Simultaneous desulfurization and denitrification of sintering flue gas via composite absorbent☆
Jie Wang ,Wenqi Zhong ,*
1 Key Laboratory of Energy Thermal Conversion and Control of Ministry of Education,School of Energy and Environment,Southeast University,Nanjing 210096,China
2 Centre for Simulation and Modelling of Particulate Systems,Southeast University-Monash University Joint Research School,Suzhou 215123,China
1.Introduction
Among the various processes in iron and steel industry,the emission of NOXfrom the sintering process accounts for 50%of the total NOXemission.Additionally,the SO2discharging from the sintering process is about 80%.These gases are the main pollutant harmful to human and other living beings,the reduction of which is an important work of the iron and steel production enterprises[1].Along with the fact that the emission standard of NOXand SO2is more and more rigorous,the development ofnoveltechnologies for simultaneous desulfurization and denitrification of sintering flue gas will be another challenging and urgent issue following the flue gas treatment of power plant[2].At present,wet flue gas desulfurization(FGD)is a relatively mature and most widely applied technology in iron and steelindustry to controlthe SO2emission.However,It is notmuch effective For the absorption ofNOXbecause NOXfrom the sintering flue gas mainly exists in the form of NO which is poorly soluble in water[3].The common technologies for denitrification used in coalfired power plant are the selective catalytic reduction(SCR)and selective non-catalytic reduction(SNCR)methods[4-6],butneitherof them can be applied to sintering flue gas directly due to the special characteristics of sintering flue gas whose gas temperature is relative low(80-160°C),gas flow rate is too large(average 2.5 × 106m3·h-1),and the concentration of NOXis low(100-400 mg·m-3).What is more,establishment of completely new equipment and technology to remove SO2and NOXsimultaneously requires too much economic budget and thus is hardly accepted by most iron and steel industries.An alternative way is to add NO removal function to the existing FGD system to reach the goal of simultaneous absorption of SO2and NOX[7].In other words,the aim of this technique is to use proper oxidize agents to react NO into NO2which ultimately can be absorbed by water.
Currently,commonly used oxidizing agents include ClO2[8,9],O3[10,11],KMnO4[12-15],H2O2[16-18],NaClO2[19-27],NaClO[28,29]and so on.For the wet FGD,the reaction mainly occurs in the liquid phase,so ClO2,O3etc.will not be taken into consideration.KMnO4,and H2O2could be effective for simultaneous absorption of SO2and NOXin alkaline conditions,and the removal effects improve with the increase of pH.However,the pH of actual desulfurization slurry is between 5 and 7,KMnO4,H2O2will not be primarily considered.Moreover,the reaction products of KMnO4may contaminate the FGD gypsum and causing secondary pollution.And the chemical performance of H2O2is unstable,so It is difficultto be transported for long distance and reserved for long time.In general,NaClO2is the oxidizing agents which had been researched a lot and its effect is great for simultaneous desulfurization and denitrification.For example,Sadaet al.[19-21]used NaClO2/NaOH solution as the absorbent to study the removal efficiency of NO under various operating parameters.They found that adding NaOH to NaClO2would decrease the absorption rate of NO.The removal efficiency is mainly affected by the L/G ratio and the concentration of NaClO2in the solution.Next,Chienetal.[22-24]did a series of similarex periments in a bench-scale spraying scrubber system in order to investigate the absorption kinetics of SO2and NO and the reaction mechanism was revealed.Unlike previous research,Yanget al.[25,26]performed the absorption experiments of NO by nitric acid solution of NaClO2in a packed bed scrubber and found the color of solution turned greenish yellow due to the presence of ClO2,they also used bubble column and spary chamber scrubber to absorb NO in acidic NaClO or Cl2solution and obtained the similar results.Deshwalet al.[27]revealed that NaClO2could decomposed into ClO2gas in acidic solution,which was believed to participate in denitrification and determined the removal efficiency of NO.In addition,researches on NaClO are relatively rare due to the fact that the Oxidation of NaClO is weak compared with NaClO2.Chenet al.[28,29]developed a two-stage chemical scrubbing system to removal NO,the efficiency is obvious but the process is complicated.
According to the previous studies,NaClO2was demonstrated to be comparatively effective among all kinds of oxidizing agents in desulfurization and denitrification,but it is still difficult to be applied extensively in industry because of the high cost.In order to solve such problems,a compound absorbent containing NaClO2and NaClO was used to investigate simultaneous removal of SO2and NO in this study,and the key point of this art is to determine the amount of oxidizing agents and the optimal experimental conditions.At last,the success of engineering test proved that this novel method has advantages of both higher efficiency and lower cost compared with the NaClO2oxidation absorption method.
2.Experimental
The experimental device is illustrated in Fig.1 which includes four main parts:a flue gas simulation system,an absorption reactor system,a gas sampling and analyzing system and an off-gas treatment system.The flue gas simulation system mainly consists of a pure N2gas cylinder(Shangyuan Gas,purity 99.9%),a pure SO2gas cylinder(Shangyuan Gas,purity 99.9%),a pure NO gas cylinder(Shangyuan Gas,purity 99.9%),a gas buffertank and fourmass flow controllers(MFC).The absorption reactor unit mainly includes a self-designed lab-scale bubbling reactor and a digital heating and temperature control device.Besides,the reactor is made of stainless steel with a diameter D1of 320 mm and a height H1of 240 mm.There are 4 intake-tubes with a diameter D2of 14 mm and 1 exhaust tube with a diameter D3of 80 mm.The gas sampling and analyzing system contain a sample conditioner(Juchuang,JCGH-2)and a flue gas analyzer(MRU,Germany).The off-gas treatment system is a tank saturated with aqueous alkaline solution.The grade of sodium chlorite is analytical-pure(available chlorine≥80%,Aladdin IndustrialCorporation,Shanghai,China).The grade of sodium hypochlorite is technical-pure(available chlorine≥30%,Nanjing Chemical Reagent Co.,Ltd.,Nanjing,China).The grade of sodium hydroxide(≥96%)and phosphoric acid(65%-68%)is analytical-pure(BOLT Chemical Trading Co.,Ltd.,Tianjin,China).

