Synthesis and characterization of poppy seed oil methyl esters
Umer Rashid ,Muhammad Ibrahim ,Imededdine Arbi Nehdi,Saud Ibrahim Al-Resayes ,Sammi Ullah ,Muhammad Aamer Mehmood ,Saira Shahzadi
1 Institute of Advanced Technology,Universiti Putra Malaysia,43400 UPM Serdang,Selangor,Malaysia
2 Chemistry Department,College of Science,King Saud University,Riyadh 1145,Saudi Arabia
3 Department of Environmental Sciences&Engineering,Faculty of Engineering,Government College University Faisalabad,Faisalabad 38000,Pakistan
4 Department of Chemistry,Government College University Faisalabad,Faisalabad 38000,Pakistan
5 Department of Bioinformatics&Biotechnology,Government College University Faisalabad,Faisalabad 38000,Pakistan
6 Department of Chemistry,Quaid-i-Azam University,Islamabad,Pakistan
1.Introduction
Global warming,the uncertain political condition of the oil producing countries,progression in industrialization and transportation have moved the researcher to develop renewable energy sources to fossil fuels[1,2].Biodiesel is regarded as an alternative fuel because it is technically feasible,economically competitive,environmentally friendly and readily available[3,4].
Biodiesel may be produced from either edible or non-edible oils.Chemically,biodiesel is produced through the transesterification of oils where a triglyceride reacts with an alcohol in the presence of a catalyst(acid or base),yielding a mixture of fatty acids,alkyl esters and glycerol as a by-product[3].Stoichio metrically,the reaction requires 1 mol of triglyceride and 3 mol of alcohol.However,an excess amount of alcohol is used to increase the yield of the alkyl esters in the forward reaction and for effective glycerol separation[5,6].Biodiesel characteristics are almost similar to the petroleum based fossil diesel.It can be used directly or in any ratio in an ordinary diesel engine without any amendments[7,8].
Biodiesel sources should satisfy two requirements as far as possible:lower production costs and have a large production scale[9].Hence,the selection of the feedstock for biodiesel and its oil content is very crucial as 60%-70%of the total production cost depends on the feedstock chosen[9].Almost 95%of the total biodiesel is produced using edible oil as feedstock.Among these soybean,sun flower,rapeseed,palm oil,coconut linseedetc.are the most studied oils in this context[3,9].However,non-edible vegetable oils obtained from various sources have also been studied as a source of biodiesel[7].Algae and many halo phytic plants are also being studied nowadays as oil sources for bio diesel production[8].Alternatively,waste oils derived from the food processing industry,such as kernels and seeds of various fruits from the production of juices,and non-food oil could be a good promising option instead of edible oils.
Poppy plants(Papaver somniferumL.)belong to the subfamilyPapaveroideaeof the familyPapaveraceaeand include 44 genera and 760 species.Most of these plant species are herbaceous plants with some exceptions of woody shrubs.The poppy plants are commonly grown in India and some parts of Pakistan for morphine and the seeds are used as medicine,traditionally[10].These plants are commonly grown in the wild,in Pakistan and Afghanistan.In most parts of Europe these plants are grown for seed oil and edible seeds[11].Poppy seeds contain up to 50%oil and Indian cultivars have higher ratios of oleic and linoleic acids as reported by Singhetal.[12].The family is outstanding for its ornamental and pharmaceutical importance.Most species are found in the Northern hemisphere[13].These plants grow under a wide range of soil and climatic conditions and cannot endure extreme cold.The poppy plants like medium textured soils treated with manure or well fertilized.The yield potential of the poppy seed is impressive,for example,about 1.8 tons/ha seed(cf.2.4 for canola).The oil content of the poppy seed is also exceptional at 45%-50%oil content per unit seed mass(cf.40%in canola).Ityields about0.8 tons of oil perha[14,15].
The present study was carried out to demonstrate the feasibility of biodiesel production from poppy oil seeds and the characterization of the fuel properties as per ASTM specifications.In this study,the application of the response surface methodology(RSM)using the central composite design(CCD)technique for modeling and optimization of the influence of several operating variables on the transesterification reaction of poppy seed oil was investigated.The fuel properties of poppy seed oil methyl esters(PSOMEs)were also appraised.
2.Experimental
2.1.Material and reagents
The poppy seeds(P.somniferum)were bought from a local market,Faisalabad and the authentication of the seeds was obtained from the Department of Botany,GC University Faisalabad,Pakistan.It was purified by filtration before further study.All reagents for the preliminary analysis,tranesterification reaction and chemicals used as standard for gas chromatography and FTIR investigation were of analytical grade.
