparticles with differentenvironments:powder,colloidal solution,and zinc ferrite-silica core-shell nanoparticles,Langmuir18(21)(2002)8209-8216.
[7]F.F.Liu,X.Y.Li,Q.D.Zhao,Y.Hou,X.Quan,G.H.Chen,Structural and photovoltaic properties of highly ordered ZnFe2O4nanotube arrays fabricated by a facile solgel template method,Acta Mater.57(9)(2009)2684-2690.
[8]M.K.Roy,H.C.Verma,Magnetization anomalies of nanosize zinc ferrite particles prepared using electrodeposition,J.Magn.Magn.Mater.306(1)(2006)98-102.
[9]J.Haetge,C.Suchomski,T.Brezesinski,Ordered mesoporous MFe2O4(M=Co,Cu,Mg,Ni,Zn)thin films with nanocrystalline walls,uniform 16 nm diameter pores and high thermal stability:Template-directed synthesis and characterization of redox active trevorite,Inorg.Chem.49(24)(2010)11619-11626.
[10]C.Yao,Q.Zeng,G.F.Goya,T.Torres,J.Liu,H.Wu,M.Ge,Y.Zeng,Y.Wang,J.Z.Jiang,ZnFe2O4nanocrystals:Synthesis and magnetic properties,J.Phys.Chem.C111(33)(2007)12274-12278.
[11]M.R.Anantharaman,S.Jagatheesan,K.A.Malini,S.Sindhu,A.Narayanasamy,C.N.Chinnasamy,J.P.Jacobs,S.Reijne,K.Seshan,R.H.H.Smits,H.H.Brongersma,On the magnetic properties of ultra- fine zinc ferrites,J.Magn.Magn.Mater.189(1)(1998)83-88.
[12]Y.Sharma,N.Sharma,G.V.S.Rao,B.V.R.Chowdari,Li-storage and cyclability of urea combustion derived ZnFe2O4as anode for Li-ion batteries,Electrochim.Acta53(5)(2008)2380-2385.
[13]N.S.Chen,X.J.Yang,E.S.Liu,J.L.Huang,Reducing gas-sensing properties of ferrite compounds MFe2O4(M=Cu,Zn,Cd and Mg),Sensors Actuators B Chem.66(1-3)(2000)178-180.
[14]C.Xiangfeng,L.Xingqin,M.Guangyao,Preparation and gas sensitivity properties of ZnFe2O4semiconductors,Sensors Actuators B Chem.55(1)(1999)19-22.
[15]A.A.Tahir,K.G.U.Wijayantha,Photoelectrochemical water splitting at nanostructured ZnFe2O4electrodes,J.Photochem.Photobiol.A Chem.216(2-3)(2010)119-125.
[16]C.G.Anchieta,D.Sallet,E.L.Foletto,S.S.da Silva,O.Chiavone,C.A.O.do Nascimento,Synthesis of ternary zinc spinel oxides and their application in the photodegradation of organic pollutant,Ceram.Int.40(3)(2014)4173-4178.
[17]S.M.Masoudpanah,S.A.S.Ebrahimi,M.Derakhshani,S.M.Mirkazemi,Structure and magnetic properties of La substituted ZnFe2O4nanoparticles synthesized by sol-gel autocombustion method,J.Magn.Magn.Mater.370(2014)122-126.
[18]X.F.Jing,Q.L.Meng,D.L.Zou,W.Feng,X.K.Han,Visible light photochromism of polyoxometalates-based composite film with deposition of ZnFe2O4nanoparticles,Mater.Lett.136(2014)229-232.
[19]Y.N.Nuli,Y.Q.Chu,Q.Z.Qin,Nanocrystalline ZnFe2O4and Ag-doped ZnFe2O4films used as new anode materials for Li-ion batteries,J.Electrochem.Soc.151(7)(2004)A1077-A1083.
[20]J.X.Qiu,C.Y.Wang,M.Y.Gu,Photocatalytic properties and optical absorption of zinc ferrite nanometer films,Mater.Sci.Eng.B112(1)(2004)1-4.
[21]M.A.Valenzuela,P.Bosch,J.Jim Nez-Becerrill,O.Quiroz,A.I.Páez,Preparation,characterization and photocatalytic activity of ZnO,Fe2O3and ZnFe2O4,J.Photochem.Photobiol.A Chem.148(1-3)(2002)177-182.
[22]Z.H.Yuan,L.D.Zhang,Synthesis,characterization and photocatalytic activity of ZnFeO/TiO nanocomposite,J.Mater.Chem.11(4)(2001)1265-1268.
[23]P.V.Kamat,Meeting the clean energy demand:Nanostructure architectures for solar energy conversion,J.Phys.Chem.C111(7)(2007)2834-2860.
[24]Z.X.Yu,Z.P.Yao,N.Zhang,Z.J.Wang,C.X.Li,X.J.Han,X.H.Wu,Z.H.Jiang,Electric field-induced synthesis of dendritic nanostructured alpha-Fe for electromagnetic absorption application,J.Mater.Chem.A1(14)(2013)4571-4576.
[25]R.Qiu,H.G.Cha,H.B.Noh,Y.B.Shim,X.L.Zhang,R.Qiao,D.Zhang,Y.Il Kim,U.Pal,Y.S.Kang,Preparation of dendritic copper nanostructures and their characterization for electroreduction,J.Phys.Chem.C113(36)(2009)15891-15896.
[26]H.Y,N.Pan,K.Zhang,Z.Wang,H.Hu,X.Wang,Fabrication of dendrite-like Au nanostructures and their enhanced photolumineseence emission,Phys.Status Solidi A204(10)(2007)3398-3404.
[27]C.L.Kuo,T.J.Kuo,M.H.Huang,Hydrothermal synthesis of ZnO microspheres and hexagonal microrods with sheetlike and platelike nanostructures,J.Phys.Chem.B109(43)(2005)20115-20121.
[28]J.Tang,J.Ye,Correlation of crystalstructures and electronic structures and photocatalytic properties of the W-containing oxides,J.Mater.Chem.15(39)(2005)4246-4251.
[29]S.Boumaza,A.Boudjemaa,A.Bouguelia,R.Bouarab,M.Trari,Visible light induced hydrogen evolution on new hetero-system ZnFe2O4/SrTiO3,Appl.Energy87(7)(2010)2230-2236.
[30]W.Zhang,M.Wang,W.Zhao,B.Wang,Magnetic composite photocatalyst ZnFe2O4/BiVO4:synthesis,characterization,and visible-light photocatalytic activity,Dalton Trans.42(43)(2013)15464-15474.
[31]H.Zeng,G.Duan,Y.Li,S.Yang,X.Xu,W.Cai,Blue luminescence of ZnO nanoparticles based on non-equilibrium processes:defect origins and emission controls,Adv.Funct.Mater.20(4)(2010)561-572.
[32]Z.W.Pan,Z.R.Dai,Z.L.Wang,Nanobelts of semiconducting oxides,Science291(5510)(2001)1947-1949.
 Chinese Journal of Chemical Engineering2016年8期
Chinese Journal of Chemical Engineering2016年8期
- Chinese Journal of Chemical Engineering的其它文章
- Computational chemical engineering - Towards thorough understanding and precise application☆
- A review of control loop monitoring and diagnosis:Prospects of controller maintenance in big data era☆
- Experimental and numerical investigations of scale-up effects on the hydrodynamics of slurry bubble columns☆
- The heat transfer optimization of conical fin by shape modification
- The steady-state and dynamic simulation of cascade distillation system for the production of oxygen-18 isotope from water☆
- Experimental mass transfer coefficients in a pilot plant multistage column extractor