Fig.1.Schematic diagram of experimental system.
During the experiment,the flow rates of N2,SO2and NO were controlled through the mass flow meters and mixed into desired concentrations in a buffer tank firstly.10 L aqueous solution with a required amountof NaClO2/NaClO in the reactorwas heated and then kept in desired temperatures by a digital heating and temperature control device.Thereafter,the simulated gas continuously flowed through the reactor with a flow rate and was absorbed by the solution.After reaction,the simulated flue gas was sampled by a sample conditioner and analyzed through a flue gas analyzer so that the concentration of NO and SO2would be monitored in real time.Meanwhile,the testdata was recorded in every 2 min,so the removalefficiency ofNOand SO2can be calculated through Eqs.(1)to(2).

In addition,the initial pH of absorption solution in the bubbling reactor could be adjusted by adding acid or alkaline solution(H3PO4and NaOH)and detected by the LEICI pH meter.Continuous stirring was provided by a mechanical agitator.The operating conditions are shown in Table 1.Finally,the simulated flue gas before being released into atmosphere would be post-processed by the off-gas treatment system.

Table 1Operating conditions
3.Results and Discussion
3.1.Effect of the concentration of NaClO2(ms)and NaClO(mp)

Fig.2.Effect of the concentration ofNaClO2/NaClOon the removalefficiencies(T R=25°C,pH=6,V g=30 L·h-1,C N=350 mg·m-3,C S=1000 mg·m-3).
Fig.2 depicts the variation in NO and SO2removal efficiencies with the concentration of absorbent,it can be seen that the removal efficiency of NO increases at first and then slow down withmsand the SO2removal efficiency almost remains constant.Whenmsincreases from1 to 3 mmol·L-1,the removalefficiency ofNOis sharply improved by 11%.Whilemschanges from 4 to 6 mmol·L-1,the removalefficiency increases by 4%only.Ifmsis constant,the removal efficiency of NO inmp=3 mmol·L-1is higher thanmp=0 mmol·L-1.It means that adding NaClOto NaClO2aqueous solution hasobvious promotion on denitrification but the effect would be weakened if excess quantities of NaClO were used.
Moreover,considering that the oxidation properties of the solution may be different under different molar ratios of NaClO2/NaClO(M)which iscalculated by using Eq.(3),experiments were carried out in different molar ratios of NaClO2/NaClO to check the oxidization of the solution.Due to the fact that the removal efficiency of SO2is less affected by the concentration of oxidizing agent,we focus on the removal efficiency of NO here.

As shown in Fig.3,the removal efficiency of NO shows an upward trend with the increase ofM.WhenMis above 1.3,the removal efficiency of NO begins to be stable.These results indicate that NaClO2plays an important role in the process of oxidative absorption,slight change of its concentration can lead to significant increase of the removal efficiency of NO.From the aspect of economy that the price of NaClO2is much higher than NaClO,the cost of oxidizing agent can be reduced by decreasing NaClO2concentration while increasing NaClO concentration.In this study,based on the efficiency and the cost consideration,the optimalMwas chosen as 1.3.