2.2.Extraction of oil
The poppy(P.somniferum)seeds were blown to remove dust,dirt and immature broken seeds.The seeds were crushed and fed into a locally made oilexpellerto extractthe oil.The oilafterextraction was subjected to filtration for purification using a vacuum filtration apparatus.The purified oil was stored in a clean glass bottle and then used for further experiments.
2.3.Characterization of poppy seed oil
The physico-chemical properties of the poppy seed oil were determined.Saponification-,peroxide-,iodine-,and acid-values were evaluated according to AOCS methods[16].
2.4.Pre-treatment
The poppy seed oil had the acid value of 6.02 mg·(g KOH)-1,consequently,the acid pre-treatment was performed as described previously[17].
2.5.transesterification reaction
transesterification was per formed asdescribed previously[18].Briefly,a 250 mlthree-necked round-bottomed reactore quipped with a thermostat,a hotplate with a stirrer and a re flux condenser at a constant stirring speed(600 r·min-1)were used for the transesterification of the poppy seed oil.100 g of poppy oil was transferred into the flask and oil was preheated at the required temperature before starting the reaction.The calculated amount of sodium methoxide was added to a predetermined amount of anhydrous methanol and the mixture was stirred until the sodium methoxide(CH3ONa)dissolved completely.The prepared methanol and catalyst solution was put into the pre-heated poppy seed oil and the reaction was started and continued for the required reaction time.Following the transesterification,the reaction mixture was left to cool down.The mixture was separated into two phases after cooling,the upper phase consisted of methyl esters,and the lower phase contained the glycerol,the excess methanol,and the un-reacted catalyst.
2.6.purification step
After separation of both layers by sedimentation,the upper methyl ester layer got rid of the impurities by distilling the residual methanol at 80°C.The left over un-reacted catalyst was removed by consecutive rinses with distilled water(45°C).Finally,the left over water was removed by treating itwith sodiumsulfate and the methylesters were filtered to purify.
2.7.Experimental design for optimization study
Response surface methodology(RSM)was applied to study the effect of the reaction parameters,i.e.,molar ratio of methanol/oil,catalyst concentration with reference to oil mass,and the reaction temperature and time,on the yield of the poppy seed oil methyl ester.The factorial level was chosen by considering the properties of the reactants.The ranges assorted for these variables were 3:1-9:1,0.5%-1.25%,20-90 min and 25-65°C for the molar ratio of methanol/oil,catalyst concentration,and the reaction time and reaction temperature,respectively.The final product was weighed to estimate the yield by using Eq.1 and then it was stored for further investigation.This yield was expressed with reference to the oil mass used for the methanolysis[17].
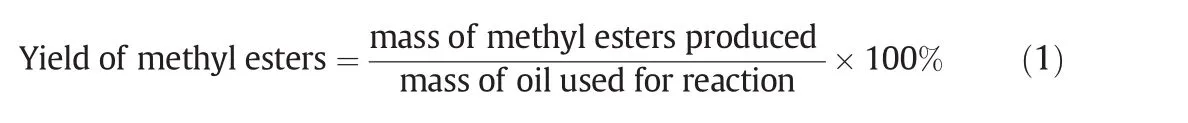
The full-factorial central composite design of 30 experiments was builtto explore the effect of the above mentioned fourreaction variables on the poppy seed oilmethylester's yield.The results obtained from the prede fined CCRD shaped the regression coefficients of the second-order multiple regression models by using Eq.2.

where,Yrepresents the predicted response variable(methyl ester yield),kis the number of factors optimized in the experiments,eis the random error andb0,bi,bii,andbijare the regression coefficients.
2.8.Statistical analysis
Data obtained from the CCRD reaction parameters'optimization were used for determining the regression coefficient of the secondorder multiple regression models[19].Design-Expert 7(Stat-Ease Inc.,Minneapolis,MN)was applied to construct the response surfaces;whereas,ANOVA was obtained to check the efficiency of the regression modelby calculating theFvalues within a level of con fidence of95%and thepvalues of<0.05.The coefficient of determination,R2,was calculated to analyze the fit of the polynomial model equation and theF-test and regression t-test were checked for the statistical coefficient significance.The optimized values for the selected variables were obtained with the help of the regression equation and by evaluation of the response surfaces and contour plots.The response surface analyses were performed by keeping two independent variables at a constant level while changing the other two independent variables.
2.9.Analytical procedure
Ester recognition and their quantitative measurements were achieved by running the sample on the Shimadzu Gas Chromatograph as per our previous reported method[3].FTIR spectrums were also analyzed for poppy seed oil and its methyl ester to check the corresponding conversion.