Fig.3.Effect of the molar ratio of NaClO2/NaClO on the removal efficiencies(T R=25°C,pH=6,V g=30 L·h-1,C N=350 mg·m-3,C S=1000 mg·m-3).
3.2.Effect of solution temperature(TR)
For the gas-liquid two phase chemical reaction,the temperature plays dual role.On one hand,the increase of temperature is beneficial to ion diffusion in solution which accelerates the rate of reaction and promotes the removal of SO2and NO.On the other hand,the increase of temperature lowers the solubility ofSO2and NOin solution which increases the mass transfer resistance between gas and liquid and restrains the oxidation and absorption of SO2and NO.Therefore,the rise of temperature has effects of promoting and inhibiting on the experiment.Theoretically,the reaction exists optimal reaction temperature.As shown in Fig.4,whenTRis between 30 °C and 50 °C,the removal efficiency of NO increases with the increase ofTR,which indicates that the positive effect of temperature is greater than its inhibition on the reaction.When the temperature is above 50°C,the removal efficiency ofNO remains almost unchanged with furtherincreasingTR,which indicates that the promotion and inhibition of temperature on the reaction are in an equilibrium state.In the actual projects,the reaction temperature is between 50 and 70 °C,so the optimalTRwas selected as 55 °C.

Fig.4.Effect of solution temperature on the removal efficiencies(pH=6,V g=30 L·h-1,m s=4 mmol·L-1,m p=3 mmol·L-1,C N=350 mg·m-3,C S=1000 mg·m-3).
3.3.Effect of initial solution pH
Previous study shows that the removal efficiencies of NO and SO2were greatly affected by the initial solution pH[27],because the variation of pH would influence the oxidative absorption characteristics of solution and lead to the change of the removal efficiencies of SO2and NO.As shown in Fig.5,the removal efficiency of SO2is slightly affected and the removal efficiency of NO decreases with increasing the initial solution pH.The main reasons are as follows:SO2is easily soluble in water to produceand H+.When the solution pH increases,the equilibrium reaction takes place in the positive direction which promotes the absorption of SO2.Furthermore,when the solution pH decreases,ClO2and Cl2are generated by the aqueous solution of NaClO2/NaClO in acidic condition,and both of the gases are beneficial to increase the removal efficiencies of NO and SO2.Therefore,the solution pH has slight effect on desulfurization,but its decrease can dramatically improve the removal efficiency of NO.Moreover,NO is insoluble in water which means that the absorption ofNOcan only rely on oxidizing agent.Therefore,the removal of efficiency of NO drops continuously when pH of the solution increases.Above analysis indicates that the lower the initial pH of the solution is,the higher removal efficiency of NO will be obtained.By considering that strong acid condition could cause severe corrosion on the experimental equipment,optimal solution pH was usually taken as 6.
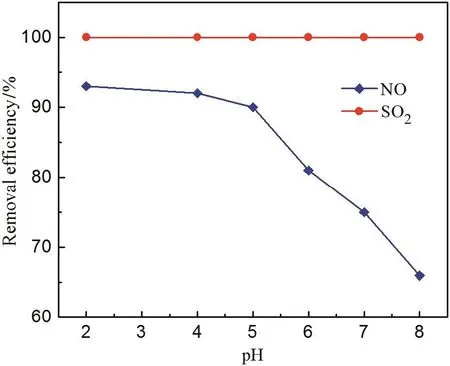
Fig.5.Effect of initial solution pH on the removal efficiencies(T R=25 °C,V g=30 L·h-1,m s=4 mmol·L-1,m p=3 mmol·L-1,C N=350 mg·m-3,C S=1000 mg·m-3).
3.4.Effect of gas flow rate(Vg)
For a specific experiment device,Vgdetermines the staying time of gas in water,influences the gas-liquid mass transfer process,and changes the removal efficiencies of NO and SO2,so it is necessary to takeVginto consideration.From Fig.6,it can be seen that the removal efficiency of SO2decreased slightly but NO drops almost linearly asVgincreases.The high removal efficiency of SO2is due to the SO2dissolved in water.While NO is not soluble in water,it must be oxidized firstly by composite absorbent,and then absorbed by water.WhenVgis large,the time of NO touching oxidizing agent will be reduced,consequently,there is notenough time for NOto be oxidized and absorbed thoroughly.The results indicate thatVgis lower,the removal efficiency of NO is higher.Considering aboutVgis constant in actual projects,we just discuss the effect of it,and the optimalVgwill not be given.
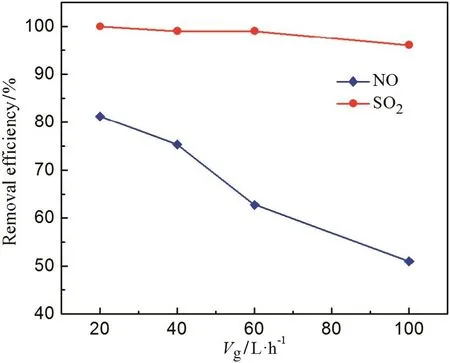
Fig.6.Effect of gas flow rate on the removal efficiencies(T R=25°C,pH=6,m s=4 mmol·L-1,m p=3 mmol·L-1,C N=350 mg·m-3,C S=1000 mg·m-3).
3.5.Effect of the inlet concentration of SO2(CS)and NO(CN)
In the practical engineering process,the content of nitrogen and sulfuris differentin various types ofcoal,which willchangeCSandCNfrom the sintering flue gas and affectthe concentration driving force between gas and liquid phases.Therefore,the removal efficiencies of SO2and NO will also change.As shown in Fig.7,the removal efficiency of SO2remains 100%and the removal efficiency of NO presents a slight upward trend with the increase ofCS.The phenomenon may be attributed to the hydrolysis of SO2,resulting in decreasing the solution pH and improving the oxidation of the absorbent.As seen from Fig.8,the removal efficiency of SO2remains stable and the removal efficiency of NO increases slightly with increasingCN.The reason might be that the increase ofCNmakes the pressure of NO in the gas phase rise and accelerates the gas-liquid mass transfer,which leads to the increase of removal efficiency of NO.
3.6.Parallel tests

Fig.7.Effect of SO2 concentration on the removal efficiencies(T R=25°C,pH=6,V g=30 L·h-1,m s=4 mmol·L-1,m p=3 mmol·L-1,C N=350 mg·m-3).

Fig.8.Effect of NO concentration on the removal efficiencies(T R=25°C,pH=6,V g=30 L·h-1,m s=4 mmol·L-1,m p=3 mmol·L-1,C S=1000 mg·m-3).
In order to check the stability and accuracy of experiment,parallel tests were carried out under the optimal conditions in whichms=4 mmol·L-1,mp=3 mmol·L-1,TR=55 °C,the initial solution pH=6,Vg=30 L·h-1,andCS=1000 mg·m-3,CN=350 mg·m-3.From Table 2,it can be seen that the average removal efficiencies of SO2and NO are 99.5%and 90.8%,respectively,and the standard deviation of removal efficiencies are 0.5 and 0.795,respectively.It indicates that the reproducibility of experiment date is good,and the performance of experiment apparatus is stable.
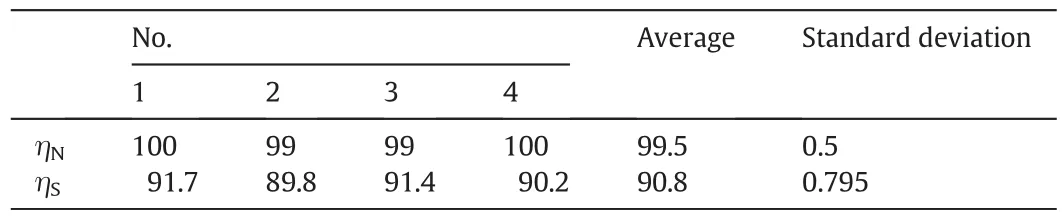
Table 2Results of parallel experiments
4.Reaction Mechanism
For revealing the reaction mechanism and the process of simultaneous removal of SO2and NO by the aqueous solution of NaClO2/NaClO,products of desulfurization and denitrificationwere qualitatively and quantitatively analyzed.As we know,the possible existence of ionand some intermediate products ClO2or Cl2[7].Based on the chemical properties of different ions,Cl-,were tested by the hydronium chromatography method and,ClO2,were detected by the electric potential titrimetric method.
4.1.Interaction mechanism of NaClO2/NaClO
By monitoring the color and smell of absorption solution and analyzing the reaction products,we can find that the oxidizability of NaClO2/NaClO may be different under various initial solution pH.As shown in Fig.9.Samples 1 and 2 are the aqueous solution containing NaClO2and NaClO,respectively.Sample 3 is the aqueous solution of NaClO2/NaClO whose pH is 9.8 without adding acid or alkaline solution to adjust,the solution is almost colorless and a little pungent odor could be smelled.Sample 4 is the solution whose initial pH is adjusted to 6 on the basis of Sample 3,the color of it turns yellowish green,pungent odor becomes more intense.The phenomenon is attributed to products from the reaction of NaClO2/NaClO in acidic condition.From a survey of previous literatures[30-32],we can speculate that the products may be ClO2and Cl2which have positive effect on desulfurization and denitrification.In order to further understand the interaction mechanism of NaClO2/NaClO,ions concentration of a single absorbents and its mixture were determined.The results are shown in Table 3.