2.10.Fuel properties
The fuel properties,such as cloud and pour points,were tested using ASTM D 2500 and ASTM D 97.The densities were determined by using the digital density analyzer(PAAR,DMA 38)with ASTM D 5002.The kinematic viscosities were determined at40°C using a Digital Viscometer(Anton Parr,Stabinger,and Model SVM 3000)by following the ASTM D 7042 procedure.The cold filter plugging point was analyzed using a CFPP apparatus(ISL,Model CPP97-2)by applying the procedure ASTM D 6371.The sulfur content was tested by using energydispersive X-ray fluorescence spectrometry(OXFORD,Model Lab-X3000)and the ASTM D 4294 procedure was applied.The Cetane number of the biodiesel was calculated by applying the procedure ASTM D 976.Each experiment was conducted in triplicate,and the data are reported as means.
3.Results and Discussion
3.1.Characteristics of the poppy seed oil
The preliminary analysis of the raw poppy seed oil was performed prior to the transesterification.The content of the cold pressed poppy oil was 46.8%on wt.basis.The iodine value(IV),peroxide value(PV),free fatty acid(FFA)content and saponi fication value(SV)of the oil under study was 138.54 g I2per 100 g of oil,1.25 mmol·L-1O2of oil,3.01 mg per g KOH and 198 mg KOH per g oil,respectively.The high SV specifies the existence of adequate saponifiable content which can be converted to biodiesel by esterification or transesterification.The FFA content indicates the presence of a significant amount of FFA,due to which,it was necessary to perform the esterification step.High FFA content in poppy seed oil leads to soap formation and diminishes the biodiesel yield if a base catalyst is used for conversion of such oils[17].The gas chromatography revealed the fatty acid pro file of the poppy oil which was as follows:linoleic acid 72.25%,followed by oleic-,palmitic-,stearic-and linolenic acids:16.15%,9.25%,3.10%and 1.20%,respectively.The acid value of the poppy seed oil was 6.02 mg of KOH per g oil,due to which,pretreatment was conducted via the esterification reaction followed by the base-catalyzed transesterification.
3.2.Biodiesel synthesis using poppy seed oil
The influence of four reaction variables,i.e.,the molar ratio of the methanol to poppy seed oil,amount of catalyst,reaction temperature and reaction time,on the yield of the poppy seed oil methyl esters(PSOMEs)via the transesterification reaction were evaluated at fixed 720 r·min-1.Response surface methodology(RSM)was applied to the predetermined experimental design points using the above four reaction variables.The experimental procedure was based on the central composite rotatable design(CCRD)template as shown in Table 1.Thirty experiments were suggested by the software and the corresponding response of each run was reported as experimental yield;whereas,it was also compared with the predicted yield(Table 1).Experimental run 5 showed the highest yield(89.1%)at the 7.5:1 molar ratio of methanol/poppy oil,0.75%catalyst concentration,45°C reaction temperature,and 90 min reaction time.However,experimental run 2 depicted the lowest yield(80%)at the 9.75:1 molar ratio of methanol/poppy oil,1%catalyst concentration,55°C reaction temperature,and 37.50 min reaction time among thirty runs.The maximum PSOME yield was greater than the rice bran oil methyl esters,84.01%[16].Although,the average yield of the PSOMEs was calculated as 85.07%.

Table 1Experimental and predicted yield of the poppy seed oil methyl esters(PSOMEs)using CCRD experimental design

Table 2Analysis of variance(ANOVA)for response surface quadratic model
The second degree polynomial model for the synthesis of the PSOMEs phase was regressed and was attained in terms of original factors,using the designed experimental data(Table 2).The equation below was used to determine the yield of the esters:

whereYis the response variable,which is,the conversion to biodiesel,andX1,X2,X3,andX4are the actual values of the predictors,explicitly,the molar ratio,catalyst concentration,reaction temperature,and reaction time,respectively.
The analysis of the variance(ANOVA)for righting the second-order response surface model by least-squares methods are presented in Table 2.The high significance of the fitted model was indicated by the high value for the model,Fvalue=173.83 with a very low probability value for the model(p<0.0001).Whereas,non-significant lack of fit(Fvalue=2.69;p<0.1429)tests also suggested that quadratic models were well fitted.The variables,i.e.,the molar ratio of the methanol to poppy oil,catalyst concentration,reaction temperature,and reaction time,showedp<0.0001 which indicates that all the variables affect the PSOME yield.At the same time,the low value of the coefficient of variation(C.V.=0.31%)indicates that the results of the fitted model were consistent.The high value ofR2(0.9939;Table 2)also indicates that the fitted model could be used for prediction with reasonable precision,and the adjustedR2value was 0.98881.