Fig.9.The color contrast of absorbents in different experiments process.
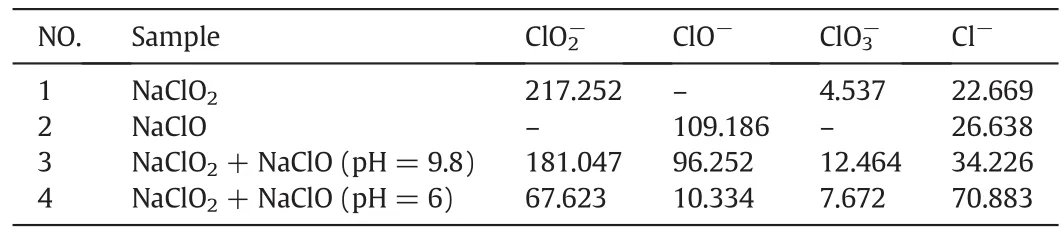
Table 3The changes of ions concentration in absorbing liquid(mg·L-1)
The possible reactions between ClO2and Cl2in alkaline solution are listed as follows:

So the existence of ClO2and Cl2can be con firmed by checking the variation ofand Cl-concentration.Comparing Samples 1 to 3,we can find that Samples 1 and 2 both contain Cl-,but ClO3-only exists in Sample 1.It can be considered that the properties of single oxidant had changed under acid condition,the reaction equations are presumed as follows[32,33]:

The concentration ofand Cl-in Sample 3 is higher than in Samples 1 and 2,indicating that ClO2and Cl2are produced.In addition,by comparing Samples 3 and 4,we can find that the concentration ofand ClO-decreases,while the concentration ofand Cl-increases,stating that a lower pH of the solution makes it more favorable for the generation of ClO2.According to the analysis of the reaction products and the reaction mechanism presumed above,the overall reaction equation of NaClO2/NaClO acid solution can be considered as follow:
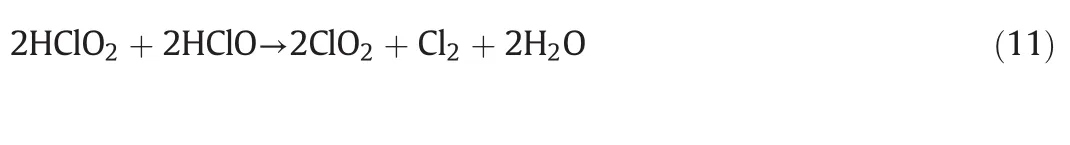
4.2.Reaction mechanism of simultaneous desulfurization and denitrification
In order to explore reaction mechanism of simultaneous absorption of NO and SO2,experiments were carried out under the optimal conditions and the reaction products were detected.The results of products analysis are listed in Table 4.
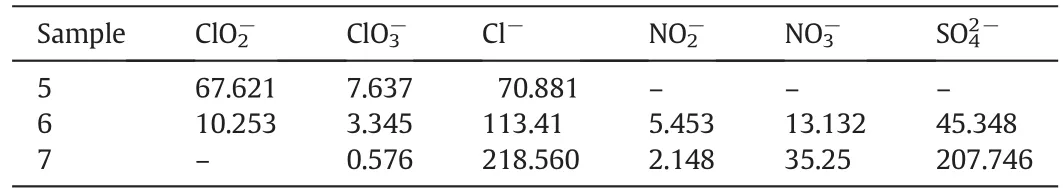
Table 4Products analysis of simultaneous desulfurization and denitrification using NaClO2/NaClO complex absorbent(mg·L-1)
Sample 5 is the initial solution containing NaClO2/NaClO which is not involved in the reaction of simultaneous removal of NO and SO2,Sample 6 is NaClO2/NaClO aqueous solution which has been in reaction for 10 min,and Sample 7 is the solution whose oxidizability has been in failure for 5 min.As shown in Table 4,both the concentration ofanddecreases,and meanwhile Cl-,,andare produced continuously by comparing Sample 5 and 6.It indicates that composite absorbent can oxidize SO2and NO to the highest state in acidic condition.After the failure ofoxidizing agent,the concentration of Cl-andis stillrising whileconcentration begins to decline,sois speculated as an intermediate product.
According to the previous analysis of interaction mechanism of NaClO2/NaClO,it was found that there exists a small quantity of ClO2and Cl2in the complex absorbent solution,and there might be a part ofClO2and Cl2from the liquid phase to the gas phase because their partial pressures in gas phase are smaller than the gas-liquid equilibrium partial pressures.Considering that SO2is soluble in water,the absorption of SO2mainly happens in the liquid phase.However,NO is poorly soluble in water,and the mass transfer resistance from liquid phase to gas phase is large,so the oxidation of NO mainly through touching with ClO2and Cl2in the interface between gas and liquid phases,then the reaction products can be absorption in the liquid phase,so the absorption of NO might happen in the gas and liquid phases at the same time.
On the basis of analysis above and the results of previous research[31-33],the possible reaction equations of simultaneous desulfurization and denitrification by NaClO2/NaClO acid solution are listed as follows:

In conclusion,the process of simultaneous removal of SO2and NO using NaClO2/NaClO aqueous solution is relatively complex,the overall reaction equations can be summarized as follows:

5.Engineering Experiments
In order to verify the availability of above experimental results and provide a solid technical support on the large-scale industrial application in the future,engineering experiments were carried out in a 2×220 m2sintering flue gas desulfurization tower at Nanjing Iron&Steel Co.The system of the wet FGD is shown in Fig.10.The experimental conditions are as follow:the flow rate of inlet gas ranges from 1.36× 106to 2.51× 106m3·h-1,the inlet gas temperature increases from 125.1 to 166.63°C,the desulfurization slurry temperature in absorber is between 50 to 70°C,the SO2concentration of inlet gas changes from 517.24 to 1128.06 mg·m-3,the NOXconcentration of inlet gas ranges from 249.75 to 340.05 mg·m-3and the molar ratio of NaClO2/NaClO is about 1.3.The variation of NO and SO2mass flux was monitored through the Pollution Source On-line Monitoring system of Nanjing City,then the removal efficiencies of NOXand SO2can be evaluated by Eqs.(26)to(27),respectively.
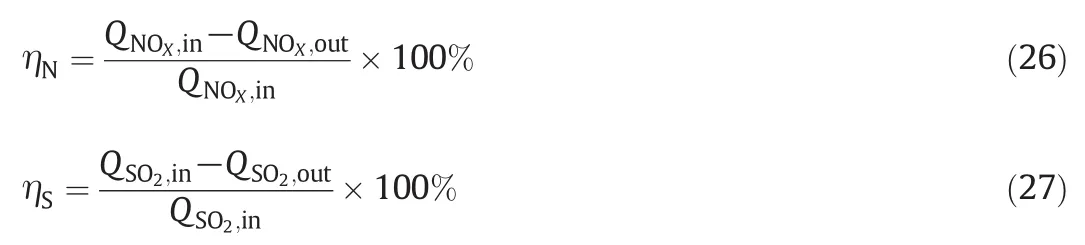
As shown in Figs.11 and 12,during the experimental period,QSdecreases from 9.8 to 6.9 kg·min-1,the removal efficiency of NOXincreases around 20%compared with ordinary times and the removal efficiency of SO2remains stable above 94%.The results meet national environmental standards and demonstrate the capability of the technology for simultaneous desulfurization and denitration.However,it can be seen in Fig.12 that the increase of NOXremoval efficiency is limited.It is speculated that when NO was oxidized to NO2which cannot be absorbed completely,because the treated flue gas with a little reddish brown color(the original flue gas color is white)and the color is similar to NO2during engineering experiment.
In terms of the present running situation of Nanjing Iron&Steel Co,the annual costs of the composite absorbent is about 5.43 million,the fee for pollutant discharge can be saved about 1.19 million a year.If the reagents are purchased in large quantities,the price could be reduced by 30%-40%.Compared with other simultaneous desulfurization and denitrification technologies,using NaClO2/NaClO as the absorbent has certain advantages in the aspect of environmental protection and economy.
6.Conclusions
A composite absorbentcontaining NaClO2and NaClO was used to simultaneous desulfurization and denitrification from flue gas.The effects of various operating parameters on the experiment were considered and the reaction products were analyzed.According to the results of discussion,following conclusions can be made:
1.Under the optimal experimental conditions,the removal efficiencies of SO2and NO reach 99.5%and 90.8%,respectively.These results indicate that the oxidizing agent,which is made of NaClO2and NaClO,has good promotion prospects in simultaneous removal of NO and SO2.
2.The removal efficiency of SO2is slightly affected by different operating parameters in the experiment and holds steady above 98%.
3.In acidic condition,high removal efficiencies of SO2and NO owe to the fact that ClO2and Cl2are produced by the reaction between NaClO2and NaClO.
4.The initial solution pH and the gas flow rate are the main factors affecting simultaneous desulfurization and denitrification.
Nomenclature
CNinitial NO inlet concentration,mg·m-3
CNO,inthe inlet concentration of NO,mg·m-3
CNO,outthe outlet concentration of NO,mg·m-3
CSinitial SO2inlet concentration,mg·m-3
CSO2,inthe inlet concentration of SO2,mg·m-3
CSO2,outthe outlet concentration of SO2,mg·m-3
Mthe molar ratio of NaClO2/NaClO
mpNaClO concentration,mmol·L-1
msNaClO2concentration,mmol·L-1
QNOX,inthe inlet mass flux of NOX,kg·min-1
QNOX,outthe outlet mass flux of NOX,kg·min-1
QSO2,inthe inlet mass flux of SO2,kg·min-1
QSO2,outthe outlet mass flux of SO2,kg·min-1
TRsolution temperature,°C
Vggas flow rate,L·h-1
η the removal efficiency
ηNremoval efficiency of NO
ηSremoval efficiency of SO2