The statistical significance of the individual parameters(molar ratio of the methanol to poppy oil,catalyst concentration,reaction temperature,and reaction time)of the fitted model for the response(conversion to esters,wt%)was evaluated by theFvalue and its correspondingpvalues,which were tabulated in Table 2.The smaller thepvalue and the greater theFvalue for the experimental parameter,the higher the significance of the parameter;hence,thepvalue reflects the relative importance of the term attached to that parameter[16].Among all the first order terms,the reaction time(F=18.11;p<0.001)was the variable that showed a more significant effect on the PSOME yield followed by the molarratio of methanol/oil(F=14.81;p<0.0001),catalyst concentration(F=6.25;p<0.0001)and reaction temperature fit(F=4.91;p<0.0001)as depicted in Table 2.Thepvalues were used as a tool to check the significance of each coefficient,indicating the interaction strength of each cross product[19].Among the first order interaction terms,molar ratio×temperature(AC)and catalyst concentration×reaction time(BD),were ascertained to be significant for the quadratic model as presented in Fig.1a and 1b,respectively;whereas,reaction temperature×time(CD)showed the least interaction(Fig.1d).The molar ratio(A2),catalyst concentration(B2),and reaction temperature(C2)were significant quadratic terms withp<0.0001 values;while,only reaction time(D2)was a non-significant quadratic term with thepvalue of 0.7870(Table 2).
3.3.Con firming the statistical model
The statistical model was verified for its precision by analyzing the residual graphs.Residuals are the deviations between the actual and predicted values of the response(PSOMEs).Fig.2(a)depicted the residuals against the predicted value explaining the lack of need fort rans formation.The random scattering of the points was observed in this case,instead of the funnel-shaped pattern sometimes observed,and this clearly suggested that the variance of the original observations is a constant for all the values for the response of the biodiesel production yield.Fig.2(a)did not reveal any obvious problem indicating the satisfactory assumption of the homogeneity of the variance.Fig.2(b)represented that the values of the output predicted from the model were in agreement with the observed values in the range of the operating variables which also showed that the majority of the points were situated in the range of 80%to 89.1%.A straight line has been coincided in the normal plot of the residual which represents a good conformity,as presented in Fig.2(b).The normal probability plot indicates that the data is almost normal(Fig.2(c)).It also shows the distribution between the predicted versus the experimental studentized residuals;whereas,the normal distribution of the studentized residuals regarding the S shape curve was not formed.Fig.2(d)shows the residual plot versus the run orders.The random pattern of the residuals would indicate the model adequacy.The out liertplot for all the runs of the biodiesel yield is shown in Fig.2(d).Most of the standard residuals should lie in the interval of±3.00.Any observation outside this interval renders an operational error in the experimental data or a potential error in the model[20].In Fig.2(d),there is no data beyond the interval±2,which reveals that the fitted model is consistent with all the data with no data recording an error.
3.4.influence of the operating parameters on the PSOME's yield

Fig.1.Interaction parameters.
Fig.2.(a)Studentized residuals and predicted biodiesel yield plot(b)Plot of predicted versus actual values for biodiesel production yield(c)Normal probability plotofresiduals(d)out lier t plot of residuals versus experimental runs.
The response surface plots of the PSOME yield acquired through Eq.2 at the center point of the CCRD are appraised in Fig.3(a,b,c,d,e,f).Fig.3(a)depicts the response surface plot of the PSOME yield for the methanol to molar ratio and catalyst concentration.Fig.3(a)displays that at a low concentration of the catalyst(NaOCH3),the yield of the PSOMEs decreased with a rise in the amount of methanol near its medium value and then decreased abruptly.The excess methanol can drive the forward reaction and synthesize more PSOME yield,the higher amount of methanol makes the PSOMEsseparation difficult,due to an increase in the glycerol solubility of the ester phase[3].At a low molar ratio,variations of the PSOME yield with an increase in the catalyst concentration are small.An increasing rate in the PSOME yield can be noticed at other molar ratio values with an increase in the catalyst concentration values.The increasing rate of the PSOME yield is very favorable at the medium molar ratio values.
The effects of the temperature and molar ratio on the PSOME yield have been shown in Fig.3(b).It was observed that at a low level of the catalyst concentration,the PSOME yield decreased with the drop in the molar ratio.The PSOME yield increased drastic changes with an increase in the molar ratio and temperature values.In fact,the biodiesel yield was enhanced by increasing the C values in all the temperature ranges.The same results were obtained by Rashidet al.[18]for the optimization of the FAME production from muskmelon using NaOCH3as the catalyst.Fig.3(c)illustrates the influence of reaction time and molar ratio on the PSOME yield.It was depicted that at low levels of the molar ratio,the PSOME yield decreased with a reduction in the reaction time.It also indicates that the PSOME yield surged with an increase in the reaction time and decrease in the molar ratio(Fig.3(c)).The surface plots of the reaction time and molar ratio parameters at the high and medium values showed almost the same behavior.The same results were obtained by Bautistaet al.[21]for the optimization of the FAME production from WCO using a solid acid catalyst.

Fig.3.3D response surfaces.
Fig.3(d)represents the influences of the catalyst concentration and temperature on the PSOME yield.At low temperature values,the PSOME yield increased gradually with the rising temperature;while,at the high catalyst concentration values,the PSOME yield changed in the opposite direction.From low to medium temperatures,the PSOME yield initially increased with the increase in the catalyst concentration and then declined after 0.9%.At a high temperature,a decrease in the PSOME yield can be observed with an increase in the catalyst concentration;while,the maximum yield was obtained at approximately 0.75%.
The same results were obtained by Bautistaetal.[21]for the optimization of the FAME production from WCOusing KOHas the catalyst.The PSOME yield increased with an increase in the reaction time and catalyst concentration as shown in Fig.3(e).At a low catalyst concentration,the PSOME yield decreased with an increasing reaction time.The maximum PSOME yield was thus attained for a higher reaction time and within the higher catalyst concentration values.Reaction time is the most significant factor which has a positive influence on the PSOME yield.The yield of the PSOMEs decreased with the increase of the temperature(Fig.3(f)).It is depicted from Fig.3(f)that the PSOME yield raised with an increase in the reaction time.at the low reaction temperature,increasing the reaction time would lead to an increase in the PSOME yield;while,at the higher temperatures,the medium temperature value is effective for achieving the higher PSOME yield.
The highest PSOME yield of89.39%was predicted from the CCRDdesign under the following conditions:molar ratio of 7.5:1,catalyst concentration of 0.75%,reaction temperature of 45°C,and reaction timeof 90 min.The experimental yield of 89.20%of PSOMEs was also observed with the above optimized parameters which were quite close to the predicted value.
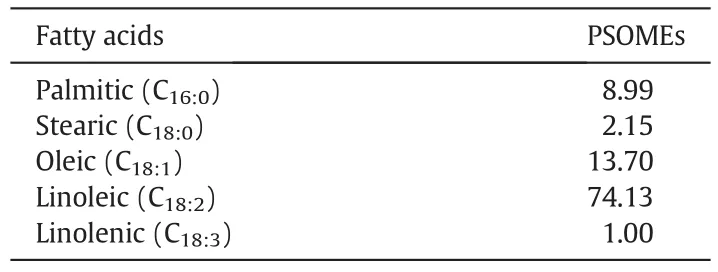
Table 3Fatty ester pro file of the poppy seed oil methyl esters(PSOMEs)
3.5.Quality of the PSOME analysis
The fatty ester pro file of the poppy seed oil used here as determined by GC is specified in Table 3.As indicated by Table 4,linoleic acid(C18:2),70.25%;oleic acid(C18:1),9.25%;palmitic acid(C16:0),9.25%;stearic acid(C18:0),3.10%and linolenic acid(C18:3),1.20%was found to be the major fatty acids in the PSOMEs.Also,considerable is the high content of linoleic(C18:1)acid in the PSOMEs compared to othermore conventional oil-seed crops;whereas,the C18:1contents were higher than the soybean oil methyl esters[18].Poppy oil also contains low amounts of saturated fatty acid methyl esters(11.4%).The contents of the unsaturated fatty acids were comparable to the rapeseed(canola),soybean and sun flower but higher than the palm oil.
The FTIR analysis was appraised to monitor the completion of the transesterification reactions of poppy seed oil.The existence of the IR band at 1435 cm-1for CH3asymmetric bending,which is the methyl ester with its deformation vibration,and 1169 for O-CH3stretching were recorded;whereas,these IR bands were not present in the parent poppy seed oil as shown in Fig.4.The band at 1377 cm-1for the O-CH2groups present in glycerol was found in the IR spectrum of the poppy seed oil;while,in the PSOMEs spectrum,this band was absent.Additionally,the regions of 1700-1800 cm-1for the C-H stretching existed in both the poppy seed oil and the PSOMEs IR spectra.These findings are in agreement with previous published works[22,23].