Fig.10.The system of wet FGD:(a)Appearance of bubbling gas absorbing tower;(b)the technological progress of sintering flue gas desulfurization.

Fig.11.The mass flux change of NO X/SO2 during experimental period.
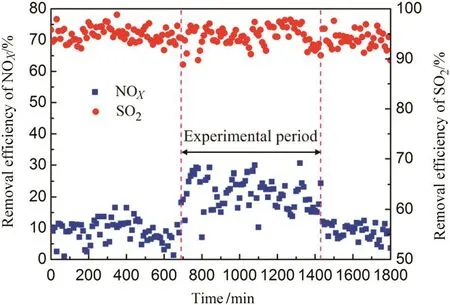
Fig.12.The change of removal efficiencies during experimental period.
[1]Y.Li,W.Q.Zhong,J.Ju,Experiment on simultaneous absorption of NO and SO2from sintering flue gas by oxidizing agents of KMnO4/NaClO,Int.J.Chem.React.Eng.12(1)(2014)1-9.
[2]D.G.Streets,S.T.Waldhoff,Present and future emissions of air pollutants in China:SO2,NOXand CO,Atmos.Environ.34(3)(2000)363-374.
[3]F.J.Gutierrez Ortiz,F.Vidal,P.Ollero,L.Salvador,V.Cortes,Pilot-plant technical assessment of wet flue gas desulfurization using limestone,Ind.Eng.Chem.Res.45(4)(2006)1466-1477.
[4]G.Qi,R.T.Yang,R.Chang,MnOX-CeO2mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3at low temperatures,Appl.Catal.B Environ.51(2)(2004)93-106.
[5]M.T.Javed,N.Irfan,B.M.Gibbs,Control of combustion-generated nitrogen oxides by selective non-catalytic reduction,J.Environ.Manag.83(3)(2007)251-289.
[6]S.W.Bae,S.A.Roh,S.D.Kim,NO removal by reducing agents and additives in the selective non-catalytic reduction(SNCR)process,Chemosphere65(1)(2006)170-175.
[7]Y.Zhao,T.Guo,Z.Chen,Y.Du,Simultaneous removal of SO2and NO using M/NaClO2complex absorbent,Chem.Eng.J.160(1)(2010)42-47.
[8]D.S.Jin,B.R.Deshwal,Y.S.Park,H.K.Lee,Simultaneous removal of SO2and NO by wet scrubbing using aqueous chlorine dioxide solution,J.Hazard.Mater.135(1-3)(2006)412-417.
[9]H.K.Lee,B.R.Deshwal,K.S.Yoo,Simultaneous removal of SO2and NO by sodium chlorite solution in Wetted-Wall column,Korean J.Chem.Eng.22(2)(2005)208-213.
[10]Y.S.Mok,H.J.Lee,Removal of sulfur dioxide and nitrogen oxides by using ozone injection and absorption reduction technique,Fuel Process.Technol.87(7)(2006)591-597.
[11]Z.Wang,J.Zhou,Y.Zhu,Simultaneous removal of NOX,SO2and Hg in nitrogen flow in a narrow reactor by ozone injection:experimental results,Fuel Process.Technol.88(8)(2007)817-823.
[12]H.Chu,T.W.Chien,S.Y.Li,Simultaneous absorption of SO2and NO from flue gas with KMnO4/NaOH solutions,Sci.Total Environ.275(1-3)(2001)127-135.
[13]H.Chu,S.Y.Li,T.W.Chien,The absorption kinetics of NO from flue gas in a stirred tank reactor with KMnO4/NaOH solutions,J.Environ.Sci.Health A33(5)(1998)801-827.
[14]C.Brogren,H.T.Karlsson,I.Bjerle,Absorption of NO in an alkaline solution of KMnO4,Chem.Eng.Technol.20(6)(1997)396-402.
[15]Z.Wei,H.Niu,Y.Ji,Simultaneous removal of SO2and NOXby microwave with potassium permanganate over zeolite,Fuel Process.Technol.90(2)(2009)324-329.
[16]D.Thomas,S.Colle,J.Vanderschuren,Kinetics of SO2absorption into fairly concentrated sulphuric acid solutions containing hydrogen peroxide,Chem.Eng.Process.42(6)(2003)487-494.
[17]S.Colle,J.Vanderschuren,D.Thomas,Pilot-scale validation of the kinetics of SO2absorption into sulphuric acid solutions containing hydrogen peroxide,Chem.Eng.Process.43(11)(2004)1397-1402.