3.6.Fuel properties of the PSOMEs
Important fuel characteristics of the optimized PSOMEs were evaluated according to the biodiesel standards ASTM D 6751 and EN 14214.

Table 4Fuel properties of the poppy seed oil methyl esters(PSOMEs)
The cetane number(CN)is the property which indicates the ignition quality of diesel[3].In the current study,the cetane number of the PSOMEs was determined as 58,which fulfilled both the ASTM D 6751 and EN 14214 standards.Gerpenet al.[24]stated that if the CN of the diesel fuel is too high,combustion can occur before the fuel and air are well mixed,which results in the incomplete combustion of the fuel,and smoke.Also,if a CN is too low,incomplete combustion occurs[22].The kinematic viscosity(KV)demonstrates the resistance of a liquid to flow caused by an internal friction of one layer of fluid moving over the other.Usually,it affects the fuel atomization when injected into the combustion chamber and results in engine deposits.The previous study depicts that the higher the viscosity,the greater the tendency of the fuel to cause such problems[24].The present study showed that PSOMEs have a higher KV(4.12 mm2·s-1)value than diesel fuel(2.65 mm2·s-1).However,there was no problem to comply with both the ASTM D 6751 and EN 14214 standards.The oxidative stability(OS)of the PSOMEs was determined by the Rancimat method EN 14112,which used 3 g of the sample per test.An oxidation stability induction(OSI)time for the PSOMEs obtained 2.08 h.The PSOME value was below the minimum times specified in ASTM D 6751(≥3 h)and EN 14214(6 h).The produced PSOMEs in this study were found to be of poor oxidative stability.The oxidative stability of the methyl esters was reduced compared to the poppy seed oil,probably a result of the loss of antioxidants during the transesterification[25].Treatment with antioxidant additives may restore the oxidative stability of the esters to an acceptable level.The cold flow properties,i.e.,cloud point(CP)and pour point(PP)are the important biodiesel characteristics.The cloud point is that point at which the first appearance of clouds in the fluid sample is observed.The temperature at which crystal formation is extensive enough to prevent free pouring of the fluid is measured by its pour point.The values of the cloud and pour points for the PSOMEs showed-8 and-18°C,respectively(Table 3).The cold flow values were considerably low compared with other biodiesel fuels,such as palm oil biodiesel,cotton seed oil biodiesel,peanut oil biodiesel,rice bran oil biodiesel[17,18,25].The flash point(FP)is the temperature at which the fuel will give off enough vapors for the production of a flammable mixture and is very important information for the storage and safe transportation of the fuel.The PSOMEs showed a flash point of 175°C which is acceptable according to both ASTM D 6751 and EN 14214.The PSOMEs analysis also showed that the sulfur content(0.014%),ash content(0.015%),density at 15°C(886 kg·m-3),acid value(0.40 mg KOH·g-1)and copper strip corrosion(1a at 50°C for 3 h)agreed with both the ASTM D 6751 and EN 14214 standards.
4.Conclusions
The crude poppy oil was synthesized into the PSOMEs/biodiesel using sodium methoxide for the transesterification reaction with the prior acid pre-treatment step.The optimum reaction conditions were opted for by using the statistical technique known as response surface methodology(RSM).The effects of the reaction variables,i.e.,methanol/oilmolarratio,catalystconcentration,reaction temperature,and reaction time were determined by RSM.The maximum conversion(89.35%)of the PSOMEs/biodiesel was gained at the 7.5:1 methanolto-oil ratio,0.75%catalyst concentration,45°C reaction temperature,and 90 min reaction time at a fixed 600 rpm agitation speed.The molar ratio and temperature were found to be more interactive parameters which affected the final yield.Validation experiments were also carried out to verify the accuracy of the model,and the result showed that the predicted value agreed well with the experimental values.The significant properties of the PSOMEs/biodiesel were determined and compared with the ASTM D 6751 and EN 1424 specifications.It was found that most of the properties were within the acceptable range of the ASTM D 6751 and EN 1424 standards.
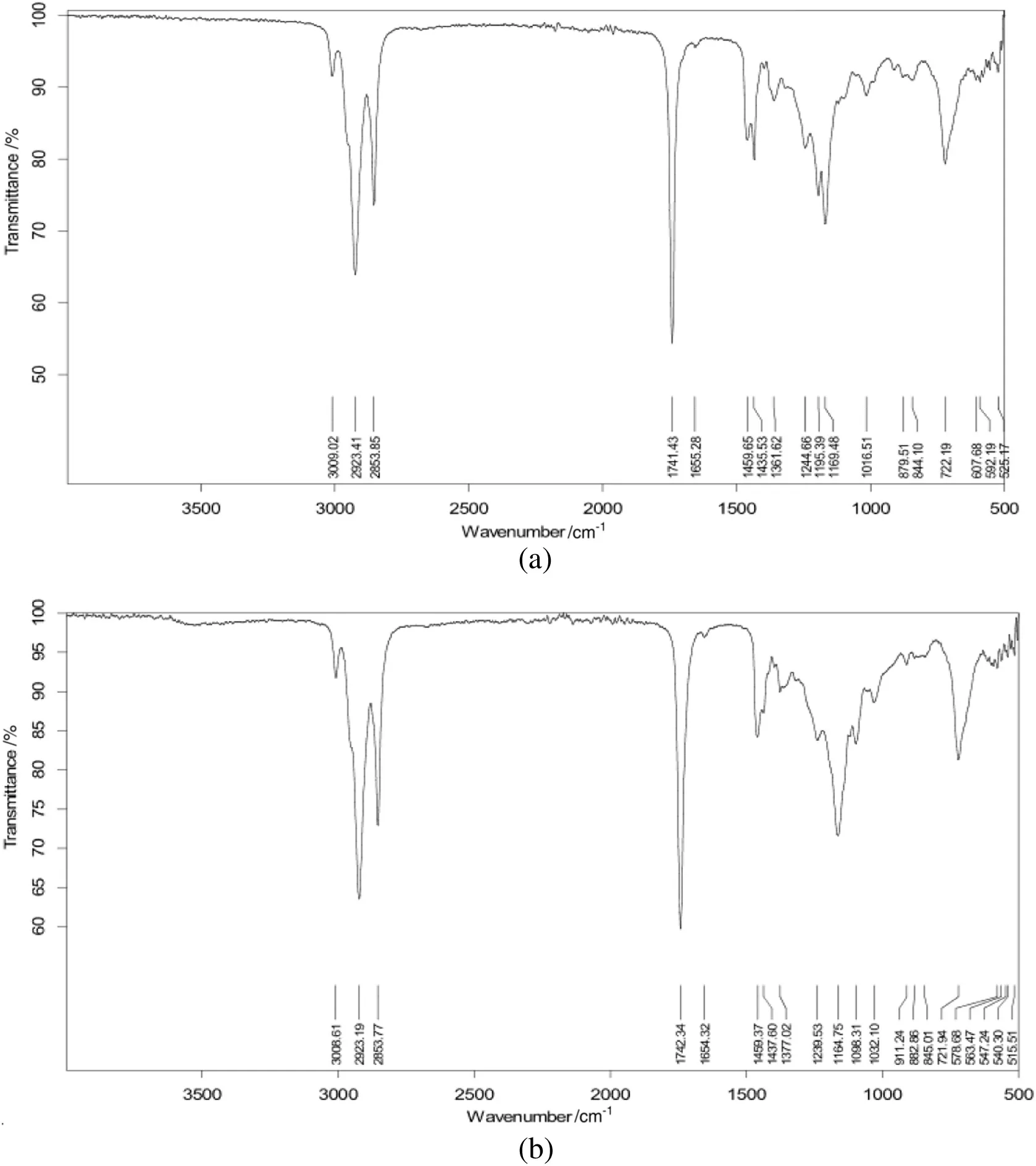
Fig.4.(a)FTIR spectra of the poppy seed oil(b)FTIR spectra of poppy seed oil methyl esters(PSOMEs).
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the research group project RGP-VPP-048.
[1]S.S.Gill,M.A.Mehmood,U.Rashid,M.Ibrahim,M.Saqib,M.R.Tabassum,Wastewater treatment coupled with biodiesel production using microalgae:A biore finery approach,Pak.J.Life Soc.Sci.11(2013)179-189.
[2]A.Saqib,M.R.Tabbssum,U.Rashid,M.Ibrahim,S.S.Gill,M.A.Mehmood,Marine macro algaeUlva:A potential feed-stock for bio-ethanol and biogas production,Asian J.Agri.Biol.1(2013)155-163.
[3]U.Rashid,F.Anwar,Production of biodiesel through optimized alkaline-catalyzed transesterification of rapeseed oil,Fuel87(2008)265-273.
[4]M.Sayadi,S.Ghatnekar,M.Kavian,Algae a promising alternative for biofuel,Proc.Int.Acad.Ecol.Environ.Sci.1(2011)112-124.
[5]D.M.Jose,R.E.Raj,B.D.Prasad,Z.R.Kennedy,A.M.Ibrahim,A multi-variant approach to optimize process parameters for biodiesel extraction from rubber seed oil,Appl.Energy88(2011)20562-22063.