[18]S.Colle,J.Vanderschuren,D.Thomas,Simulation of SO2absorption into sulfuric acid solutions containing hydrogen peroxide in the fast and moderately fast kinetic regimes,Chem.Eng.Process.60(22)(2005)6472-6479.
[19]E.Sada,H.Kumazawa,I.Kudo,T.Kondo,Absorption of NO in aqueous mixed solutions of NaClO2 of and NaOH,Chem.Eng.Sci.33(3)(1978)315-318.
[20]E.Sada,H.Kumazawa,Y.Yamanaka,I.Kudo,T.Kondo,And nitric oxide in aqueous mixed solutions of sodium chlorite and sodium hydroxide,J.Chem.Eng.Jpn11(4)(1978)276-282.
[21]E.Sada,H.Kumazawa,M.A.Butt,Single and simultaneous absorptions of lean SO2and NO2into aqueous slurries of Ca(OH)2or Mg(OH)2particles,J.Chem.Eng.Jpn12(2)(1979)111-117.
[22]T.W.Chien,H.Chu,H.T.Hsueh,Kinetic study on absorption of SO2and NOXwith acidic NaClO2solutions using the spraying column,J.Environ.Eng.129(11)(2003)967-974.
[23]H.Chu,T.W.Chien,B.W.Twu,The absorption kinetics of NO in NaClO2/NaOH solutions,J.Hazard.Mater.84(2-3)(2001)241-252.
[24]T.W.Chien,H.Chu,Removal of SO2and NO from flue gas by wet scrubbing using an aqueous NaClO2solution,J.Hazard.Mater.80(1-3)(2000)43-57.
[25]C.Yang,H.Shaw,H.Perlmutter,Absorption of NO promoted by strong oxidizing agents:1.Inorganic oxychlorites in nitric acid,Chem.Eng.Commun.143(1)(1996)23-38.
[26]C.Yang,H.Shaw,Aqueous absorption of nitric oxide induced by sodium chlorite oxidation in the presence of sulfur dioxide,Environ.Prog.17(2)(1998)80-85.
[27]B.R.Deshwal,S.H.Lee,J.H.Jung,B.H.Shon,H.K.Lee,Study on the removal of NOxfrom simulated flue gas using acidic NaClO2solution,J.Environ.Sci.20(1)(2008)33-38.
[28]L.Chen,C.Hsu,C.Yang,Oxidation and absorption of nitric oxide in a packed tower with sodium hypochlorite aqueous solutions,Environ.Prog.24(3)(2005)279-288.
[29]L.Chen,J.W.Lin,C.L.Yang,Absorption of NO2in a packed tower with Na2SO3aqueous solution,Environ.Prog.21(4)(2002)225-230.
[30]B.R.Deshwal,H.Jo,H.Lee,Reaction kinetics of decomposition of acidic sodium chlorite,Can.J.Chem.Eng.82(3)(2004)619-623.
[31]T.W.Chien,H.Chu,H.T.Hsueh,Spray scrubbing of the nitrogen oxides into NaClO2solution under acidic conditions,J.Environ.Sci.Health A36(4)(2001)403-414.
[32]B.Kormanyos,I.Nagypal,G.Peintler,A.Horvath,Effect of chloride ion on the kinetics and mechanism of the reaction between chlorite ion and hypochlorous acid,Inorg.Chem.47(17)(2008)7914-7920.
[33]T.Lehtimaa,V.Tarvo,G.Mortha,S.Kuitunen,T.Vuorinen,Reactions and kinetics of Cl(III)decomposition,Ind.Eng.Chem.Res.47(15)(2008)5284-5290.
 Chinese Journal of Chemical Engineering2016年8期
Chinese Journal of Chemical Engineering2016年8期
- Chinese Journal of Chemical Engineering的其它文章
- Computational chemical engineering - Towards thorough understanding and precise application☆
- A review of control loop monitoring and diagnosis:Prospects of controller maintenance in big data era☆
- Experimental and numerical investigations of scale-up effects on the hydrodynamics of slurry bubble columns☆
- The heat transfer optimization of conical fin by shape modification
- The steady-state and dynamic simulation of cascade distillation system for the production of oxygen-18 isotope from water☆
- Experimental mass transfer coefficients in a pilot plant multistage column extractor