[6]Y.Sharma,B.Singh,Development of biodiesel:current scenario,Renew.Sustain.Energy Rev.13(2009)1646-1651.
[7]M.M.Gui,K.T.Lee,S.Bhatia,Feasibility of edible oil vs.non-edible oil vs.waste edible oil as biodiesel feedstock,Energy33(2008)1646-1653.
[8]I.Atadashi,M.Aroua,A.A.Aziz,High quality biodiesel and its diesel engine application:A review,Renew.Sustain.Energy Rev.14(2010)1999-2008.
[9]F.Anwar,U.Rashid,M.Ashraf,M.Nadeem,Okra(Hibiscus esculentus)seed oil for biodiesel production,Appl.Energy87(2010)779-785.
[10]S.Singh,S.Shukla,K.Khanna,B.Dixit,R.Banerji,Variation of major fatty acids in F8 generation of opium poppy(Papaver somniferum×Papaver setigerum)genotypes,J.Sci.Food Agric.76(1998)168-172.
[11]E.W.Eckey,Vegetable fats and oils,Soil Sci.78(1954)83.
[12]S.Singh,K.Khanna,B.Dixit,S.Srivastava,Fatty acid composition of opium poppy(Papaver somniferum)seed oil,Ind.J.Agri.Sci.60(1990)358-359.
[13]B.Bozan,F.Temelli,Extraction of poppy seed oil using supercritical CO2,J.Food Sci.68(2003)422-426.
[14]P.Larkin,Biodiesel from Afghanistan poppies,Proposal from CSIRO Plant Industry,Canberra2007.
[15]P.Prochazka,L.Smutka,Czech Republic as an important producer of poppy seed,AGRIS on-line Papers Econ Informatics,22012 1-13.
[16]AOCS,Of ficial methods and recommended practices of the American Oil Chemists'Society(AOCS)Champaign,IL,US,1989.
[17]U.Rashid,F.Anwar,T.M.Ansari,M.Arif,M.Ahmad,Optimization of alkaline transesterification of rice bran oil for biodiesel production using response surface methodology,J.Chem.Technol.Biotechnol.84(2009)1364-1370.
[18]U.Rashid,H.A.Rehman,I.Hussain,M.Ibrahim,M.S.Haider,Muskmelon(Cucumis melo)seed oil:A potential non-food oil source for biodiesel production,Energy36(2011)5632-5639.
[19]G.A.Seber,A.J.Lee,Linear regression analysis,John Wiley&Sons,2012.
[20]I.Noshadi,N.Amin,R.S.Parnas,Continuous production of biodiesel from waste cooking oil in a reactive distillation column catalyzed by solid heteropolyacid:Optimization using response surface methodology(RSM),Fuel94(2012)156-164.
[21]L.F.Bautista,G.Vicente,R.Rodriguez,M.Pacheco,Optimisation of FAME production from waste cooking oil for biodiesel use,Biomass Bioenergy33(2009)862-872.
[22]I.A.Nehdi,H.Sbihi,C.P.Tan,S.I.Al-Resayes,Garden cress(Lepidium sativumLinn.)seed oil as a potential feedstock for biodiesel production,Bioresour.Technol.126(2012)193-197.
[23]M.A.Dube,S.Zheng,D.D.McLean,M.Kates,A comparison of attenuated total reflectance-FTIR spectroscopy and GPC for monitoring biodiesel production,J.Am.Oil Chem.Soc.81(2004)599-603.
[24]J.V.Gerpen,B.Shanks,R.Pruszko,D.Clements,G.Knothe,Biodiesel analytical methods,National Renew Ener Lab,Colorado,2004 37-47.
[25]B.R.Moser,M.J.Haas,J.K.Winkler,M.A.Jackson,S.Z.Erhan,G.R.List,Evaluation of partially hydrogenated methyl esters of soybean oil as biodiesel,Eur.J.Lipid Sci.Technol.109(2007)17-24.
 Chinese Journal of Chemical Engineering2016年8期
Chinese Journal of Chemical Engineering2016年8期
- Chinese Journal of Chemical Engineering的其它文章
- Computational chemical engineering - Towards thorough understanding and precise application☆
- A review of control loop monitoring and diagnosis:Prospects of controller maintenance in big data era☆
- Experimental and numerical investigations of scale-up effects on the hydrodynamics of slurry bubble columns☆
- The heat transfer optimization of conical fin by shape modification
- The steady-state and dynamic simulation of cascade distillation system for the production of oxygen-18 isotope from water☆
- Experimental mass transfer coefficients in a pilot plant multistage column extractor
